Abstract
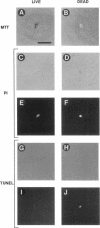
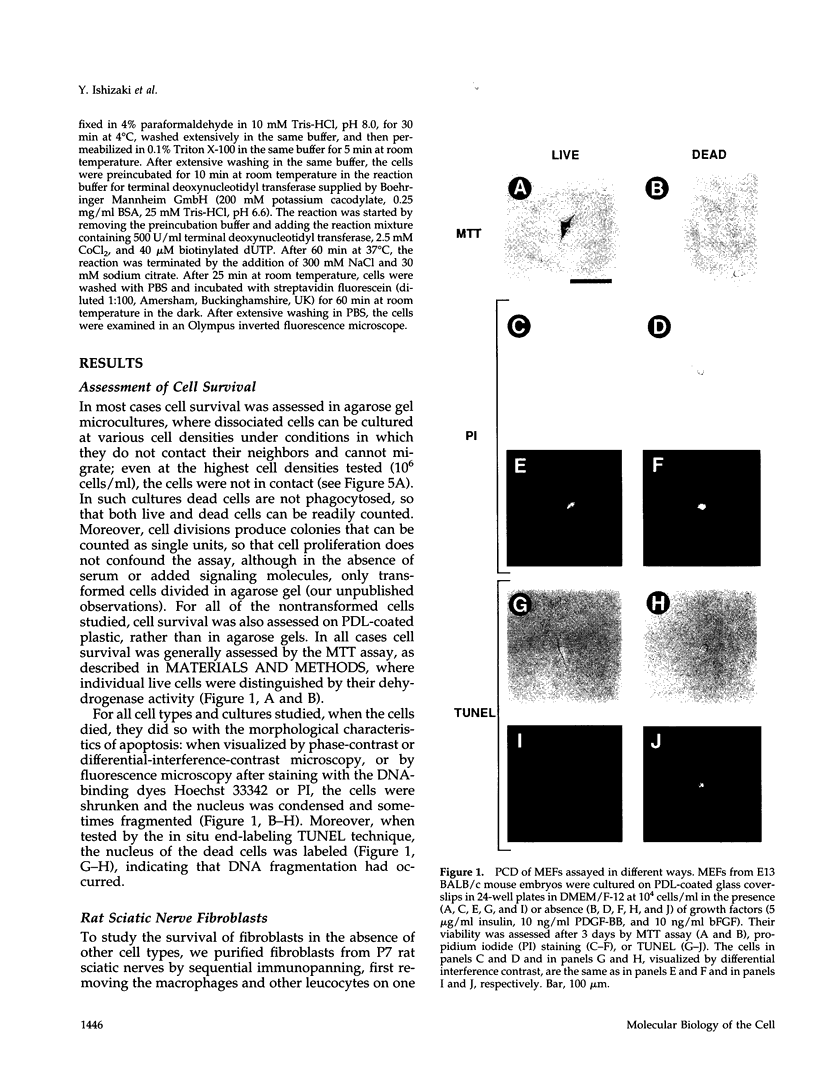
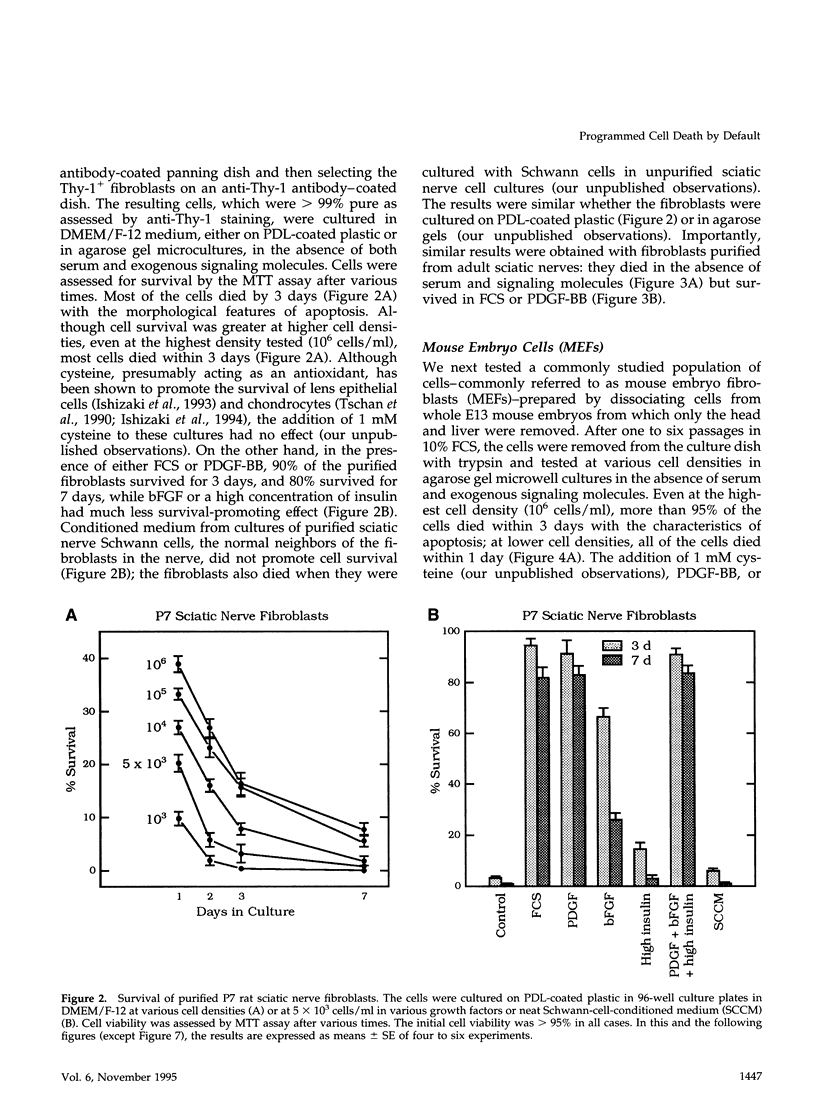
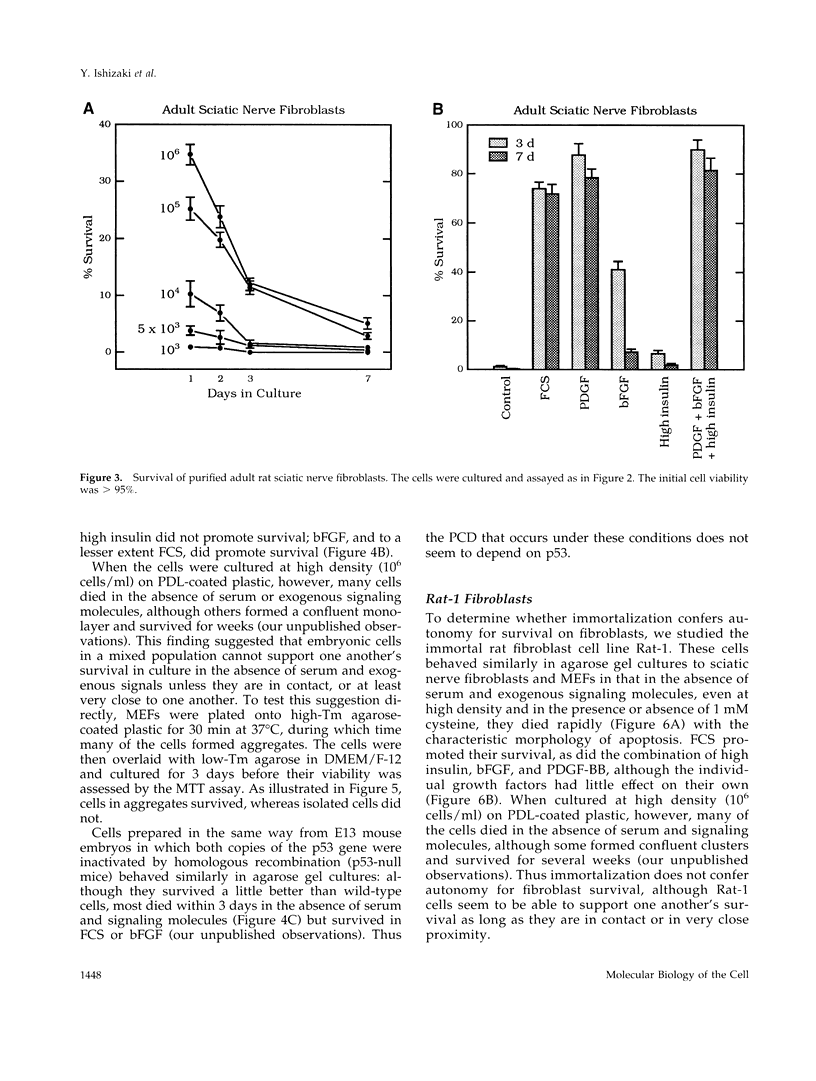
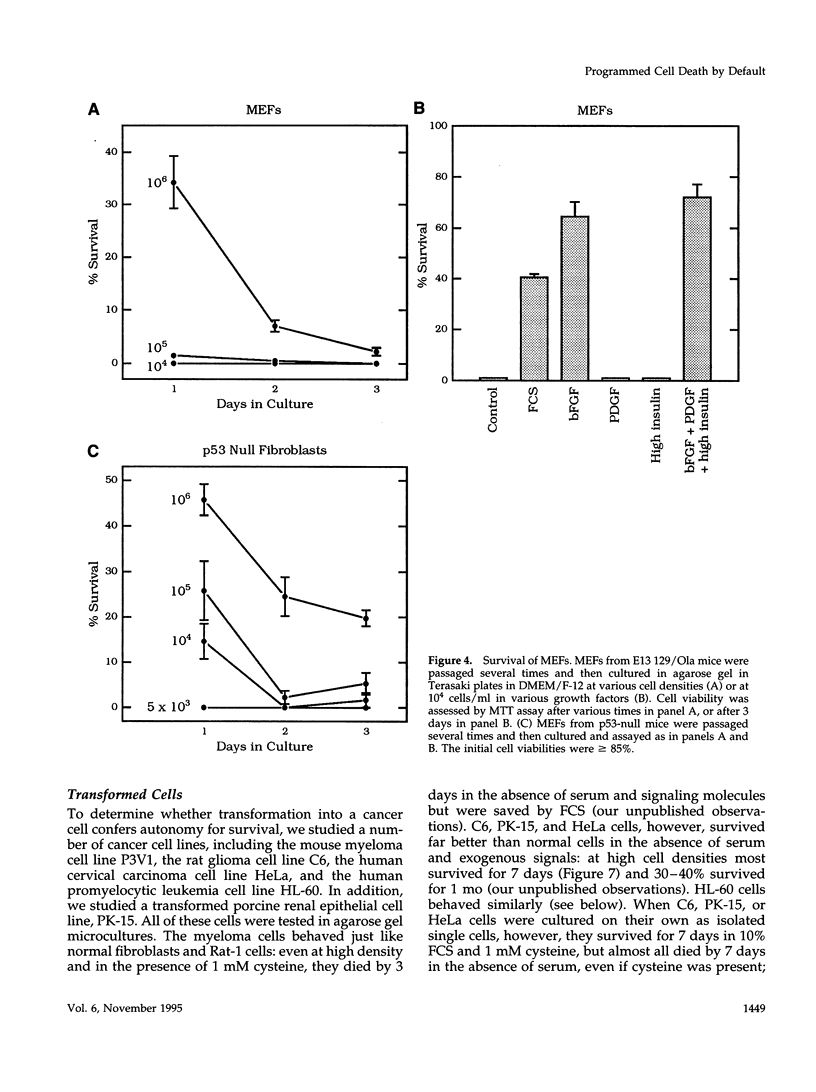
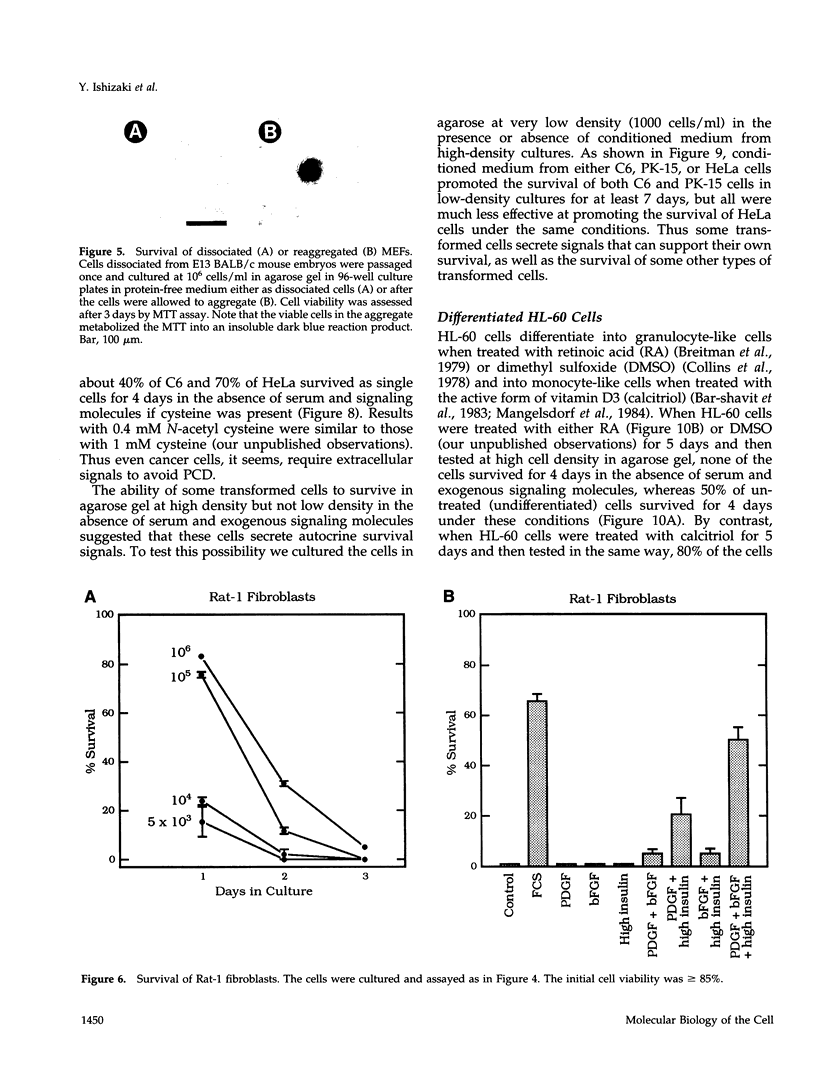
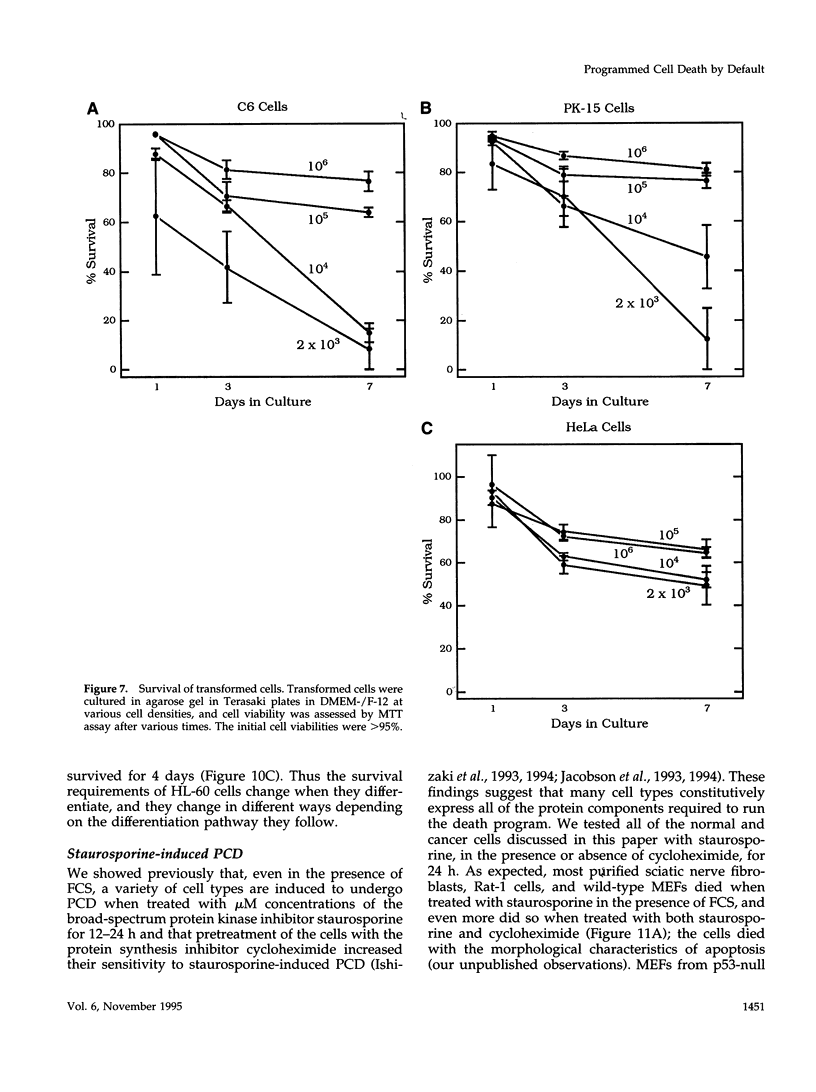
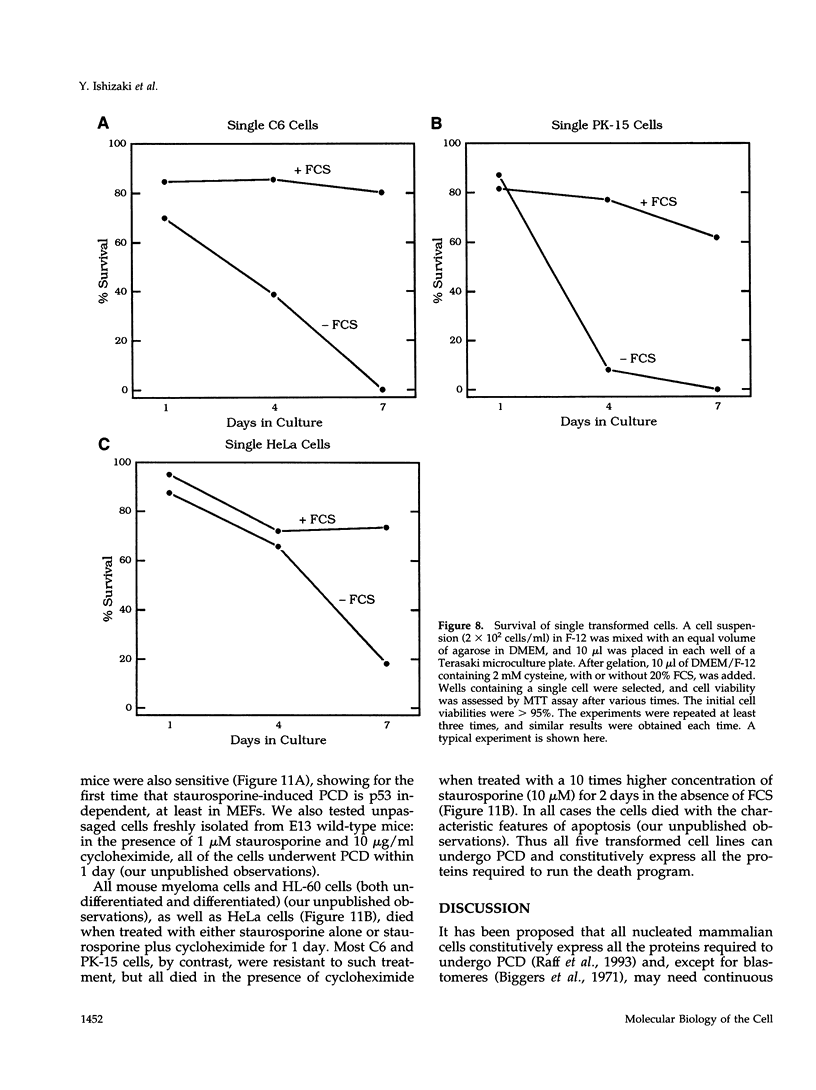
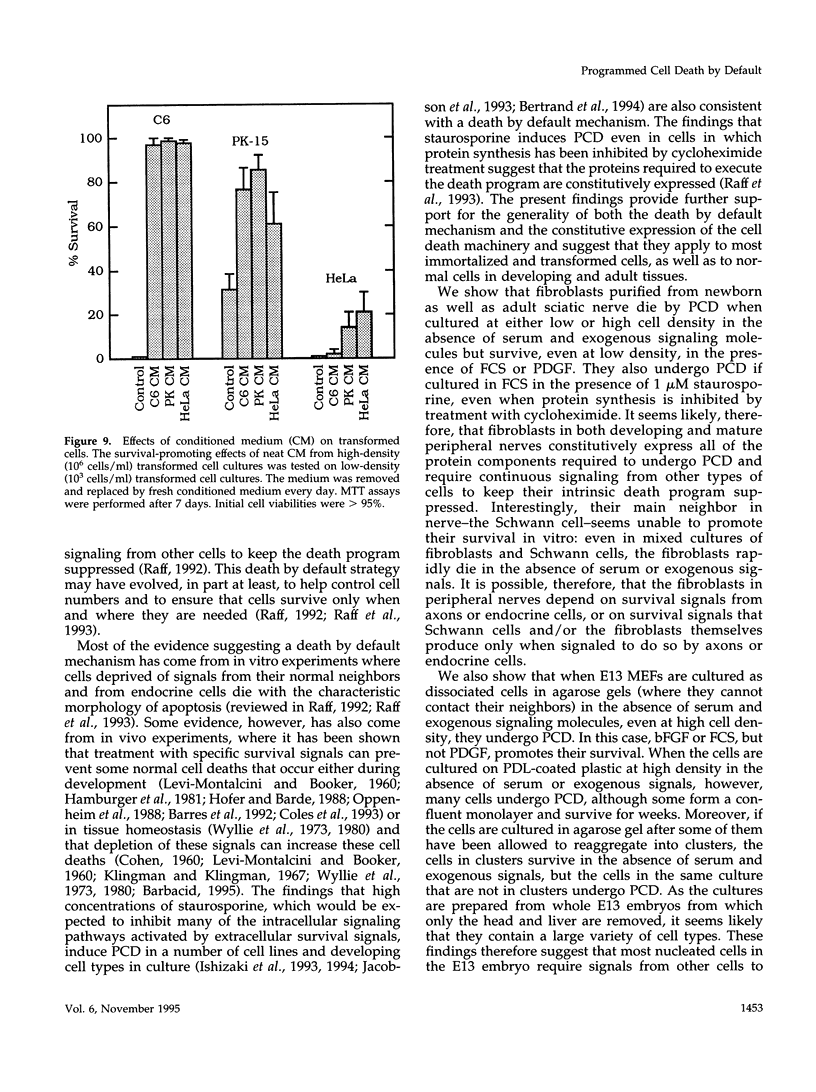
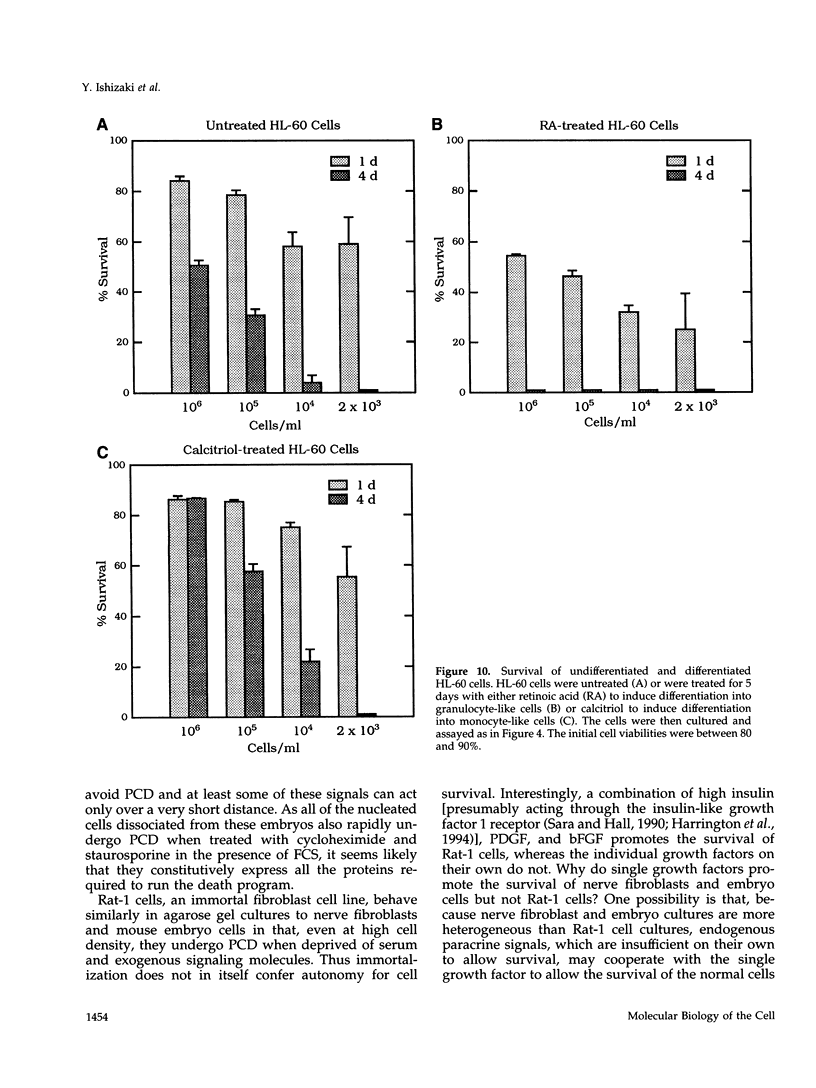
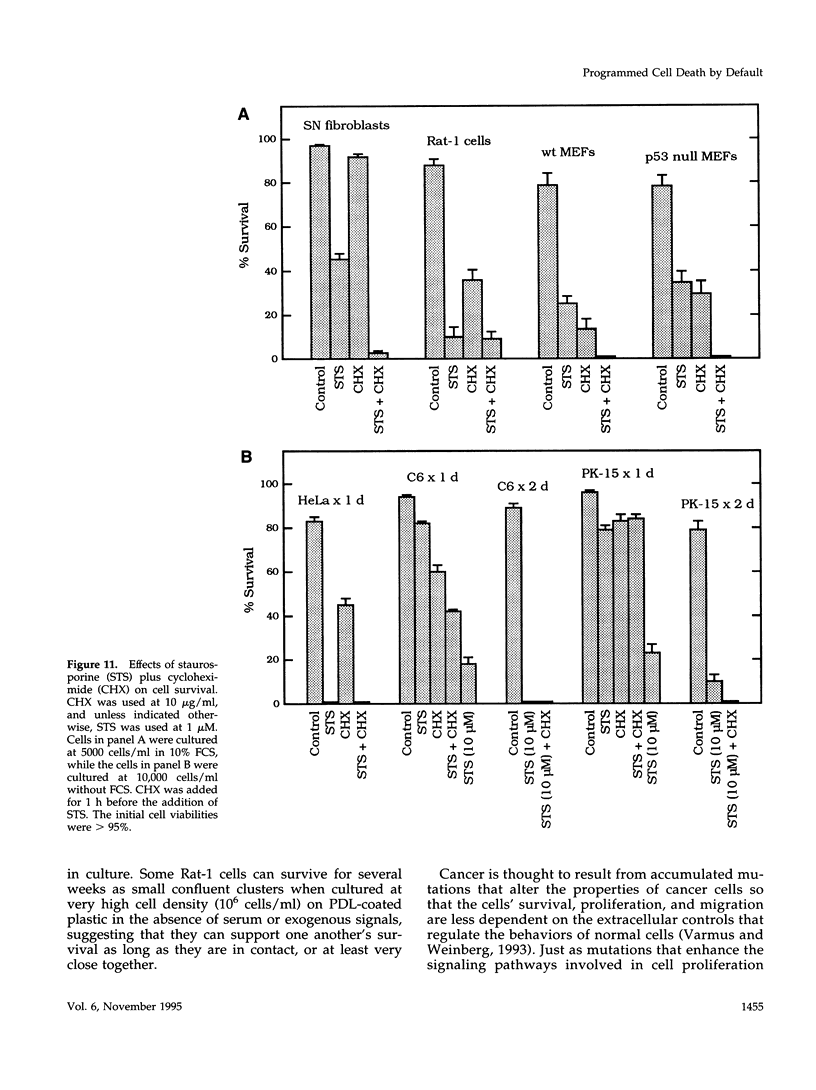
We recently proposed that most mammalian cells constitutively express all of the proteins required to undergo programmed cell death (PCD) and undergo PCD unless continuously signaled by other cells not to. Although some cells have been shown to work this way, the vast majority of cell types remain to be tested. Here we tested purified fibroblasts isolated from developing or adult rat sciatic nerve, a mixture of cell types isolated from normal or p53-null mouse embryos, an immortalized rat fibroblast cell line, and a number of cancer cell lines. We found the following: 1) All of these cells undergo PCD when cultured at low cell