Abstract
In vivo, KCNQ1 α-subunits associate with the β-subunit KCNE1 to generate the slowly activating cardiac potassium current (IKs). Structurally, they share their topology with other Kv channels and consist out of six transmembrane helices (S1–S6) with the S1–S4 segments forming the voltage-sensing domain (VSD). The opening or closure of the intracellular channel gate, which localizes at the bottom of the S6 segment, is directly controlled by the movement of the VSD via an electromechanical coupling. In other Kv channels, this electromechanical coupling is realized by an interaction between the S4-S5 linker (S4S5L) and the C-terminal end of S6 (S6T). Previously we reported that substitutions for Leu353 in S6T resulted in channels that failed to close completely. Closure could be incomplete because Leu353 itself is the pore-occluding residue of the channel gate or because of a distorted electromechanical coupling. To resolve this and to address the role of S4S5L in KCNQ1 channel gating, we performed an alanine/tryptophan substitution scan of S4S5L. The residues with a “high impact” on channel gating (when mutated) clustered on one side of the S4S5L α-helix. Hence, this side of S4S5L most likely contributes to the electromechanical coupling and finds its residue counterparts in S6T. Accordingly, substitutions for Val254 resulted in channels that were partially constitutively open and the ability to close completely was rescued by combination with substitutions for Leu353 in S6T. Double mutant cycle analysis supported this cross-talk indicating that both residues come in close contact and stabilize the closed state of the channel.
Keywords: Biophysics, Cell Surface Protein, Heart, Ion Channels, Potassium Channels, Electrophysiology
Introduction
KCNQ1 (KvLQT1) α-subunits tetramerize to create a voltage-gated K+ (Kv) channel. Like other Kv channels, each α-subunit contains six membrane spanning segments (S1–S6) with a pore loop between the fifth and sixth segment that forms the selectivity filter. The co-assembly with KCNE1 (minK) β-subunits generates the channel complex that underlies the native IKs in the heart (1, 2). A fundamental property of all Kv channels is their ability to detect a change in membrane potential (Vm) and to respond to this change by opening or closing their activation gate that seals off the ion permeation pathway in a closed configuration (3, 4). The channel activation gate is located in the C-terminal end of the S6 segment (S6T),3 whereas the voltage-sensing domain (VSD) is formed by the S1-S4 segments with the charged S4 as the main component (5). Substitution of the S4 charges in KCNQ1 perturbed channel gating (6, 7) and gating currents that originate from the redistribution of these S4 charges have recently been recorded for the KCNQ family of Kv channels (8). These data indicate that as in Shaker-type channels, the S1-S4 segment in KCNQ1 forms the channel VSD that reorients upon a change in membrane potential. This VSD reorientation is then translated into channel gate opening or closure through an electromechanical coupling. This coupling mechanism remains poorly defined but several studies suggest an interaction between the S4-S5 linker (S4S5L) and S6T (9–14). The crystal structure of Kv1.2, a mammalian Shaker-type K+ channel, indicated that residue contacts at the coupling interface between S4S5L and S6T are mainly hydrophobic, and it was suggested that they serve to transfer the energy from the VSD movement onto the channel gate (15–17). On the other hand, in slowly activating channels such as hERG (KCNH2) and Hyperpolarization-activated cyclic nucleotide-gated cation channels mainly electrostatic interactions between the S4S5L and S6T have been identified (9–11).
We previously identified in KCNQ1 several S6T residues that are involved in opening or closure of the channel gate (18). In particular, an alanine or a charged residue substitution for Leu353 precluded normal channel closure and resulted in a constitutively partial open channel. This impaired channel closure could be explained in two ways: 1) the side chain of residue Leu353 itself forms the cytoplasmic activation gate that seals off the ion permeation pathway or 2) Leu353 interacts with residues of S4S5L in the closed channel configuration and mutating Leu353 results in a loosened electromechanical coupling with a failure to close completely. To investigate the latter possibility further, we identified the residues in the S4S5L of KCNQ1 that participate in the gating machinery. These residues group together on one side of S4S5L according to a homology model based on the Kv1.2 structure. By combining specific S4S5L substitutions with Leu353 mutants, we could restore normal channel closure, suggesting that these residue pairs interact or come in close contact in the closed channel configuration and are part of the electromechanical coupling in KCNQ1 channels.
EXPERIMENTAL PROCEDURES
Molecular Biology
Human KCNQ1 was expressed using a pIRES2-EGFP expression vector (BD Biosciences, San Jose, CA). Mutations were introduced with a PCR reaction using mutant primers and the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). After PCR-based mutagenesis, a PvuI-EcoR I fragment containing the mutation was cut out of the PCR-amplified vector and ligated in KCNQ1/pIRES2-EGFP to replace the wild-type (WT) sequence. Double-strand sequencing of the exchanged fragment and the adjacent sequence confirmed the presence of the desired modification and the absence of unwanted mutations. Plasmid DNA for mammalian expression was obtained by amplification in XL2 Bluescript cells (Stratagene) and then isolated from the bacterial cells with the endotoxin-free Maxiprep kit (Sigma).
Electrophysiology
CHO-K1 cells were cultured in Ham F12 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen). The cells were transfected with 6 μg cDNA of WT or mutant KCNQ1 following the FuGENE transfection method (Roche Diagnostics). For electrophysiological experiments untagged KCNQ1 constructs were used. 16 h after transfection, the cells were trypsinized and GFP fluorescent cells were used for experiments within 12 h. Current recordings were made with a Multiclamp-700B amplifier (Axon Instruments, Foster City, CA) in the whole cell configuration of the patch clamp technique. Experiments were performed at room temperature (20–23 °C); current recordings were low pass-filtered and sampled at 2–10 kHz with a Digidata 1322A data acquisition system (Axon Instruments). Command voltages and data storage were controlled with pClamp8 software (Axon Instruments). Patch pipettes were pulled from 1.2-mm borosilicate glass capillaries (World Precision Instruments, Inc., Sarasota, Florida) with a P-2000 puller (Sutter Instruments, Novato, CA) and were heat polished afterward. The cells were perfused continuously with a bath solution containing the following: 145 mm NaCl, 4 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, 10 mm glucose, and adjusted to pH 7.35 with NaOH. The pipette solution contained the following: 110 mm KCl, 5 mm K4-BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid), 5 mm K2-ATP, 1 mm MgCl2, 10 mm HEPES, and was adjusted to pH 7.2 using KOH. Junction potentials were zeroed with the filled pipette in the bath solution. The remaining liquid junction potential was estimated to be 1.7 mV and was not corrected. The access resistance varied from 3 to 9 megohms without compensation and was below 3 megohms after whole cell compensation. Experiments were excluded from analysis if voltage error estimates exceeded 5 mV.
