Abstract
The last step of sulfur assimilation is catalyzed by O-acetylserine(thiol)lyase (OASTL) enzymes. OASTLs are encoded by a multigene family in the model plant Arabidopsis thaliana. Cytosolic OASA1 enzyme is the main source of OASTL activity and thus crucial for cysteine homeostasis. We found that nitrating conditions after exposure to peroxynitrite strongly inhibited OASTL activity. Among OASTLs, OASA1 was markedly sensitive to nitration as demonstrated by the comparative analysis of OASTL activity in nitrated crude protein extracts from wild type and different oastl mutants. Furthermore, nitration assays on purified recombinant OASA1 protein led to 90% reduction of the activity due to inhibition of the enzyme, as no degradation of the protein occurred under these conditions. The reduced activity was due to nitration of the protein because selective scavenging of peroxynitrite with epicatechin impaired OASA1 nitration and the concomitant inhibition of OASTL activity. Inhibition of OASA1 activity upon nitration correlated with the identification of a modified OASA1 protein containing 3-nitroTyr302 residue. The essential role of the Tyr302 residue for the catalytic activity was further demonstrated by the loss of OASTL activity of a Y302A-mutated version of OASA1. Inhibition caused by Tyr302 nitration on OASA1 activity seems to be due to a drastically reduced O-acetylserine substrate binding to the nitrated protein, and also to reduced stabilization of the pyridoxal-5′-phosphate cofactor through hydrogen bonds. This is the first report identifying a Tyr nitration site of a plant protein with functional effect and the first post-translational modification identified in OASA1 enzyme.
Keywords: Arabidopsis, Enzyme Catalysis, Enzyme Inactivation, Enzyme Structure, Mass Spectrometry (MS), Nitric Oxide, Oxidative Stress, Plant, Protein Chemical Modification, Tyrosine Nitration
Introduction
Sulfur is an essential nutrient for all living organisms as it is a component of the amino acids cysteine and methionine required for protein synthesis. Moreover, a major determinant in plant cellular redox control such as glutathione (GSH) also contained sulfur. Most plant sulfur-containing compounds, including GSH, are derived from Cys, which is the final product of the primary sulfate assimilation pathway. The Cys biosynthetic pathway involves two sequential reactions catalyzed by Ser acetyltransferase (SAT),4 which synthesizes the intermediary product, O-acetyl-Ser (OAS), from acetyl-CoA and Ser, and O-acetyl-Ser(thiol)lyase (OASTL), which incorporates sulfide, coming from the assimilatory reduction of sulfate, to OAS producing Cys. This reaction requires pyridoxal phosphate (PLP) as cofactor. There are nine genes coding for different isoforms of OASTL in the Arabidopsis genome (1). The most abundant OASTL transcripts correspond to the cytosolic OASA1, the plastidial OASB, and the mitochondrial OASC isoforms. Analysis of null alleles of different SAT and OASTL genes together with subcellular metabolite distributions in A. thaliana have recently shown that cysteine is predominantly formed in the cytosol, while OAS is produced in the mitochondria (2–6). The major cytosolic OASTL isoform and main responsible for cysteine biosynthesis, OASA1, is essential for heavy metal tolerance as its overexpression is sufficient to confer tolerance to elevated cadmium concentrations (7–9). By contrast, the mutant oasa1.1 shows sensitivity to heavy metals but it is due to a constitutively reduced capacity to eliminate reactive oxygen species (ROS) under non-stressed conditions (9).
The uptake and assimilation of sulfate is strongly regulated by diverse regulatory mechanisms (for a recent review, see Ref. 10). Some components of the pathway are specifically regulated at the transcriptional level in plants, mainly the sulfate uptake and the reduction of 5′-adenylylsulfate (APS). It has been characterized that OASA1 is regulated at the transcriptional level in different abiotic stresses such as salinity and the presence of heavy metal (7, 11). Besides, SAT and OASTL form the hetero-oligomeric cysteine synthase complex in such a way that SAT requires binding to OASTL for full activity, while bound OASTL becomes inactivated (12). It has been proposed this complex acting as a sensor of the sulfur status of the plant. Moreover, the activity of the cysteine synthase complex is also regulated at the level of the rate-limiting step catalyzed by SAT through cysteine-mediated inhibition of this enzyme, although depending on subcellular localization and plant species (13). Because cysteine biosynthesis requires the reduced sulfur in form of sulfide, which is exclusively produced through sulfate assimilatory pathway in plastids (14), the mitochondria provide the bulk of OAS (2), and the main site for Cys production is cytosol in Arabidopsis, an exchange of sulfide and OAS between subcellular compartments must be important in controlling the function of the enzyme components of the cysteine synthase complex.
Post-translational modification represents an increasingly interesting level of regulation of protein function in all living organisms. Among more than hundred different post-translational modifications characterized, those mediated by the action of nitric oxide (NO)-derived modifiers have attracted lately the attention of plant biology researchers. The most important post-translational modifications related to NO action are S-nitrosylation of Cys (15) and nitration of Tyr residues (16). Nitration of Tyr residues under physiological conditions is mostly the result of protein interaction with the strong nitrating agent peroxynitrite, which is formed by the reaction of NO with superoxide anion (17). There is a second alternative mechanism of tyrosine nitration based on the generation of NO2• radicals by various hemoperoxydases in the presence of hydrogen peroxide and nitrite (18). Whatsoever, the nitration of tyrosine residues is a selective process with respect to the proteins that are nitrated and the affected tyrosine residues in a given protein (19, 20).
Despite the well documented regulation of OASTL and SAT function at the protein-protein interaction level, to our knowledge nothing has been reported about post-translational regulation of those proteins. Here we address whether post-translational modification of OASA1 may modulate its activity. Peroxynitrite-mediated nitration of crude extracts and purified recombinant protein as well as plant treatments with peroxynitrite inhibited OASTL activity through nitration of tyrosine residues. Mass spectrometry analysis of nitrated recombinant OASA1 protein allowed the identification of nitrated Tyr302, a catalytically essential amino acid residue that is close to the previously reported key Asn77 in O-acetylserine binding site of OASA1 (21).