density in the absence of serum and exogenous signaling molecules but can be rescued by serum or specific growth factors, suggesting that they need extracellular signals to avoid PCD. (2) The mixed cell types dissociated from normal mouse embryos can only support one another's survival in culture if they are in aggregates, suggesting that cell survival in embryos may depend on short-range signals. (3) Some cancer cells secrete factors that support their own survival. (4) The survival requirements of a human leukemia cell line change when the cells differentiate. (5) All of the cells studied can undergo PCD in the presence of cycloheximide, suggesting that they constitutively express all of the protein components required to execute the death program.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Teitelbaum S. L., Reitsma P., Hall A., Pegg L. E., Trial J., Kahn A. J. Induction of monocytic differentiation and bone resorption by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5907–5911. doi: 10.1073/pnas.80.19.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995 Apr;7(2):148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992 Jul 10;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Jacobson M. D., Schmid R., Sendtner M., Raff M. C. Does oligodendrocyte survival depend on axons? Curr Biol. 1993 Aug 1;3(8):489–497. doi: 10.1016/0960-9822(93)90039-q. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bertrand R., Solary E., O'Connor P., Kohn K. W., Pommier Y. Induction of a common pathway of apoptosis by staurosporine. Exp Cell Res. 1994 Apr;211(2):314–321. doi: 10.1006/excr.1994.1093. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner P., Hörler I., Mendler M., Houze Y., Winterhalter K. H., Eich-Bender S. G., Spycher M. A. Induction and prevention of chondrocyte hypertrophy in culture. J Cell Biol. 1989 Nov;109(5):2537–2545. doi: 10.1083/jcb.109.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Khan M., Mudge A. W. Calcitonin gene-related peptide promotes Schwann cell proliferation. J Cell Biol. 1995 May;129(3):789–796. doi: 10.1083/jcb.129.