Data Analysis
The holding potential was set to −80 mV, and the interpulse interval was set to at least 15 s. The voltage protocols were adjusted to determine the biophysical properties of WT and mutant channels adequately. Time constants of activation and deactivation were determined by fitting the current recordings with a single or double exponential function. The voltage dependence of channel activation was fitted with a Boltzmann equation: y = 1/(1 + exp(−(E − V½)/k)), in which k represents the slope factor, E represents the applied voltage, and V½ the voltage at which 50% of the channels are activated and referred to as the midpoint potential. Both V½ and the slope factor k were used to calculate the Gibbs free energy of activation at 0 mV (ΔG0): ΔG0 = 0.0002389zFV½, with the factor 0.0002389 to express the values in kcal/mol. ΔΔG0 was calculated as (ΔG0mutant − ΔG0WT). Standard errors of ΔG0 and ΔΔG0 were calculated using linear error propagation (19). Results are expressed as mean ± S.E. with n the number of cells analyzed.
Confocal Imaging
WT and mutant KCNQ1 constructs were tagged with GFP at their carboxyl terminus. CHO-K1 cells were grown on coverslips and transfected with 2 μg of WT KCNQ1-GFP or mutant cDNA. 48 h after transfection, confocal images were obtained on a Zeiss CLSM 510 confocal microscope, equipped with an argon laser (excitation, 488 nm) for the visualization of GFP.
RESULTS
Alanine/Tryptophan Scanning Mutagenesis of S4S5L in KCNQ1
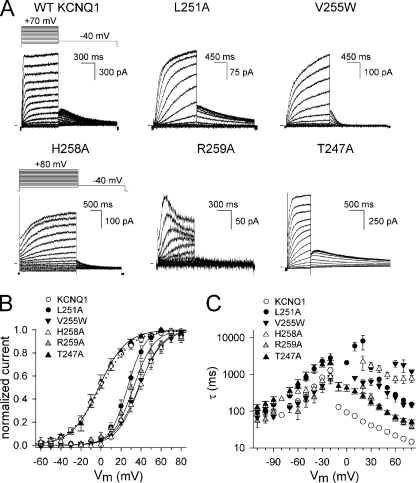
To determine which residues in S4S5L are involved in KCNQ1 channel gating, we performed an alanine substitution scan combined with selected tryptophan mutations. The S4S5L of KCNQ1 is mainly hydrophobic with on both ends an arginine residue (Arg249 and Arg259) (Table 1). All residues from Thr247 to Gln260 were mutated to an alanine to reduce the volume of the side chain, except in the case of a native glycine. Small residues like Gly252, Ser253, Val254, and Val255 were also mutated to a tryptophan to increase the volume of the side chain substantially. Alanine and tryptophan perturbation mutagenesis has been used previously to explore secondary structure and protein-facing residues of both water-exposed and membrane-embedded domains in several voltage-gated K+ channels (20–22). WT KCNQ1 channels displayed currents that were characterized by a single exponential time course of activation with an apparent threshold of approximately −40 mV. Upon membrane repolarization, a slow deactivation process was preceded by a transient increase in current, a “hooked tail,” resulting from a rapid recovery of channel inactivation that occurred during the preceding depolarization (Fig. 1) (1, 2, 23).
TABLE 1.
Biophysical characteristics of WT KCNQ1 and S4S5L residue mutants
Values are means ± S.E. V½ is the midpoint of activation, and k is the slope of the activation curve fitted with the Boltzmann equation. Time constants were derived from monoexponential fits to activating or deactivating currents. τ+60 mV is the time constant for activation at the potential V½ + 60 mV. τ−50 mV and τ−100 mV are the time constants for deactivation at the potentials V½ − 50 mV and V½ − 100 mV. ΔG0 represents the Gibbs free energy of activation at 0 mV. ΔΔG0 was calculated as (ΔG0mutant − ΔG0WT). ΔG0 and ΔΔG0 are expressed in kcal/mol. S.E. of ΔG0 and ΔΔG0 were calculated using linear error propagation in accordance to Yifrach et al. (19). The number of cells analyzed is represented by n. NE, not expressed in the plasma membrane; NF, nonfunctional (but in the plasma membrane).
| Activation |
Deactivation |
ΔG0 | ΔΔG0 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| V1/2 | k | τV1/2+60 | n | τV1/2−100 | τV1/2−50 | n | |||
| mV | mV | ms | ms | ms | |||||
| KCNQ1 | −0.2 ± 2.5 | 16 ± 0.9 | 21 ± 1.9 | 15 | 97 ± 15 | 354 ± 69 | 8 | −0.01 ± 0.10 | |
| T247A | −2.2 ± 3.3 | 14 ± 1.1 | 69 ± 8.5 | 5 | 156 ± 29 | 573 ± 153 | 5 | −0.09 ± 0.14 | −0.08 ± 0.17 |
| W248A | 5.9 ± 1.7 | 9.6 ± 1.6 | 30 ± 5.5 | 6 | 57 ± 11 | 175 ± 28 | 6 | 0.36 ± 0.12 | 0.37 ± 0.15 |
| R249A | 4.1 ± 1.4 | 17 ± 0.9 | 45 ± 5.9 | 4 | 95 ± 4 | 386 ± 72 | 5 | 0.14 ± 0.05 | 0.15 ± 0.10 |
| L250A | NF | ||||||||
| L251A | 27 ± 2.3 | 9.2 ± 0.9 | 150 ± 9 | 7 | 310 ± 24 | 3600 ± 490 | 5 | 1.72 ± 0.22 | 1.73 ± 0.23 |
| G252A | −8.2 ± 3.3 | 13 ± 0.9 | 51 ± 8.4 | 6 | 150 ± 5.0 | 660 ± 37 | 4 | −0.37 ± 0.15 | −0.36 ± 0.17 |
| G252W | −14 ± 2.4 | 9.2 ± 1.0 | 16 ± 1.3 | 4 | 93 ± 20 | 130 ± 20 | 4 | −0.89 ± 0.18 | −0.88 ± 0.20 |
| S253A | −0.4 ± 4.6 | 17 ± 1.6 | 20 ± 1.4 | 5 | 120 ± 10 | 480 ± 43 | 5 | −0.01 ± 0.15 | 0.00 ± 0.18 |
| S253W | 2.7 ± 3.2 | 17 ± 1.3 | 32 ± 6.0 | 5 | 130 ± 30 | 400 ± 70 | 4 | 0.09 ± 0.11 | 0.10 ± 0.14 |
| V254A | 4.7 ± 2.7 | 14 ± 1.2 | 51 ± 8.0 | 6 | 220 ± 28 | 1000 ± 140 | 4 | 0.18 ± 0.14 | 0.19 ± 0.17 |
| V254W | NE | ||||||||
| V255A | 3.4 ± 3.1 | 15 ± 1.0 | 54 ± 11 | 6 | 130 ± 18 | 490 ± 80 | 3 | 0.13 ± 0.12 | 0.14 ± 0.15 |
| V255W | 34 ± 1.4 | 11 ± 0.2 | 150 ± 17a | 4 | 100 ± 33 | 720 ± 130 | 5 | 1.81 ± 0.08 | 1.82 ± 0.12 |
| F256A | 2.8 ± 3.5 | 12 ± 0.6 | 38 ± 6 | 6 | 110 ± 11 | 320 ± 18 | 6 | 0.22 ± 0.11 | 0.23 ± 0.14 |
| I257A | −6.8 ± 3.0 | 10 ± 0.6 | 38 ± 5 | 5 | 81 ± 14 | 290 ± 34 | 5 | −0.40 ± 0.17 | −0.39 ± 0.20 |
| H258A | 38 ± 2.4 | 12 ± 1.2 | 770 ± 150 | 5 | 339 ± 16 | 868 ± 51 | 4 | 1.86 ± 0.22 | 1.87 ± 0.47 |
| R259A | 32 ± 3.0 | 13 ± 2.5 | 58 ± 5.8 | 5 | 95 ± 37 | 730 ± 170 | 4 | 1.44 ± 0.30 | 1.45 ± 0.32 |
| Q260A | 0.7 ± 3.0 | 12 ± 1.8 | 38 ± 3.0 | 8 | 120 ± 0.6 | 260 ± 27 | 3 | 0.03 ± 0.14 | 0.04 ± 0.17 |
a For the activation of the V255W mutant, a double exponential fit was used, and the time constant of the fast component is shown (for the slow component, see Fig. 1C).