EXPERIMENTAL PROCEDURES
Plant Growth Conditions and Treatments
A. thaliana seeds of Col-0 wild type or different oastl mutants (9) were sown in moistened soil and grown under photoperiodic conditions (cycles of 16 h day and 8 h night for long days, at 22 °C and 20 °C). Plants were illuminated with 150 μE m−2 s−1 cool-white fluorescent lamps and grown in 60% relative humidity. Alternatively, surface sterilized seeds were germinated and grown in agar-supplemented Murashige and Skoog (MS) medium (Duchefa, Haarlem, The Netherlands).
Expression, Extraction, and Purification of Recombinant His-tagged OASA1
The complete cDNA of OASA1 was cloned in pDEST17 vector (Invitrogen) to express a hexahistidine-tagged version of OASA1 by transformation of BL21-AI Escherichia coli competent cells (Invitrogen). Site-directed mutagenesis to generate Y203A and Y302A versions of OASA1 was performed as previously reported (22) with slight modifications. For protein induction, cell cultures with an OD of 0.4 were treated with 0.1% l-arabinose overnight at 22°. Recombinant protein production was checked by SDS-PAGE and Western blot analysis. Recombinant protein purification was carried out with the Ni-NTA purification system (Invitrogen) following manufacturers recommendations. His-tagged proteins were detected by Western blot with a polyclonal anti-His6 antibody (Santa Cruz Biotechnology).
Protein Extraction, Immunoprecipitation, and Nitrating Treatments
For activity assays, around 100 mg of Col-0 and oastl mutant leaves were frozen and ground in liquid nitrogen and then extracted by adding extraction buffer (50 mm phosphate buffer, pH 7.5, 1 mm EDTA, 10 μm PLP, 0.5 mm PMSF, 1% (v/v) protease inhibitor mixture from Sigma) with or without 1 mm DTT, as described in each case, and briefly vortexing. Protein extracts were obtained by 13,000 × g centrifugation at 4 °C. For immunoprecipitation purposes, proteins were extracted in (10 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% (v/v) protease inhibitor mixture from Sigma) buffer. Protein extracts (1 mg) were precleared with 50 μl of protein A-agarose (EZView Sigma) for 8 h at 4 °C. The unbound fractions were incubated overnight with 0.1 μg of monoclonal anti-3-NY antibody (Cayman) at 4 °C. To recover 3-NY-containing proteins, 60 μl of protein A-agarose were added and incubated for 8 h at 4 °C. After extensive washing with extraction buffer, proteins were eluted at 95 °C with elution buffer (1% SDS, 100 mm DTT, 50 mm Tris-HCl, pH 7.6) three times. Protein was quantified by Bradford′s method (23).
Nitrating treatments on crude extracts or purified recombinant protein was performed by treatment with peroxynitrite generated from sodium nitrite plus hydrogen peroxide, or 3-morpholinosydnonimine hydrochloride (SIN-1; Invitrogen) at the indicated concentrations, as previously reported (24, 25). In planta peroxynitrite treatments were performed by infiltrating leaves with SIN-1. Selective scavenging of peroxynitrite with epicatechin (26) was used to assess the specificity of protein nitration and not oxidation and the subsequent inhibition of OASTL activity.
Measurement of OASTL Activity
OASTL activity was measured using the method described previously (27) in protein extracts obtained as described above. Cysteine was determined by measuring optical density at 560 nm after the formation of a complex with nynhydrin (28).
Western Blots
Protein extracts were separated by 10% SDS-PAGE, blotted onto nitrocellulose membrane, stained with Ponceau-S and probed with antibodies at the followed dilutions: monoclonal anti-3-NY (Cayman Chemicals) 1:1000, anti-His (Santa Cruz Biotechnology) 1:500, and custom-made polyclonal anti-recombinant OAS-A1 antibody (Biomedal S.L.) 1:10000. Secondary antibody was anti-mouse or anti-rabbit, for monoclonal or polyclonal primary antibodies, respectively, coupled to horseradish peroxidase (GE) at 1:10000 dilution, and ECL or ECL advance kit (GE) was used for immunoreactive protein detection.
MS Analysis
Samples were digested with sequencing grade trypsin, chymotrypsin, or the glutamic acid specific V8 protease (Promega) to improve peptide sequence coverage. Peptide separation by LC-MS/MS was performed using an Ultimate nano-LC system (LC Packings) and a QSTAR XL Q-TOF hybrid mass spectrometer (MDS Sciex-Applied Biosystems). Samples (5 μl) were delivered to the system using a FAMOS autosampler (LC Packings) at 40 μl min−1, and the peptides were trapped onto a PepMap C18 pre-column (5 mm, 300 m i.d; LC Packings). Peptides were then eluted onto the PepMap C18 analytical column (15 cm, 75 m i.d; LC Packings) at 200 nl min-1 and separated using a 55 min gradient of 15–50% ACN (120 min for the mixtures). The QSTAR XL was operated in information-dependent acquisition mode, in which a 1-s TOF MS scan from 400–2000 m/z, was performed, followed by 3-s product ion scans from 65–2000 m/z on the three most intense doubly or triply charged ions. Database search on Swiss-Prot and NCBInr databases was performed using MASCOT search engine (Matrix- Science). Searches were done with the different proteases specificity allowing one missed cleavage and a tolerance on the mass measurement of 100 ppm in MS mode and 0.8 Da for MS/MS ions. Carbamidomethylation of Cys was used as a fixed modification and oxidation of Met, deamidation of Asn and Gln, and nitration of Tyr as variable modifications.
Structural Analysis of OASA1
Three-dimensional structure of OASA1 (At4g14880) was obtained from the Protein Data Bank (PDB code access 1Z7W). The structure was visualized with Yasara or PyMol software. The distance between residues in Angstroms (Å) and the presence of hydrogen bonds were carried out with both programs with default settings.