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. PURIFICATION OF A NERVE-GROWTH PROMOTING PROTEIN FROM THE MOUSE SALIVARY GLAND AND ITS NEURO-CYTOTOXIC ANTISERUM. Proc Natl Acad Sci U S A. 1960 Mar;46(3):302–311. doi: 10.1073/pnas.46.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles H. S., Burne J. F., Raff M. C. Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development. 1993 Jul;118(3):777–784. doi: 10.1242/dev.118.3.777. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. M. Neurotrophic factors. Switching neurotrophin dependence. Curr Biol. 1994 Mar 1;4(3):273–276. doi: 10.1016/s0960-9822(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Dolci S., Williams D. E., Ernst M. K., Resnick J. L., Brannan C. I., Lock L. F., Lyman S. D., Boswell H. S., Donovan P. J. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature. 1991 Aug 29;352(6338):809–811. doi: 10.1038/352809a0. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Chan D. C., Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991 Mar 8;64(5):1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994 Feb;124(4):619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V., Brunso-Bechtold J. K., Yip J. W. Neuronal death in the spinal ganglia of the chick embryo and its reduction by nerve growth factor. J Neurosci. 1981 Jan;1(1):60–71. doi: 10.1523/JNEUROSCI.01-01-00060.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington E. A., Bennett M. R., Fanidi A., Evan G. I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994 Jul 15;13(14):3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M. M., Barde Y. A. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988 Jan 21;331(6153):261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y., Burne J. F., Raff M. C. Autocrine signals enable chondrocytes to survive in culture. J Cell Biol. 1994 Aug;126(4):1069–1077. doi: 10.1083/jcb.126.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki Y., Voyvodic J. T., Burne J. F., Raff M. C. Control of lens epithelial cell survival. J Cell Biol. 1993 May;121(4):899–908. doi: 10.1083/jcb.121.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., Raff M. C. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J. 1994 Apr 15;13(8):1899–1910. doi: 10.1002/j.1460-2075.1994.