FIGURE 1.
Biophysical properties of WT KCNQ1 and mutant channels in the S4-S5 linker region. A, representative current traces for the WT KCNQ1 channel and several mutants expressed in CHO-K1 cells. Cells were clamped at a holding potential of −80 mV, and 800–2500 ms pulses to voltages between −60 to +80 mV were imposed in steps of +10 mV. Tail currents were recorded by stepping to −40 mV. (Note the differences in scale bars.) B, voltage dependence of activation. Activation curves were obtained by plotting the normalized tail currents as a function of the prepulse potential. The solid lines represent the average Boltzmann fits and were compared with WT (dotted line). Parameters are shown in Table 1. Besides T247A, the other mutations resulted in channels that displayed a voltage dependence that was shifted to more positive potentials compared with WT. C, activation and deactivation time constants derived from monoexponential fits (biexponential in the case of V255W) to the raw current traces were plotted as a function of applied voltage. All mutants resulted in markedly slowed activation kinetics.
Based on the impact of the substitution on the channel gating properties compared with WT, the residues were categorized in two groups: 1) “high impact” residue positions where a mutation had a marked effect on channel gating and 2) “low impact” positions where a substitution was well tolerated and resulted in channels with biophysical properties fairly similar to WT KCNQ1. First, the effect of the mutation on the voltage dependence of activation was determined by calculating ΔG0 and subsequently ΔΔG0 (Table 1; for details on the calculations, see “Experimental Procedures”). To quantify the relevance of the perturbation, we used a cut-off value of 1 kcal/mol. Using this cut-off value four mutants (L251A, V255W, H258A, and R259A) scored as high impact (Fig. 1 and Table 1), and they all displayed a strong positive shift in the voltage dependence of activation. Taking these shifts into account, the time constants of channel opening and closure for R259A were similar to WT, suggesting that this substitution only changed the energy level between the open and closed state (ΔG0). The other substitutions (L251A, V255W and H258A) not only altered ΔG0, but also slowed the kinetics of channel gating: we observed a more than 3-fold slowing of either the activation kinetics alone (V255W) or of both activation and deactivation kinetics (L251A and H258A). In addition, T247A also resulted in a 3-fold change in activation kinetics. Although T247A had a lower impact on ΔG0 than the other substitutions, it was included in the group of high impact residues because of the marked change in activation kinetics.
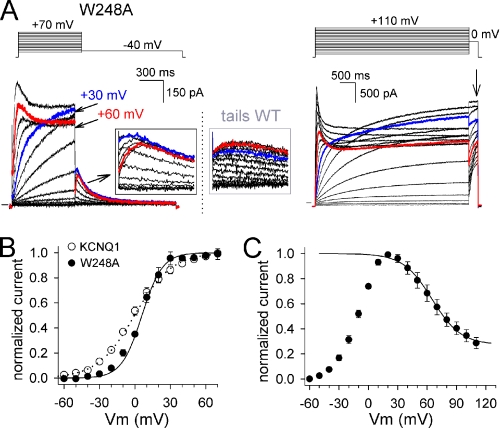
Although the effect of the W248A and V254A substitutions was not reflected in an altered ΔG0, they were categorized as high impact positions because they resulted in channels with an otherwise abnormal phenotype. The W248A mutant had a voltage dependence of channel activation, activation and deactivation time constants similar to WT. However, the voltage dependence of channel inactivation was markedly shifted toward more positive potentials compared with WT (Fig. 2). Because channels started to inactivate at potentials where steady-state activation was complete, a crossover of the current traces was observed at more depolarized potentials (from +30 to +70 mV). Consequently, the “hooked” tail currents appeared at these potentials only (Fig. 2). To explore whether inactivation occurred in the lower voltage range of −40 to +20 mV, we used a triple pulse protocol as shown in supplemental data. The results showed that this was not the case, suggesting that no inactivation occurred at voltages below +20 mV (supplemental Fig. 1).
FIGURE 2.
The W248A mutant displays enhanced channel inactivation. A, representative current traces for the mutant W248A. The left panel shows the current activations obtained with 800-ms depolarization steps, and the right panel shows those with 5 s pulses. In each case, the voltage protocol used is represented on top. Note the “crossing” of the current recordings at +60 mV (red trace) and +30 mV (blue trace), respectively. Also, the hooked tail currents, typical for recovery from the inactivated state, only appeared with more depolarized potentials (see black boxed inset). For comparison, a blow-up of the tail currents of WT KCNQ1 are provided in the gray box. B, voltage dependence of activation determined by plotting the normalized tail current amplitudes, that were obtained after 800-ms depolarizations (left panel A), as a function of prepulse potential. The solid line represents the average Boltzmann fit for W248A (circles) and the dotted line represents the fit of WT (open circles). C, normalized current voltage curve obtained by normalizing the steady-state current amplitude after 5-s prepulses (A, right panel, indicated with an arrow). Note that there is no current inactivation in the voltage range of −40 to +20 mV. However, above +20 mV, the channels displayed a pronounced inactivation behavior that resulted in a steady-state current decrease. The apparent inactivation curve had a midpoint of 64.9 ± 3.2 mV with a slope of 14.7 ± 0.8 mV (n = 5).