RESULTS
Inhibition of OASTL Activity under Nitrating Conditions
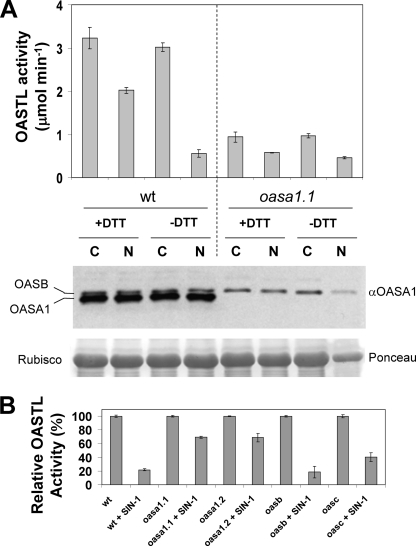
Nitration of many different cellular acceptors including tyrosine residues of proteins is the result of either peroxynitrite action or NO2• radicals attack on the corresponding targets. Peroxynitrite can be produced in situ by different donors including 3-morpholinosydnonimine (SIN-1). Similarly, NO2• radicals can be produced in vitro by treatment with nitrite and hydrogen peroxide. We have tested whether nitrating treatments altered the OASTL activity of crude protein extracts from Arabidopsis. Fig. 1A shows that nitration with 1 mm nitrite/peroxide treatment led to a reduction of 81% in OASTL activity levels in wild-type extracts without DTT, and to around 37% in the presence of DTT, commonly used in the extraction buffer for OASTL activity (7–9). OASTL activity associated to OASA1 represented around 65–70% of total activity present in wild-type Arabidopsis crude extracts, as oasa1.1 extracts had 30–35% of the activity present in wild-type plants (Fig. 1A) (9). Similar treatments on oasa1.1 extracts led to a reduction of OASTL activity of 38 and 53% with and without DTT, respectively (Fig. 1A). The reduction of OASTL activity upon nitration must be due to inactivation of the enzyme as no changes in the OASA1 protein levels could be detected in any of the described treatments (Fig. 1A). Moreover, crude extracts from wild type and mutants of the most abundant OASTLs were also nitrated with 0.5 mm SIN-1 in the absence of DTT. Nitrated wild type, oasb, and oasc mutant extracts retained 22, 19, and 40%, respectively, of the OASTL activity present in non-nitrated controls (Fig. 1B). However, when nitrated the two allele mutants oasa1.1 and oasa1.2, extracts retained 69% of the activity of its corresponding control (Fig. 1B). As shown with nitrite/peroxide-treated samples, SIN-1 treatment did not produce OASA1 protein degradation as tested by Western blot (data not shown). These data suggest that OASA1 is more sensitive to nitration-mediated inhibition than other OASTLs. Based on wild type and oasa1 allele activities upon nitration, SIN-1, and nitrite/peroxide treatments inhibited 37 and 47% of the OASTL activity catalyzed by non OASA1 proteins (Table 1). In turn, OASA1 activity was 88 and 95% inhibited by both nitrating treatments (Table 1). Differential effect of nitration in inhibiting activity of OASA1 and other OASTLs moved us to analyze the effects of nitrating treatment on purified recombinant OASA1.
FIGURE 1.
Effect of nitration on OASTL activity and OASA1 protein levels of wild type and mutant extracts. Total OASTL activity and OASA1 protein levels were analyzed in wild type and oasa1.1 extracts obtained either in the presence (+DTT) or absence of (−DTT), and either non-nitrated as control (C) or nitrated (N) by treatment with 1 mm nitrite/hydrogen peroxide mixture for 1 h. Anti-OASA1 antibody crossreacted preferentially with OASA1 but showed also a weaker crossreaction with OASB isoform. Ponceau S staining of Rubisco is shown as loading control. OASTL activities are the mean values of three replicates ± S.D. (A). OASTL activity in 0.5 mm SIN-1 treated extracts from wild type, oasa1.1, oas1.2, oasb, and oasc mutants was measured after 1 h. After SIN-1 removal, activity was measured along with non-treated samples as controls. Values are the mean ± S.D. of three replicates and are expressed relative to the corresponding non-nitrated control for every genotype (B).
TABLE 1.
Sensitivity of OASA1 and other OASTL enzymes to nitration-mediated inhibition
OASTL activity associated to OASA1 was estimated by the difference between the total activity measured in wild-type seedlings and that measured in oasa1.1 mutant. Nitration (N) was performed by treatments for 1 h with nitrite/peroxide or SIN-1, and no DTT as indicated. Untreated samples were used as controls (C). Values (nmol min−1) are the mean of three replicates ± standard deviation. Relative percentages of inhibition were calculated for OASA1 and the rest of OASTLs after nitration by both treatments.