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingman G. I., Klingman J. D. Catecholamines in peripheral tissues of mice and cell counts of sympathetic ganglia after the prenatal and postnatal administration of the nerve growth factor antiserum. Int J Neuropharmacol. 1967 Nov;6(6):501–508. doi: 10.1016/0028-3908(67)90050-0. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Koeffler H. P., Donaldson C. A., Pike J. W., Haussler M. R. 1,25-Dihydroxyvitamin D3-induced differentiation in a human promyelocytic leukemia cell line (HL-60): receptor-mediated maturation to macrophage-like cells. J Cell Biol. 1984 Feb;98(2):391–398. doi: 10.1083/jcb.98.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J. Apoptosis: suicide, execution or murder? Trends Cell Biol. 1993 May;3(5):141–144. doi: 10.1016/0962-8924(93)90128-n. [DOI] [PubMed] [Google Scholar]
- Meredith J. E., Jr, Fazeli B., Schwartz M. A. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993 Sep;4(9):953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Haverkamp L. J., Prevette D., McManaman J. L., Appel S. H. Reduction of naturally occurring motoneuron death in vivo by a target-derived neurotrophic factor. Science. 1988 May 13;240(4854):919–922. doi: 10.1126/science.3363373. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Barres B. A., Burne J. F., Coles H. S., Ishizaki Y., Jacobson M. D. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993 Oct 29;262(5134):695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. L., Philpott K. L., Brooks S. F. Apoptosis: the cell cycle and cell death. Curr Biol. 1993 Jun 1;3(6):391–394. doi: 10.1016/0960-9822(93)90211-6. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Reed J. C. Anchorage dependence, integrins, and apoptosis. Cell. 1994 May 20;77(4):477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Sara V. R., Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990 Jul;70(3):591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- Shi L., Nishioka W. K., Th'ng J., Bradbury E. M., Litchfield D. W., Greenberg A. H. Premature p34cdc2 activation required for apoptosis. Science. 1994 Feb 25;263(5150):1143–1145. doi: 10.1126/science.8108732. [DOI] [PubMed] [Google Scholar]
- Solary E., Bertrand R., Kohn K. W., Pommier Y. Differential induction of apoptosis in undifferentiated and differentiated HL-60 cells by DNA topoisomerase I and II inhibitors. Blood. 1993 Mar 1;81(5):1359–1368. [PubMed] [Google Scholar]
- Tschan T., Höerler I., Houze Y., Winterhalter K. H., Richter C., Bruckner P. Resting chondrocytes in culture survive without growth factors, but are sensitive to toxic oxygen metabolites. J Cell Biol. 1990 Jul;111(1):257–260. doi: 10.1083/jcb.111.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Ucker D. S. Death by suicide: one way to go in mammalian cellular development? New Biol. 1991 Feb;3(2):103–109. [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Macaskill I. A., Currie A. R. Adrenocortical cell deletion: the role of ACTH. J Pathol. 1973 Oct;111(2):85–94. doi: 10.1002/path.1711110203. [DOI] [PubMed] [Google Scholar]
- Zambetti G. P., Levine A. J. A comparison of the biological activities of wild-type and mutant p53. FASEB J. 1993 Jul;7(10):855–865. doi: 10.1096/fasebj.7.10.8344485. [DOI] [PubMed] [Google Scholar]