The V254A mutation slowed down channel closure by 2-fold but did not affect the activation kinetics or the voltage dependence of channel activation (Table 1). Similar to W248A, the V254A mutant also displayed pronounced channel inactivation, and the steady-state current level at the end of the pulse was comparable with the instantaneous one (Fig. 3A). Furthermore, analysis of the instantaneous current amplitude as a function of applied voltage showed constitutive conduction at hyperpolarized potentials (membrane potentials where the channels should be closed); this component (without leak correction) crossed the zero current level at −64 mV, which is close to the reversal potential for K+ with the solutions used (Fig. 3D). The presence of this constitutive current component was not observed for WT KCNQ1 channels or the other channel mutants. To further investigate the contribution of residue Val254 in the channel gating mechanism, we mutated this residue to a negatively charged aspartate (V254E) and a positively charged lysine (V254K). Similar to V254A, the V254E mutation displayed a clear constitutive current component at hyperpolarized potentials (Fig. 3). In contrast to the alanine substitution, the voltage-dependent current component of V254E was drastically shifted to more positive potentials compared with WT KCNQ1 (Fig. 3, Table 2). A lysine substitution for Val254 (V254K) was not expressed at the plasma membrane (not expressed).
FIGURE 3.
Val254 mutants display a constitutive conducting phenotype. A, representative current trace for the mutant V254A and V254E with the voltage protocol shown on top. Note the pronounced channel inactivation of the V254A mutant and the instantaneous current component of both mutants. B, the voltage-dependent component of the V254A mutant displayed a similar voltage dependence as WT KCNQ1. For V254E, the activation curve was at least +70 mV shifted toward positive potentials compared with WT (parameters are shown in Tables 1 and 2). C, the activation and deactivation time constants of V254A were slightly slowed compared with WT KCNQ1. Taking into account the positively shifted voltage dependence of channel activation, V254E displayed markedly faster activation time constants than WT, whereas channel closure was slowed. D, instantaneous current amplitudes (determined after the capacitive transient) as a function of pulse potential for KCNQ1 WT, V254A, and V254E.
TABLE 2.
Biophysical properties of S4S5L mutants alone and in combination with S6T substitutions
For definitions of abbreviations, see the legend to Table 1. NF, nonfunctional; ND, not determined.
| Activation |
Deactivation |
ΔG0 | ΔΔG0 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| V1/2 | k | τV1/2+60) | n | τV1/2−100) | τV1/2−50) | n | |||
| mV | mV | ms | ms | ms | |||||
| KCNQ1 | −0.2 ± 2.5 | 16 ± 0.9 | 21 ± 1.9 | 15 | 97 ± 15 | 354 ± 69 | 8 | −0.01 ± 0.09 | |
| V254Aa | 4.7 ± 2.7 | 14 ± 1.2 | 51 ± 8.0 | 6 | 220 ± 28 | 1000 ± 140 | 4 | 0.18 ± 0.14 | 0.19 ± 0.17 |
| V254La | −3.1 ± 4.9 | 18 ± 1.9 | 22 ± 2.7 | 6 | ND | ND | −0.10 ± 0.16 | −0.09 ± 0.18 | |
| V254Ea | 73 ± 5.0 | 17 ± 3.5 | 28 ± 1.3b | 5 | 150 ± 15c | 300 ± 25d | 4 | 2.52 ± 0.54 | 2.53 ± 0.55 |
| V254K | NE | ||||||||
| L353Aa | −1.0 ± 2.1 | 18 ± 2.0 | 28 ± 2.1 | 10 | 150 ± 12 | 570 ± 34 | 9 | −0.03 ± 0.07 | 0.04 ± 0.11 |
| L353Ea | −22 ± 3.0 | 17 ± 1.1 | 28 ± 6.6 | 5 | 170 ± 40 | 280 ± 78 | 4 | −0.76 ± 0.11 | −0.69 ± 0.15 |
| L353Ka | 2.7 ± 3.8 | 19 ± 2.1 | 10 ± 2.1 | 5 | 320 ± 50 | 1800 ± 170 | 7 | 0.08 ± 0.12 | 0.09 ± 0.15 |
| V254L/L353A | 24.7 ± 2.7 | 17 ± 0.9 | 204 ± 13 | 7 | 117 ± 12 | 336 ± 15 | 5 | 0.85 ± 0.10 | 0.86 ± 0.14 |
| V254K/L353E | NE | ||||||||
| V254E/L353K | NE | ||||||||
| L250K/L353E | 16.4 ± 1.3 | 22 ± 2.2 | 172 ± 10 | 6 | 64 ± 7.0 | 208 ± 22 | 6 | 0.43 ± 0.06 | 0.44 ± 0.11 |
| H258K/L353E | 13.3 ± 2.6 | 16 ± 1.0 | 109 ± 6 | 5 | 204 ± 24 | 2020 ± 240 | 5 | 0.48 ± 0.10 | 0.49 ± 0.13 |
| L250K | NF | ||||||||
| H258K | 30 ± 2.3 | 15 ± 1.0 | 237 ± 24b | 3 | 402 ± 60 | 1790 ± 90 | 4 | 1.16 ± 0.13 | 1.17 ± 0.16 |
a Values that displayed a partial open phenotype are shown.
b Values determined at +80 mV are shown.
c Values determined at −120 mV are shown.
d Values determined at −70 mV are shown.
The residues Arg249, Gly252, Ser253, Phe256, Ile257, and Gln260 belonged to the low impact group of substitutions that resulted in mutant channels with gating kinetics similar to WT (Table 1). On the other hand, some mutants like L250A and V254W did not generate any current in the voltage range from −130 to +100 mV. To determine what caused the lack of current, these subunits were tagged with GFP, and their subcellular localization was determined with confocal microscopy. Fig. 4 shows that mutant V254W was retained intracellularly, indicating that this mutant did not pass the quality control of the endoplasmic reticulum and did not translocate to the plasma membrane (not expressed). In contrast, mutant L250A was located at the plasma membrane indicating that the surface expression of this mutant channel was not significantly affected. Rather, the mutation caused a severe disturbance of the gating process rendering the mutant channels nonfunctional or stabilized the closed channel configuration such that depolarizations up to +110 mV were insufficient to open the mutant channels. Therefore, L250A was also categorized as high impact.