| wt C | oasa1.1 C | OASA1 C | Other OASTLs C | wt N | oasa1.1 N | OASA1 N | Other OASTLs N | Inhibition OASA1 | Inhibition other OASTLs | |
|---|---|---|---|---|---|---|---|---|---|---|
| % | % | |||||||||
| Nitrite/H2O2 | 3020 ± 99 | 970 ± 49 | 2050 | 970 | 559 ± 85 | 458 ± 22 | 101 | 458 | 95 | 47 |
| SIN-1 | 603 ± 3 | 231 ± 3 | 372 | 231 | 189 ± 25 | 145 ± 30 | 44 | 145 | 88 | 37 |
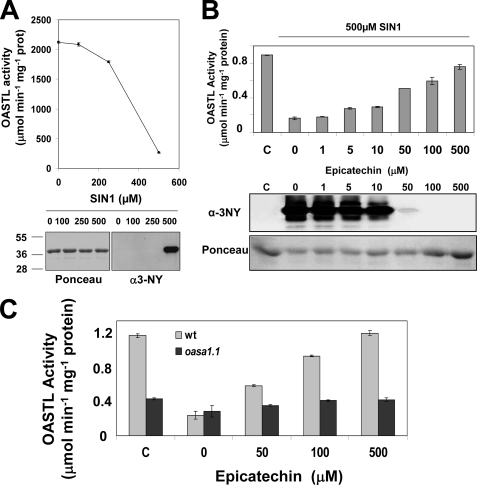
Samples of purified recombinant OASA1 obtained from E. coli expressing an N-terminal hexahistidine-tagged version were tested for OASTL activity after treatment with increasing concentrations of SIN-1. At 250 μm SIN-1 significant inhibition was already detected and 87% inhibition occurred at 500 μm (Fig. 2A). This percentage of inhibition is fully consistent with the estimation of inhibition of the OASTL activity corresponding to OASA1 detected in nitrated crude extracts (Table 1). Reduction of OASTL activity under these nitrating conditions was not due to degradation of protein as demonstrated by unaltered levels of proteins present in samples treated with increasing SIN-1 concentrations (Fig. 2B). Correlating with the strong inhibition of OASTL activity of OASA1 the corresponding Tyr-nitrated form of OASA1 was detected by Western blot using an anti-3-nitroY antibody (Fig. 2A). We checked that inhibition of OASA1 activity was specifically associated to nitration and not to oxidation by using the peroxynitrite scavenger epicatechin (26). Increasing concentrations of epicatechin blocked SIN-1 mediated OASA1 nitration and the concomitant inhibition of OASTL activity (Fig. 2B). Moreover, the protective effect of epicatechin specifically on OASA1 was supported by the negligible effect caused by epicatechin on crude extracts from the oasa1.1 mutant (Fig. 2C).
FIGURE 2.
OASTL activity and nitrated OASA1 protein levels in purified OASA1 protein treated with increasing concentrations of the nitrating reagent SIN-1. Samples of 12 μg of His-tagged purified recombinant OASA1 protein were treated with the indicated concentration of SIN-1 for 1 h. After SIN-1 removal, OASTL activity, and total and nitrated protein levels were quantified (A). Activity and protein levels in samples treated with 0.5 mm SIN-1 or untreated as a control (c) and epicatechin at the indicated concentrations (B). Western blot with anti-3nitroY antibody (α-3NY) and Ponceau staining as loading control are shown in panels A and B. Scavenging effect of epicatechin on the OASTL activity of SIN-1 (0.5 mm)-nitrated crude extracts from wild type (clear gray bars) and oasa1.1 (dark gray bars) leaves (C). OASTL activities are the mean values of three replicates ± S.D.
Identification of Tyr-nitration Site in Nitrated OASA1
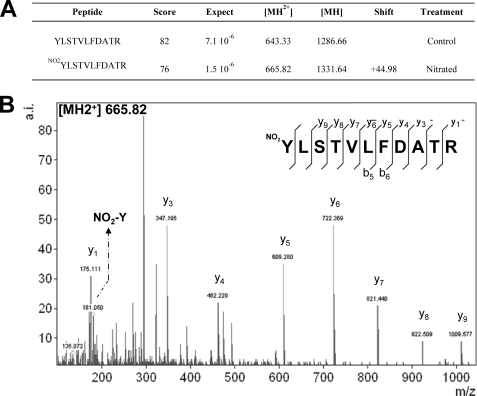
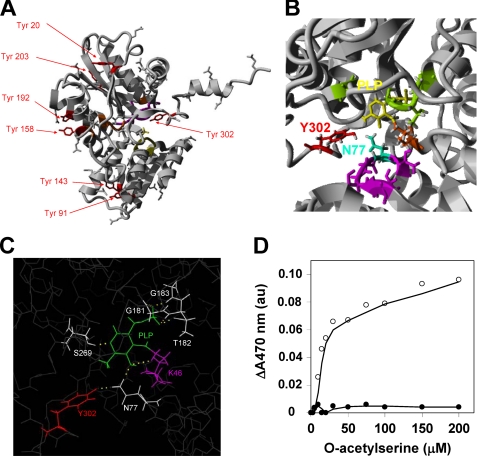
Nitrated and non-nitrated samples of purified OASA1 used for inhibition assays described above were further analyzed by mass spectrometry. Samples were digested with chymotrypsin or trypsin and analyzed by LC-MS/MS. Nitrated and non-nitrated samples were confirmed to be OASA1 with MASCOT scores (sequence coverages) of 725 (79%) and 1207 (88%) for trypsin-digested samples and 248 (24%) and 163 (40%) for chymotrypsin-digested samples, respectively. By LC-MS/MS, the nitrated peptide Y(NO2)LSTVLFDATR (Z = 2; m/z = 665.828096; MASCOT Ion Score: 76; Expect: 1.5e-006; Monoisotopic mass of neutral peptide: 1329.6565) was identified with an increase mass of 44.99 Da compatible with acquisition of a nitro group in Tyr302 (Fig. 3). OASA1 contains 7 Tyr residues distributed all along the amino acid sequence but most of them are located far away from the active site in the three-dimensional structure, except Tyr302, which is directed to the active site (Fig. 4A). Tyr302 is located at 4.7 Å from the Asn77 (Fig. 4B), and forming a hydrogen bond with this amino acid (Fig. 4C), which has been previously reported to be essential for the activity (21). Asn77 interacts with the O-acetylserine and SAT binding site and through hydrogen bond stabilizes pyridoxal phosphate (PLP) cofactor anchored by Lys46 (21). Changes in spatial conformation of PLP and substrate microenvironment sites may also alter the efficiency of substrate binding to the active site. Upon binding of O-acetylserine to the active site of the enzyme, the substrate reacts with PLP yielding the α-aminoacrylate intermediate with an absorbance maximum at 470 nm (21, 29). We measured the increase in absorbance at 470 nm of purified OASA1 in the presence of increasing concentrations of O-acetylserine and compared with the values obtained using the same amount of nitrated protein. The non-nitrated OASA1 increases its absorbance at 470 nm with increasing OAS concentration as expected. By contrast, no increase in absorbance was detected in nitrated OASA1 (Fig. 4D), suggesting that either binding of O-acetylserine is severely hindered by nitration of Tyr302 or binding occurs but far enough from the PLP site to avoid productive interaction between substrate and cofactor.