FIGURE 4.
Subcellular localization of KCNQ1 WT and mutants determined by confocal microscopy. The α-subunits were tagged with GFP at their C-terminal end, and typical pictures of CHO-K1 cells expressing the channel complexes are represented on top. To determine the distribution of the GFP fluorescence over the cell, the GFP emission profile at a cross-section of the cell was determined by plotting the light intensity as a function of the cross-section distance. These profiles are shown below with the cross-section itself indicated in the picture above by the red line. Note the increased GFP emission intensity at the cell boundaries in the profile of the KCNQ1 WT channel and the mutants L250A and L250K, highlighting the robust plasma membrane expression of these channels. In contrast, the V254W, V254K, and V254C-L353C mutants displayed no marked membrane associated fluorescence, and the profile showed that more GFP emission was observed intracellularly. On top of each panel is indicated the total number of cells examined (n) with different independent transfections (#) all confirming the representative GFP-channel pattern.
Restoring Channel Closure of S4S5L and S6T Mutants by Combination
Mutating residue Val254 in S4S5L to an alanine resulted in a channel with a deactivation failure leading to a constitutive current component (Fig. 3). This phenotype was reminiscent of the effect of a similar alanine substitution for residue Leu353 in S6T (18). If a disrupted coupling is the cause for the partial open phenotype of the Leu353 mutants, then Val254 would be a good candidate to be the interacting partner of Leu353 in the closed configuration. To explore this possibility, we combined the L353A mutation with a leucine substitution at position Val254 (V254L), testing whether the increase in side chain volume at position Val254 might compensate for the loss at Leu353. Indeed, the V254L/L353A double mutant resulted in functional channels that did not display a constitutively current component at hyperpolarized potentials, indicating that the channels could close completely (Fig. 5). However, the voltage dependence of activation was markedly shifted toward positive potentials compared with WT KCNQ1 and both individual mutations V254L and L353A (Table 2). In addition, the activation kinetics were 10-fold slower compared with WT even after correcting for the shifted voltage dependence, whereas the deactivation time constants were similar to WT. Thus, the effect of the L353A mutation on pore closure could be rescued by increasing the side chain volume at residue position Val254 in S4S5L. Furthermore, a double mutant cycle analysis for V254L/L353A gave a ΔΔG0 value of 0.98 kcal/mol. This value is close to the cut-off value of 1 kcal/mol, indicating that, besides the rescue of the partial open phenotype, both residues are also coupled in energetic terms.
FIGURE 5.
Combinations of S4S5L and S6T substitutions. A, representative currents for the mutants V254L, L353A, and the double mutant V254L/L353A. In the case of V254L, the instantaneous current level at +70 mV depolarization (gray current trace) is indicated with dashed line. B, instantaneous current amplitudes for WT, V254L, L353A, and the double mutant V254L/L353A. Note that the double mutant V254L/L353A did not display an instantaneous current component as was the case for the single V254L and L353A mutation.
To investigate further the possible coupling between Val254 and residue Leu353 of S6T in the closed state, we attempted to cross-link both residues using cysteine substitutions. Although the L353C mutation was functional, the double mutant V254C/L353C construct displayed impaired trafficking (Fig. 4). Because this precluded the cross-linking approach, we combined the different charged aspartate and lysine substitutions at positions Val254 and Leu353 in the hypothesis that a complementary charge at position 254 might rescue the charge mutation at 353. Unfortunately, both double combinations V254E/L353K and V254K/L353E were not tolerated, and subcellular localization indicated that the channels did not reach the plasma membrane. Because combining the charge substitutions of Val254 and Leu353 was inconclusive, we investigated whether other positions might rescue the partial open phenotype of the L353E mutant. Therefore, we introduced in S4S5L a lysine at the position one helical turn upward or downward with respect to Val254. The double mutants L250K/L353E and H258K/L353E resulted in functional channels that displayed robust current activation, which was not the case for the individual L250K mutant that was nonfunctional. Furthermore, introduction of positive charges in S4S5L rescued the failure of L353E to close completely, and both double mutants L250K/L353E and H258K/L353E did not display a constitutive current component at hyperpolarized potentials (supplemental Fig. 2). Thus, in the case of the double mutant L250K/L353E, both nonfunctionality (L250K) and the failure to close completely (L353E) were rescued by the combination. Assuming that L250K alone stabilizes the closed state such that the threshold for channel opening is >+110 mV, double mutant cycle analysis then results in a ΔΔG0 value of at least −3.0 kcal/mol indicative for both residues being coupled. However, although the H258K mutation rescued the constitutive partial open phenotype of L353E, the double mutant cycle analysis yielded a ΔΔG0 value of 0.07 kcal/mol for the H258K/L353E combination. This indicates that both residues are not energetically coupled and affect the same rate-limiting or consecutive transition step(s) independently (24).
DISCUSSION
S4S5L of KCNQ1 Adopts an α-Helix and Constitutes Part of the Electromechanical Coupling
In this study, we performed an alanine/tryptophan perturbation scan of the S4S5L in KCNQ1 to determine the relevant residues in channel gating. The S4S5L is in KCNQ1 largely hydrophobic with both ends marked by a positively charged arginine residue (Arg249 and Arg259). Alanine substitutions for these two flanking residues have been described previously (6), and our observations are in agreement with this study. The R249A mutant appeared to have no effect on channel gating, whereas the R259A mutation shifted the voltage dependence of activation toward positive potentials by ∼+30 mV (Table 1). Besides this R259A mutation, we identified several other residues that altered channel gating upon mutation and categorized them in two groups: (i) residue positions that were defined as having a “high impact” on channel gating when mutated (Thr247, Trp248, Leu250, Leu251, Val254, Val255, His258, and Arg259) and (ii) residues that tolerated substitutions well and were therefore marked as having a “low impact” (Arg249, Gly252, Ser253, Phe256, Ile257, and Gln260).
On an α-helical wheel representation, the high and low impact residues each cluster at opposite sides of the helix (Fig. 6). Such a separation pattern is a strong indication for an α-helical structure of the S4S5L in KCNQ1, similar to the secondary structure proposed for the related Shaker-type channels (15, 25). Interestingly, known LQT1 disease mutations in this segment appear to target the high impact residues (Trp248, Leu250, Leu251, Val254, His258, and Arg259) (26–32), further strengthening the idea that this side of S4S5L constitutes an important part of the gating machinery.
FIGURE 6.
α-Helical projection of the S4S5L region in KCNQ1. α-Helical wheel representation of the S4S5L (top) and the S6T (bottom) sequence. The high impact positions in S4S5L are indicated with filled gray circles and include Thr247, Trp248, Leu250, Leu251, Val254, Val255, His258, and Arg259, respectively. The registered LQT1 mutations in S4S5L are represented in boldface and apparently involve substitutions of the high impact residues. Note that the pattern of these residues concurs with a α-helical structure and cluster on one side of the helix. Because these high impact positions alter channel gating upon mutation, this side of the helix presumably interacts with other segments of the KCNQ1 protein presumably the S6T region for which the sequence is shown below. In case of S6T, residue Leu353 is marked with a gray circle. Note that both helices run antiparallel with respect to each other.
Contacts between S4S5L and S6T Stabilize Closed Channel Conformation
The crystal structure of rKv1.2 showed that the S4S5L of Shaker-type K+ channels adopts an amphipathic α-helix that runs parallel to the lipid/cytoplasmic interface and is positioned over S6T of the same α-subunit (15, 16). In Shaker-type Kv channels, the direct contacts between S4S5L and S6T are mainly of a hydrophobic nature, and flanking noninteracting residues serve a stabilizing role by orienting S4S5L and S6T correctly (33). On the other hand, in the slowly gating hERG and HCN channels, electrostatic interactions between S4S5L residues and S6T have been identified (9–11). In KCNQ1, direct electrostatic interactions seem unlikely, as the high impact side on the S4S5L is mainly hydrophobic. Furthermore, we showed previously that the residues in the S6T that altered channel gating upon mutation are all uncharged residues (18). Therefore, the contacts between S4S5L and S6T are most likely of a hydrophobic nature in KCNQ1, similar to the other fast activating Shaker-type Kv channels.
A previous substitution scan of S6T in KCNQ1 showed that mutating Leu353 to an alanine or a charged residue resulted in a channel that failed to close completely. We observed a similar phenotype for the V254A mutation in this study. Two different mechanisms could explain the constitutive partially open phenotype of the Leu353 mutations: 1) the residue Leu353 forms the cytoplasmic activation gate that seals off the ion permeation pathway or 2) Leu353 comes in close proximity to residues of the S4S5L in the closed channel configuration, and mutation of Leu353 results in a loosened electromechanical coupling by disrupting subdomain contacts (18). Here, we show that mutations at position Val254 resulted in a similar phenotype and, more importantly, that the failure to close completely was rescued by combination (double mutant V254L/L353A). Furthermore, double mutant cycle analysis was also suggestive for both residues being coupled. These results are in support of the second hypothesis, suggesting that Val254 is the interacting partner of Leu353 and that their contact (which would be of a hydrophobic nature) stabilizes the closed state in the WT channel.
Electromechanical Coupling Consists of Multiple S4S5L-S6T Interactions
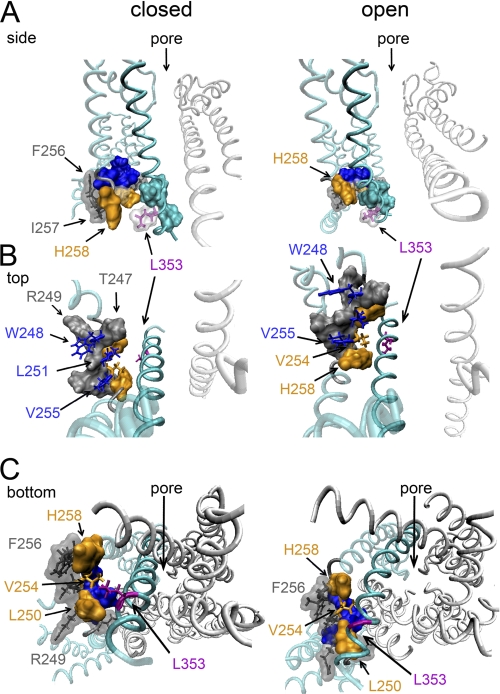
To interpret the results on a molecular level, we used the recently published KCNQ1 three-dimensional homology models for both open and closed channel configurations (34). In the closed state model, the high impact positions Leu250, Val254, and His258 are indeed located at the contact interface with S6T (Fig. 7). Furthermore, residue Val254 comes close enough to residue Leu353 to allow for van der Waals interactions (34). This proximity between Val254 and Leu353 in a closed channel conformation fits with the hypothesis that the interaction of both residues stabilizes the closed state. On the contrary, in the open state model, the distance between Val254 and Leu353 increases and becomes too large for both residues to maintain a physical contact. The orientation of both residues in this model is similar to our previously described homology model (18), and Leu353 points downward to the intracellular mouth of the permeation pore. Apparently, the residues of S4S5L that contact S6T in the open conformation appear to be the same as those in the closed conformation, namely Leu250, Val254, and His258. Although caution is needed with interpretations of homology models, this suggests that the side on S4S5L that makes contact with S6T is largely the same in both the open and closed states, whereas this does not hold for S6T, which adopts a different orientation. Consequently, the electromechanical coupling between S4S5L and S6T relies on different residue contacts for the closed and open channel conformations.
FIGURE 7.
KCNQ1 three-dimensional model showing the S4S5L/S6T interactions in both the open and closed state. Illustrations were produced using the recently published KCNQ1 homology models for both the closed and open state (34) and the program Visual Molecular Dynamics (44). A, side view on the pore domain with the closed state on the left and the open state on the right. For clarity, the front and back subunit were omitted. In cyan, the sequence of one subunit displayed in ribbon representation, with all segments besides S6 shown in transparency. The important residues are shown with their solvent-accessible surface. The high impact residues in S4S5L that according to the model are in close contact to residues in S6T (Leu250, Val254, and His258) are colored orange. The S6T residue Leu353 is colored purple. Note that in the closed state, residue Leu353 comes in close proximity to residue Val254 in S4S5L. The high impact residues Trp248, Leu251, and Val255 in S4S5L are highlighted in blue. B, similar representation as in A but now with a top view on the interaction between S4S5L and S6T. Residues Trp248, Leu251, and Val255 (blue side chains) are directed toward the membrane interface whereas residues Leu250, Val254, and His258 (in orange space fill, except Val254) are facing S6T. Note that the relative orientation of the blue and orange-colored residues of S4S5L is the same in both the closed and open channel conformation. However, the side on S6T that contacts S4S5L is different in the closed versus the open state. Apparently, Leu353 rotates toward the cytoplasmic mouth of the pore and away from Val254 in S4S5L when transitioning from the closed to the open channel conformation. C, viewed from the internal side using the same color coding as in A but now showing all four subunits. The low impact residues Phe256, Ile257, and Arg249 (displayed in gray with their solvent-accessible surface as transparent) locate on the side of S4S5L that faces away from S6T and are apparently not making protein contacts in either the closed or open conformation.