FIGURE 3.
Identification of a Tyr-nitration site in OASA1. LC-MS/MS analysis of nitrated OASA1 and non-nitrated control allowed the identification of Y*LSTVLFDATR peptide with nitrated or non-nitrated Tyr302, respectively (A). The 22.49 Da shift of the double charged peptide indicates nitration of Tyr302. Ion score (MASCOT) of the nitrated and non-nitrated peptides are shown. The MS spectra corresponding to Y(NO2)LSTVLFDATR (Z = 2; m/z = 665.82) is shown (B). NO2-Y indicates the presence of the nitrated tyrosine immonium ion mass in the LC-MS/MS spectra. Identified y and b ion series are pointed out.
FIGURE 4.
Three-dimensional model of OASA1 showing the position and potential interactions of Tyr residues. The conformation of OASA1 molecule showing the position of the seven Tyr residues (in red), the PLP binding site (in orange), O-acetylserine substrate binding site (in yellow) and SAT protein interaction site (in purple) are shown (A). Detail of the three-dimensional structure showing Tyr302 and the Asn77 residues at a 4.7 Å distance, the amino acid residues interacting with the Lys46 (in orange)-linked PLP (in yellow) through hydrogen bonds (in green), and the OAS and SAT binding site (in purple) (B). In silico analysis, using PyMol software, of the potential hydrogen bonds (yellow dashed line) between amino acid residues surrounding PLP (C). Spectrophotometric detection of the reaction of OASA1-bound O-acetylserine with PLP to form an α-aminoacrylate intermediate. Samples of 200 μg of non-nitrated (open circles) or nitrated (close circles) purified OASA1 were incubated with increasing concentrations of O-acetylserine as indicated, and the increase in the absorbance at 470 nm registered (D).
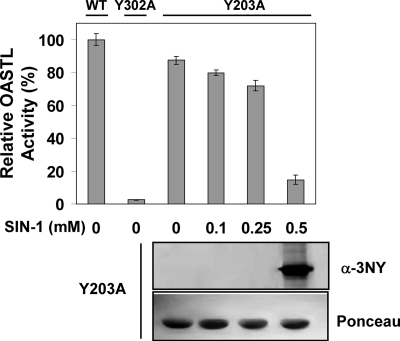
We have further confirmed the essential role of Tyr302 for OASTL activity of OASA1 by constructing a site-directed Y302A mutant version that retained less than 3% of the activity of the unmodified recombinant OASA1 protein (Fig. 5). This effect was specific for Tyr302 as another mutated version Y203A, with a mutation in a Tyr residue that was not identified above as a functional target of nitration, retained 88% of the activity and responded to SIN-1-nitrating treatment with nitration of the mutated protein and consequent inhibition of the activity (Fig. 5) similarly to that observed for unmodified OASA1 (Fig. 2A).
FIGURE 5.
OASTL activity of mutated recombinant versions of OASA1. Purified samples of WT and mutated (Y302A and Y203A) versions of OASA1 were assayed for OASTL activity in the absence or presence of the indicated concentrations of SIN-1. OASTL activities are the mean values of three replicates ± standard deviation. Western blot with anti-3nitroY antibody (α-3NY) and Ponceau staining as loading control (Ponceau) are shown for nitrated Y203A samples.
In Vivo Detection of Nitrated OASA1
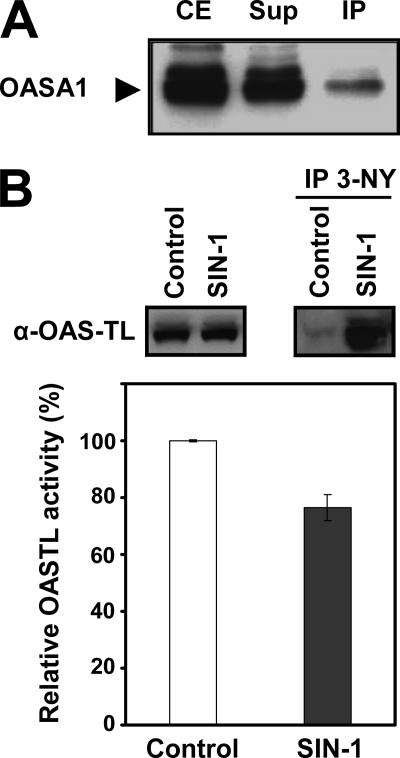
To test whether the nitration of Tyr detected in OASA1 in vitro could be physiologically relevant, we performed an immunoprecipitation assay of wild-type protein extracts with anti-3-nitroY antibody. The immunoprecipitated proteins were analyzed by Western blot with an antibody against OASA1. Fig. 6A shows that a small fraction of the OASA1 protein present in the crude extract was detected in the immunoprecipitated fraction, suggesting that OASA1 may be Tyr-nitrated under physiological conditions in the absence of an exogenous nitrating treatment. Despite efforts to identify in vivo Tyr-nitrated peptides we did not success, likely because of the low amounts of nitrated protein that are expected under physiological conditions. We then proceed to perform in planta nitration experiments by infiltrating leaves with SIN-1 and further protein extraction and OASTL activity analysis. Fig. 6B shows that by 90 min after treatment with 2 mm SIN-1, OASTL activity was 34% inhibited. Inhibition of activity correlated with detection of a significant fraction of nitrated OASA1 in SIN-1-treated leaf samples (Fig. 6B).
FIGURE 6.
Analysis of in vivo Tyr-nitration of OASA1. Crude extracts from wild-type A. thaliana seedlings were immunoprecipitated with anti-3-nitroY antibody, and the presence of OASA1 in crude extract (CE) input and the resulting supernatant (Sup) and immunoprecipitate (IP) was analyzed by Western blot with anti-OASA1 antibody (A). Leaves were infiltrated with 2 mm SIN-1 for 90 min and then crude protein extracts were prepared for OASTL activity measurement (bottom panel) and for immunoprecipitation with 3-nitroTyr (IP 3-NY) antibodies. Western blot with anti-OASA1 (α-OASA1) was performed with protein extracts (left up panel) or IP (right up panel). OASTL activity values are the mean values of three replicates ± standard deviation (B).
DISCUSSION
Cysteine biosynthesis is a crucial process because this amino acid is a constituent of proteins. Moreover, Cys is a precursor for a huge number of essential bio-molecules, such as many plant defense compounds formed in response to different environmental adverse conditions. Among them, glutathione is the most determinant molecule in controlling the redox state of the cells. The main site of cysteine biosynthesis is the cytosol in A. thaliana, and therefore the major contributor to this synthesis is OASA1, but in its unbound form, when it is fully active. Association and dissociation of the cysteine synthase complex appears to be a level of regulation of cysteine biosynthesis in response to sulfur nutrition perturbations (12). The gene coding for OASA1 is also transcriptionally regulated by stress conditions (7, 11). Sulfate assimilation has been proposed to be extensively regulated by abiotic stress at the post-transcriptional level acting on plant adenosine 5′-phosphosulfate reductase (APR) through a complex network of multiple signals (30). However, besides transcriptional control of OASTL genes and interaction with SAT, no other level of regulation has been reported for OASTL function. A rapid way to modulate the function of OASTL may be through post-translational modification of the protein. However, to date no such modification has been identified in plants. Many stress activated responses have in common the participation of redox processes. Under oxidative, nitrosative, or nitrative conditions plant proteins can undergo several modifications including oxidation of Met, Cys, or Trp residues, nitration of Tyr, and nitrosylation of Cys (31). The main physiological nitrating molecules are the radicals NO2• and ONOO−, which are in turn produced from nitric oxide by further oxidation (32). Although NO2• and ONOO− are extremely reactive, their interactions with proteins do not lead to stochastic nitration of Tyr residues of the target protein. Factors determining the selectivity of Tyr nitration in proteins include the exposure of the aromatic ring to the surface of the protein, the location of the Tyr on a loop structure, its association with a neighboring negative charge, the proximity of the proteins to the site of generation of nitrating agents, their abundance and the local environments of the Tyr residues (19, 33). Moreover, the physiological significance of a regulatory mechanism based on the differential effect of a post-translationally modified protein relies on the conservation of that residue in others proteins with the same activity. This work reports the negative effect of Tyr302 nitration on the OASTL activity of OASA1. This protein, which represents the main isoform in Arabidopsis, contains 7 Tyr residues spanning the whole primary sequence of the protein. Three-dimensional structure of OASA1 allowed the spatial location of different Tyr residues respect to the surface molecule and proximity to the catalytic site (Fig. 4). Table 2 summarizes the parameters defining the position and the microenvironment of every Tyr residue in OASA1 protein. Among seven Tyr residues of OASA1 only two, Tyr91 and Tyr302, are located in loops, are conserved in the family of OASTL Arabidopsis proteins (3) and have both negatively and positively charged amino acids close enough (Table 2) to fulfill theoretical requirements for being targets of nitration (19, 33). In our approach using purified recombinant OASA1 protein nitrated in vitro, only Tyr302 was identified as 3-nitroTyr (Fig. 3). The identification of Tyr302 in nitrated OASA1 that lost most of its OASTL activity is consistent with the location of this residue very close to the active site of the enzyme (Fig. 4). In fact, Tyr302 is only 4.7 Å from the Asn77, which is a key amino acid for the efficient binding of the substrate O-acetylserine to OASA1 (21). Moreover, Asn77 and other closely located amino acids including Ser269, Gly181, Gly183, Thr183, and Thr185 are all involved in stabilizing PLP through hydrogen bonds with the phosphate group of the cofactor (21). It is tempting to propose that 3-nitroY302 might introduce a perturbation in the microenvironment of PLP cofactor leading to a more restricted accessibility of the substrates to the catalytic site or to unproductive substrate binding. Productive O-acetylserine binding to OASA1 can be easily tested because the conversion to cysteine is mediated by the formation of an α-aminoacrylate intermediate with PLP that show a maximum of absorbance at 470 nm. An increase in A470 should be detected when appropriate O-acetylserine binding occurs and that is what we observed with purified OASA1 (Fig. 4C). However, a similar assay with the nitrated Tyr302-containing protein led to no increase in A470 (Fig. 4C), suggesting that modification of this amino acid either drastically hinder binding of the substrate to the enzyme, or the conformation of the active site has been altered enough to allow binding of the substrate but not close enough to PLP to form the α-aminoacrylate intermediate required to complete the transformation of O-acetylserine to cysteine. By contrast, Tyr91 is located in a surface loop far from the catalytic site of the enzyme (Fig. 3A) and thus, even if nitrated under certain conditions, less effect on catalytic activity should be expected. Because phosphorylation of Tyr is a post-translational modification with a big impact on general plant signaling (34), and particularly in hormone-derived signaling (35–37), nitration of Tyr may have not only an effect itself but may interfere also with phosphorylation, thus being doubly important in regulating function of the modified protein. Interestingly, prediction of Tyr phosphorylation sites in OASA1 gave a high score only to Tyr158 and Tyr302 (Table 2). The potential modification of Tyr302 by either phosphorylation or nitration may represent also a new still unexplored regulatory mechanism in controlling OASA1 function. Moreover, it has been demonstrated that O-acetylserine and the C terminus of SAT compete for the same binding site (38), and the modification of Tyr302 (Fig. 4B) may have influence on the formation or disruption of the Cys synthase complex. It is clear whatsoever that Tyr302 is an essential residue for OASTL activity of OASA1 as demonstrated by the drastically reduced activity detected in a Y302A-mutated version of OASA1 (Fig. 5).