The observation that H258K and L353E are not energetically coupled indicates that they do not interact directly but that they affect the rate-limiting transition or consecutive gating steps independently (24). This would mean that both residues have different residue counterparts during the transition from the closed to the open state. For Leu353, the most likely interaction partners are Leu250 and Val254. However, the fact that H258K could recue the failure of L353E to close completely suggests that there is a certain degree of flexibility in the coupling. To explain these results mechanistically, we propose that introduction of a negative charged residue at position 353 (L353E) is not favored by its normal hydrophobic interacting partners (Leu250 and Val254). Thus, the L353E side chain adopts most likely a different orientation, thereby slightly changing the S6T position preventing full channel closure. Introducing a positive charge (H258K) in S4S5L would by itself affect the position of S4S5L, and as H258K comes in the vicinity of L353E (Fig. 7), the imposed structural rearrangement is apparently such that it compensates for the disruption of L353E.
The idea that S4S5L-S6T coupling consists of multiple contacts that have a certain degree of flexibility is quite conceivable for moving parts of the channel protein. Indeed, a rather loose S4S5L-S6T coupling has been suggested by Choveau et al. (accompanying article (45)); they showed that peptides containing the S4S5L or S6T sequence fragments compete for the S4-S5 linker or S6T site of the channel. This suggests that the S4S5L can be compared with a ligand that binds to S6T and locks the channel gate in the closed conformation. Consistent with our results, S4S5L peptide fragments were effective when they contained all the high impact residues Leu250, Val254, and His258, shown here to contact the S6T in the closed channel conformation. Likewise, only the S6T fragments containing Leu353 were effective. The observation that disturbing this interaction results in a constitutive partial open phenotype fits the coupling mechanism in which the binding of S4S5L to S6T stabilizes the closed state (accompanying article (45)). A dynamic coupling between the S4S5L and S6T has also been suggested recently for the closed state inactivation mechanism of Kv4.2 channels (35). In this case, it was proposed that reaching closed state inactivation was due to an uncoupling of the S4S5L from S6T.
KCNQ1 Mutations Have Tendency to Impair Channel Closure and to Enhance the Inactivation Process at Same Time
The behavior of the V254A mutation is similar to what has been reported for substitutions at position Val310 that result in a reduction of side chain volume. Val310 is located at the base of the selectivity filter and mutating it to a glycine or an alanine resulted in markedly enhanced inactivation and in the inability to close completely, similar to the phenotype of V254A (36). In the case of Val310, the impaired channel closure probably involves contacts with other S5 and S6 residues. The observation that the S4S5L mutation V254A resulted in a similar phenotype and destabilized the electromechanical coupling in the closed state shows that several KCNQ1 mutations that affect the overall gating process have a tendency to result in an open channel conformation. The study by Choveau et al. (accompanying article (45)) shows that the S4S5L-S6T interaction indeed stabilizes the closed state. This is comparable with the observations in hERG channels that suggest that the interaction between the S4S5L and S6T stabilizes the channels closed state (10, 11).
Many Kv channels display C-type inactivation, and it has been shown to be a process that involves conformational changes at the level of the selectivity filter (37–41). At least two mechanisms have been proposed for constriction of the selectivity filter: 1) a reorientation of the VSD (42, 43) or 2) a rearrangement in S6 after channel opening that is transmitted to the selectivity filter destabilizing its conducting conformation (41). Possibly, both pathways exist and depending on the type of Kv channel one predominates. In the case of the Val310 mutation that locates at the base of the selectivity filter, the enhanced inactivation can be caused by directly destabilizing the conducting state of the selectivity filter. In the case of the S4S5L mutations at position Trp248 and Val254, the origin of the pronounced inactivation process is less clear. In both homology models for the open and closed channel conformation, residues Leu250, Val254, and His258 are facing the bottom part of the S6 segment, whereas other high impact residues Trp248, Leu251, and Val255 are oriented toward the lipid-membrane interface. If this orientation is correct, this suggests that substitutions at the latter positions result in altered gating kinetics by disturbing the anchoring of the S4S5L to the plasma membrane. However, caution is needed with this interpretation because S4S5L might be oriented differently with respect to S6T in the open and closed conformations, making the contact interface with the S6T quite broad (on the α-helical wheel representation in Fig. 6 stretching from Leu250 to Arg259). In the case of the W248A mutation, the impact was mainly on the voltage dependence of inactivation. If the inactivation process in KCNQ1 involves a S4 movement, this would mean that the S4S5L reorientation restricts the movement of the voltage-sensing domain. On the other hand, the V254A mutation that, like W248A, induces an enhanced inactivation rate but disturbs the coupling with S6T supports the idea that inactivation is linked to reorientations within the S6 segment itself.
In conclusion, by performing a residue substitution scan of the S4S5L region in KCNQ1, we established that residue Val254 of the S4S5L comes in close proximity to Leu353 in S6T upon channel deactivation, stabilizing the closed conformation. In addition to this specific residue pair, the electromechanical coupling is most likely formed by multiple additional contacts, which, in concert, directly influence gate opening and closure and indirectly also influence the inactivation process of KCNQ1 channels.
Supplementary Material
Acknowledgments
We thank Jean-Pierre Timmermans for the use of the confocal microscope and Isabelle Baró and Jean Mérot for helpful discussion on the manuscript.
This work was supported by Research Foundation Flanders (Fonds voor Wetenschappelijk Onderzoek Vlaanderen) Grants G.0152.06 and G.0256.08 (to D. J. S.) and 1.5.044.07 (to A. J. L.), the Interuniversity Attraction Poles program P6/31 of the Belgian Federal Science Policy Office, GOA/TOP41.3016 (concerted action fund University of Antwerp), and Tournesol T2010.13 (to D. J. S. and G. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- S6T
- C-terminal end of the S6 segment
- S4S5L
- S4-S5 linker
- VSD
- voltage-sensing domain.