TABLE 2.
Theoretical requirements of Tyr residues of OASA1 to be nitrated and in silico prediction of phosphorylation potential
Distances between residues were calculated from the three-dimensional structure model shown in Fig. 4 with the analysis performed with Yasara software as indicated under “Experimental Procedures.” Prediction of phosphorylation potential was analyzed with the NetPhos 2.0 software from CBS.
| Tyr | Conserved | Distance to Asp/Glu | Proximal basic amino acids in primary sequence | Location in loop | Tyr phosphorylation score |
|---|---|---|---|---|---|
| 20 | No | - | No | No | 0.156 |
| 91 | Yes | 3.9 Å to Glu58 | Lys92 | Yes | 0.043 |
| 143 | Yes | 8.1 Å to Asp56 | No | No | 0.050 |
| 158 | No | 4.3 Å to Glu195 | His157 | No | 0.912a |
| 192 | Yes | 7.6 Å to Glu195 | Lys191 | No | 0.138 |
| 203 | No | 5.7 Å to Glu242 | Lys201 | No | 0.032 |
| 302 | Yes | 11.2 Å to Glu300 | Arg301 | Yes | 0.718a |
a Significant score for Tyr phosphorylation site prediction.
The fact that even under an extensive in vitro nitration assay on OASA1 only one of the Tyr residues of the protein has been identified as nitrated tells about the selectivity of the Tyr nitration process. Alternatively, the ratio of nitrated molecules to the non-nitrated protein molecules present in the sample may be very low thus making the MS-based identification difficult. This may explain why we could not identify the 3-nitro Tyr302 modification in vivo. Nevertheless, crude extracts that were not exogenously nitrated should contain nitrated forms of OASA1, as immunoprecipitation with anti-3-nitroTyr antibody and further detection by Western blot with a specific anti-OASA1 antibody allowed the recovery and detection of OASA1 protein in the immunoprecipitated fraction (Fig. 6A). Because immunoprecipitation did not certainly led to an OASA1 enrichment (Fig. 6A) compared with levels detected in the crude extracts, it is likely that the amount of nitrated forms in vivo are far below the levels of non-nitrated form of the protein. We have anyway confirmed the in vivo effect of nitration on OASA1 by infiltration of leaves with SIN-1 and further protein and activity analysis.
Post-translational modification of OASA1 by Y-nitration may represent a rapid and efficient regulatory mechanism to control the biosynthesis of cysteine and glutathione in response to stress factors. Both abiotic and biotic stress factors lead often to the production of reactive oxygen and nitrogen species in the stressed plants. Simultaneous production of NO (39) and superoxide anion in cells of stressed plants correlates with the production of strong nitrating peroxynitrite, which can nitrate Tyr residues of proteins. Cytosolic OASA1 is the main OASTL enzyme so an efficient control of its activity is crucial for controlling cysteine homeostasis under stress conditions. Recently, new insights on the function of this enzyme as a determinant of the antioxidative capacity of the cytosol have been proposed (9). Heavy metals have been characterized as inducers of cysteine biosynthesis (7, 8), and also as activators of NO production in eukaryotic algae and plants (40, 41). NO-derived and peroxynitrite-mediated Tyr-nitration of OASA1 may represent a rapid mechanism to control the amplitude and duration of the responses triggered by heavy metals or any other stress factors in plants. Alternatively, this sort of rapid inactivation mechanism could be operative only in tissues undergoing the direct effect of the stress factor, thus allowing the execution of the stress-activated responses in the rest of the plant. This local inactivation mechanism of OASA1 could be useful to avoid an extra production of cysteine and/or glutathione, which may act as a strong scavenger of the reactive oxygen and nitrogen species in the stressed area, thus limiting the activation of downstream signaling processes required for an efficient stress-related response in the whole plant. Rapid and local inactivation of enzymes could represent not only an efficient mechanism to modulate responses to stress, but also as a way to restrict enzyme activities in certain plant organs or under specific developmental stages, what makes this post-translational modification a relevant level for the regulatory effects exerted by sulfur-containing compounds on development and responses to stress. The identification of the Tyr nitration site in OASA1 protein and the subsequent inhibition of enzyme activity represent, to our knowledge, the first report identifying a post-translational modification of plant OASTLs and the first identification and further characterization of a nitration site in a plant protein with strong impact on its function.
Acknowledgments
We thank Rafael Ruiz-Partida from Centro de Investigación Principe Felipe (CIPF) for helpful assistance with three dimensional protein structure analysis. Proteomic analysis was performed in the Laboratorio de Proteómica from CIPF.
This work was supported by Grants BIO2008-00839 and CONSOLIDER TRANSPLANTA CSD2007-00057 (to J. L.) and BIO2010-15201 (to C. G.).
- SAT
- serine acetyltransferase
- APS
- 5′-adenylsulfate
- APR
- adenosine 5′-phosphosulfate reductase
- CE
- crude extract
- IP
- immunoprecipitate
- LC-MS/MS
- liquid chromatography tandem mass spectrometry
- MS
- Murashige and Skoog medium
- NO
- nitric oxide
- OAS
- O-acetyl-Ser
- OASTL
- O-acetylserine(thiol)lyase
- PLP
- pyridoxal-5′-phosphate
- PMSF
- phenylmethanesulfonylfluoride
- ROS
- reactive oxygen species
- SIN-1
- 3-morpholinosydnonimine hydrochloride
- Sup
- supernatant
- TCA
- trichloroacetic acid.