REFERENCES
- 1. Barhanin J., Lesage F., Guillemare E., Fink M., Lazdunski M., Romey G. (1996) Nature 384, 78–80 [DOI] [PubMed] [Google Scholar]
- 2. Sanguinetti M. C., Curran M. E., Zou A., Shen J., Spector P. S., Atkinson D. L., Keating M. T. (1996) Nature 384, 80–83 [DOI] [PubMed] [Google Scholar]
- 3. del Camino D., Yellen G. (2001) Neuron 32, 649–656 [DOI] [PubMed] [Google Scholar]
- 4. Liu Y., Holmgren M., Jurman M. E., Yellen G. (1997) Neuron 19, 175–184 [DOI] [PubMed] [Google Scholar]
- 5. Bezanilla F. (2008) Nat. Rev. Mol. Cell Biol. 9, 323–332 [DOI] [PubMed] [Google Scholar]
- 6. Panaghie G., Abbott G. W. (2007) J. Gen. Physiol. 129, 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shamgar L., Haitin Y., Yisharel I., Malka E., Schottelndreier H., Peretz A., Paas Y., Attali B. (2008) PLoS ONE 3, e1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miceli F., Cilio M. R., Taglialatela M., Bezanilla F. (2009) Channels 3, 274–283 [PubMed] [Google Scholar]
- 9. Decher N., Chen J., Sanguinetti M. C. (2004) J. Biol. Chem. 279, 13859–13865 [DOI] [PubMed] [Google Scholar]
- 10. Ferrer T., Rupp J., Piper D. R., Tristani-Firouzi M. (2006) J. Biol. Chem. 281, 12858–12864 [DOI] [PubMed] [Google Scholar]
- 11. Tristani-Firouzi M., Chen J., Sanguinetti M. C. (2002) J. Biol. Chem. 277, 18994–19000 [DOI] [PubMed] [Google Scholar]
- 12. Lu Z., Klem A. M., Ramu Y. (2002) J. Gen. Physiol. 120, 663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prole D. L., Yellen G. (2006) J. Gen. Physiol. 128, 273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Batulan Z., Haddad G. A., Blunck R. (2010) J. Biol. Chem. 285, 14005–14019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long S. B., Campbell E. B., Mackinnon R. (2005) Science 309, 897–903 [DOI] [PubMed] [Google Scholar]
- 16. Long S. B., Campbell E. B., Mackinnon R. (2005) Science 309, 903–908 [DOI] [PubMed] [Google Scholar]
- 17. Pathak M. M., Yarov-Yarovoy V., Agarwal G., Roux B., Barth P., Kohout S., Tombola F., Isacoff E. Y. (2007) Neuron 56, 124–140 [DOI] [PubMed] [Google Scholar]
- 18. Boulet I. R., Labro A. J., Raes A. L., Snyders D. J. (2007) J. Physiol. 585, 325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yifrach O., MacKinnon R. (2002) Cell 111, 231–239 [DOI] [PubMed] [Google Scholar]
- 20. Hong K. H., Miller C. (2000) J. Gen. Physiol. 115, 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li-Smerin Y., Hackos D. H., Swartz K. J. (2000) Neuron 25, 411–423 [DOI] [PubMed] [Google Scholar]
- 22. Monks S. A., Needleman D. J., Miller C. (1999) J. Gen. Physiol. 113, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pusch M., Magrassi R., Wollnik B., Conti F. (1998) Biophys. J. 75, 785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mildvan A. S., Weber D. J., Kuliopulos A. (1992) Arch. Biochem. Biophys. 294, 327–340 [DOI] [PubMed] [Google Scholar]
- 25. Ohlenschläger O., Hojo H., Ramachandran R., Görlach M., Haris P. I. (2002) Biophys. J. 82, 2995–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deschênes D., Acharfi S., Pouliot V., Hegele R., Krahn A., Daleau P., Chahine M. (2003) Can. J. Physiol. Pharmacol. 81, 129–134 [DOI] [PubMed] [Google Scholar]
- 27. Franqueza L., Lin M., Shen J., Splawski I., Keating M. T., Sanguinetti M. C. (1999) J. Biol. Chem. 274, 21063–21070 [DOI] [PubMed] [Google Scholar]
- 28. Wang Z., Tristani-Firouzi M., Xu Q., Lin M., Keating M. T., Sanguinetti M. C. (1999) J. Cardiovasc. Electrophysiol. 10, 817–826 [DOI] [PubMed] [Google Scholar]
- 29. Kubota T., Shimizu W., Kamakura S., Horie M. (2000) J. Cardiovasc. Electrophysiol. 11, 1048–1054 [DOI] [PubMed] [Google Scholar]
- 30. Napolitano C., Priori S. G., Schwartz P. J., Bloise R., Ronchetti E., Nastoli J., Bottelli G., Cerrone M., Leonardi S. (2005) JAMA 294, 2975–2980 [DOI] [PubMed] [Google Scholar]
- 31. Itoh T., Tanaka T., Nagai R., Kikuchi K., Ogawa S., Okada S., Yamagata S., Yano K., Yazaki Y., Nakamura Y. (1998) Hum. Genet. 103, 290–294 [DOI] [PubMed] [Google Scholar]
- 32. Labro A. J., Boulet I. R., Timmermans J. P., Ottschytsch N., Snyders D. J. (2010) J. Mol. Cell Cardiol. 48, 1096–1104 [DOI] [PubMed] [Google Scholar]
- 33. Labro A. J., Raes A. L., Grottesi A., Van Hoorick D., Sansom M. S., Snyders D. J. (2008) J. Gen. Physiol. 132, 667–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith J. A., Vanoye C. G., George A. L., Jr., Meiler J., Sanders C. R. (2007) Biochemistry 46, 14141–14152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barghaan J., Bähring R. (2009) J. Gen. Physiol 133, 205–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seebohm G., Westenskow P., Lang F., Sanguinetti M. C. (2005) J. Physiol. 563, 359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kiss L., LoTurco J., Korn S. J. (1999) Biophys. J. 76, 253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kurata H. T., Fedida D. (2006) Prog. Biophys. Mol. Biol. 92, 185–208 [DOI] [PubMed] [Google Scholar]
- 39. Gibor G., Yakubovich D., Rosenhouse-Dantsker A., Peretz A., Schottelndreier H., Seebohm G., Dascal N., Logothetis D. E., Paas Y., Attali B. (2007) Biophys. J. 93, 4159–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cordero-Morales J. F., Cuello L. G., Zhao Y., Jogini V., Cortes D. M., Roux B., Perozo E. (2006) Nat. Struct. Mol. Biol. 13, 311–318 [DOI] [PubMed] [Google Scholar]
- 41. Cuello L. G., Jogini V., Cortes D. M., Perozo E. (2010) Nature 466, 203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Panaghie G., Purtell K., Tai K. K., Abbott G. W. (2008) Biophys. J. 95, 2759–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Olcese R., Latorre R., Toro L., Bezanilla F., Stefani E. (1997) J. Gen. Physiol. 110, 579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Humphrey W., Dalke A., Schulten K. (1996) J. Mol. Graph. 14, 33–38 [DOI] [PubMed] [Google Scholar]
- 45. Choveau F., Rodriguez N., Abderemane Ali F., Labro A. J., Rose T., Dahimene S., Boudin H., Le Henaff C., Escande D., Snyders D. J., Charpentier F., Merot J., Baro I., Loussouarn G. (2010) J. Biol. Chem. 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.