REFERENCES
- 1. Wirtz M., Droux M., Hell R. (2004) J. Exp. Bot. 55, 1785–1798 [DOI] [PubMed] [Google Scholar]
- 2. Haas F. H., Heeg C., Queiroz R., Bauer A., Wirtz M., Hell R. (2008) Plant Physiol. 148, 1055–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heeg C., Kruse C., Jost R., Gutensohn M., Ruppert T., Wirtz M., Hell R. (2008) Plant Cell 20, 168–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krueger S., Niehl A., Lopez Martin M. C., Steinhauser D., Donath A., Hildebrandt T., Romero L. C., Hoefgen R., Gotor C., Hesse H. (2009) Plant Cell Environ. 32, 349–367 [DOI] [PubMed] [Google Scholar]
- 5. Watanabe M., Kusano M., Oikawa A., Fukushima A., Noji M., Saito K. (2008) Plant Physiol. 146, 310–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watanabe M., Mochida K., Kato T., Tabata S., Yoshimoto N., Noji M., Saito K. (2008) Plant Cell 20, 2484–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dominguez-Solís J. R., Gutierrez-Alcalá G., Vega J. M., Romero L. C., Gotor C. (2001) J. Biol. Chem. 276, 9297–9302 [DOI] [PubMed] [Google Scholar]
- 8. Dominguez-Solís J. R., López-Martin M. C., Ager F. J., Ynsa M. D., Romero L. C., Gotor C. (2004) Plant Biotechnol. J. 2, 469–476 [DOI] [PubMed] [Google Scholar]
- 9. López-Martín M. C., Becana M., Romero L. C., Gotor C. (2008) Plant Physiol. 147, 562–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yi H., Galant A., Ravilious G. E., Preuss M. L., Jez J. M. (2010) Mol. Plant 3, 269–279 [DOI] [PubMed] [Google Scholar]
- 11. Barroso C., Romero L. C., Cejudo F. J., Vega J. M., Gotor C. (1999) Plant Mol. Biol. 40, 729–736 [DOI] [PubMed] [Google Scholar]
- 12. Wirtz M., Hell R. (2006) J. Plant Physiol. 163, 273–286 [DOI] [PubMed] [Google Scholar]
- 13. Droux M. (2004) Photosynth. Res. 79, 331–348 [DOI] [PubMed] [Google Scholar]
- 14. Leustek T., Martin M. N., Bick J. A., Davies J. P. (2000) Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 141–165 [DOI] [PubMed] [Google Scholar]
- 15. Lindermayr C., Durner J. (2009) J. Proteomics 73, 1–9 [DOI] [PubMed] [Google Scholar]
- 16. Corpas F. J., Chaki M., Leterrier M., Barroso J. B. (2009) Plant Signal. Behav. 4, 920–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szabó C., Ischiropoulos H., Radi R. (2007) Nat. Rev. Drug Discov. 6, 662–680 [DOI] [PubMed] [Google Scholar]
- 18. Thomas D. D., Espey M. G., Vitek M. P., Miranda K. M., Wink D. A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 12691–12696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ischiropoulos H. (2003) Biochem. Biophys. Res. Commun. 305, 776–783 [DOI] [PubMed] [Google Scholar]
- 20. Abello N., Kerstjens H. A., Postma D. S., Bischoff R. (2009) J. Proteome Res. 8, 3222–3238 [DOI] [PubMed] [Google Scholar]
- 21. Bonner E. R., Cahoon R. E., Knapke S. M., Jez J. M. (2005) J. Biol. Chem. 280, 38803–38813 [DOI] [PubMed] [Google Scholar]
- 22. Atanassov I. I., Atanassov I. I., Etchells J. P., Turner S. R. (2009) Plant Methods 5, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 24. Radi R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y., Lu N., Gao Z. (2009) Int. J. Biochem. Cell Biol. 41, 907–915 [DOI] [PubMed] [Google Scholar]
- 26. Schroeder P., Klotz L. O., Buchczyk D. P., Sadik C. D., Schewe T., Sies H. (2001) Biochem. Biophys. Res. Commun. 285, 782–787 [DOI] [PubMed] [Google Scholar]
- 27. Barroso C., Vega J. M., Gotor C. (1995) FEBS Lett. 363, 1–5 [DOI] [PubMed] [Google Scholar]
- 28. Gaitonde M. K. (1967) Biochem. J. 104, 627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tai C. H., Cook P. F. (2001) Acc. Chem. Res. 34, 49–59 [DOI] [PubMed] [Google Scholar]
- 30. Koprivova A., North K. A., Kopriva S. (2008) Plant Physiol. 146, 1408–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rinalducci S., Murgiano L., Zolla L. (2008) J. Exp. Bot. 59, 3781–3801 [DOI] [PubMed] [Google Scholar]
- 32. Durzan D. J., Pedroso M. C. (2002) Biotechnol. Genet. Eng. Rev. 19, 293–337 [DOI] [PubMed] [Google Scholar]
- 33. Souza J. M., Daikhin E., Yudkoff M., Raman C. S., Ischiropoulos H. (1999) Arch. Biochem. Biophys. 371, 169–178 [DOI] [PubMed] [Google Scholar]
- 34. de la Fuente van Bentem S., Hirt H. (2009) Trends Plant Sci. 14, 71–76 [DOI] [PubMed] [Google Scholar]
- 35. Ghelis T., Bolbach G., Clodic G., Habricot Y., Miginiac E., Sotta B., Jeannette E. (2008) Plant Physiol. 148, 1668–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oh M. H., Wang X., Kota U., Goshe M. B., Clouse S. D., Huber S. C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim T. W., Guan S., Sun Y., Deng Z., Tang W., Shang J. X., Sun Y., Burlingame A. L., Wang Z. Y. (2009) Nat. Cell Biol. 11, 1254–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Francois J. A., Kumaran S., Jez J. M. (2006) Plant Cell 18, 3647–3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lozano-Juste J., León J. (2010) Plant Physiol. 152, 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang L. P., Mehta S. K., Liu Z. P., Yang Z. M. (2008) Plant Cell Physiol. 49, 411–419 [DOI] [PubMed] [Google Scholar]
- 41. De Michele R., Vurro E., Rigo C., Costa A., Elviri L., Di Valentin M., Careri M., Zottini M., Sanità di Toppi L., Lo Schiavo F. (2009) Plant Physiol. 150, 217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]