Abstract
Myelin formation is a multistep process that is controlled by a number of different extracellular factors. During the development of the central nervous system (CNS), oligodendrocyte progenitor cells differentiate into mature oligodendrocytes that start to enwrap axons with myelin membrane sheaths after receiving the appropriate signal(s) from the axon or its microenvironment. The signals required to initiate this process are unknown. Here, we show that oligodendrocytes secrete small membrane vesicles, exosome-like vesicles, into the extracellular space that inhibit both the morphological differentiation of oligodendrocytes and myelin formation. The inhibitory effects of exosome-like vesicles were prevented by treatment with inhibitors of actomyosin contractility. Importantly, secretion of exosome-like vesicles from oligodendrocytes was dramatically reduced when cells were incubated by conditioned neuronal medium.
In conclusion, our results provide new evidence for small and diffusible oligodendroglial-derived vesicular carriers within the extracellular space that have inhibitory properties on cellular growth. We propose that neurons control the secretion of autoinhibitory oligodendroglial-derived exosomes to coordinate myelin membrane biogenesis.
Keywords: Development, Exocytosis, Membrane, Neurobiology, Neurodifferentiation
Introduction
During the development of the CNS, oligodendrocyte precursor cells (OPCs)2 undergo major changes in cell morphology when they start to differentiate into myelin-forming oligodendrocytes (1–3). First, a large network of branching processes is formed, and then oligodendrocytes start to extend massive amounts of myelin membrane sheaths (4, 5). The generation of myelinating oligodendrocytes from proliferating, immature OPCs is complex and depends on an intrinsic genetic differentiation program that is regulated in part by an intracellular molecular clock determining how many times an OPC can divide before differentiating (6–8). This intrinsic program is under the control of various extrinsic factors present within the microenvironment of oligodendrocytes. Many of these factors such as Notch1, Wnt, LINGO-1 (leucine-rich repeat, and Ig domain-containing, Nogo receptor-interacting protein 1), or the polysialylated neural cell adhesion molecule are inhibitory in nature and prevent OPCs from differentiating into mature oligodendrocytes (9–14). Down-regulation of these inhibitory cues seems to be an important mechanism in triggering the differentiation of oligodendrocytes. Once differentiation of oligodendrocytes is induced, the cells start to synthesize large amount of myelin membrane components including lipids and several myelin-specific proteins, the major ones of these being the proteolipid protein (PLP) and the myelin basic proteins (MBP). Curiously, the biosynthesis of the major myelin membrane components occurs before the assembly of myelin is initiated, most likely by neuronal factor(s). In fact, such premyelinating oligodendrocytes that have started to synthesize myelin lipids and proteins but have not yet wrapped myelin around axons are only found for a relatively short period during the development of the CNS (15). Importantly, these premyelinating oligodendrocytes seem to separate the different myelin membrane components at distinct spatial localization, possibly to prevent premature and inappropriate assembly. It is therefore possible that the regulation of myelin membrane trafficking is one important mechanism that induces myelin membrane assembly at the appropriate time during development. Indeed, previous work has provided evidence that the trafficking of PLP is influenced by neurons (16, 17). A relatively large fraction of PLP is delivered to late endosomes/lysosomes in cultured oligodendrocytes, whereas the localization of PLP to late endosomes/lysosomes is reduced after coculture with neurons (17). These late endosomal multivesicular bodies (MVBs) can either be transported down the endosomal pathway for lysosomal degradation, or they can fuse with the plasma membrane to release their intraluminal vesicles into the extracellular space and are then termed exosomes (18, 19). Exosomes are membrane vesicles with a diameter of 50–100 nm that are secreted into the extracellular space by many different cells, where they play an important role in processes such as protein turnover, signal transduction, transfer of mRNA, induction of immune responses, and tumor spreading (19, 20). Previous studies have shown that oligodendrocytes secrete relatively large amounts of exosomes, but the physiological function of these vesicles has remained obscure (21, 22). Here, we studied the function of oligodendrocyte-derived exosomes in primary cell culture.
EXPERIMENTAL PROCEDURES
Inhibitors and Antibodies
The following inhibitors were purchased from Sigma; Y27632, blebbistatin, and PP2. The following primary antibodies were used: mouse anti-PLP (antibody no. 3F4), PLP (antibody no. 431A), and anti-Olig-2 (kind gift from K. Nave); mouse anti-NogoA (antibody no. 11C7, a kind gift from M. Schwab); mouse anti-flotillin-2, mouse anti-Alix (BD Biosciences); mouse anti-TSG101 (antitumor susceptibility gene 101) (Abcam) mouse anti-α-actin (AC-40, Sigma-Aldrich); mouse anti-CNPase (Sigma); mouse anti-A2B5, mouse anti-MAG, mouse anti-MOG (Millipore); rabbit anti-SFK (phospho-Tyr-529) (Calbiochem); rabbit anti-SFK (phospho-Tyr-418), rabbit anti-phospho-FAK (focal adhesion kinase; phospho-Tyr-397) (Invitrogen); mouse anti-FAK (BD Transduction Laboratories); rabbit anti-Fyn (H-80, Santa Cruz Biotechnology); mouse anti-Lamp1 (CD107a) (Santa Cruz Biotechnology); rabbit anti-phospho-Akt (phospho-Ser-473), rabbit anti-Akt (pan), rabbit anti-phospho ribosomal subunit 6 (phospho-Ser-235/236), rabbit anti-RhoA, rabbit anti-phospho-MLC2 (phospho-Ser-19), and rabbit anti-MLC2 (Cell Signaling); mouse anti-β-III-tubulin (Promega); rabbit anti-calnexin (Sressgen Bioreagents); rabbit anti-MBP (DakoCytomation); mouse anti-O1; secondary antibodies were purchased from Dianova, Germany.
Preparation of Brain Sections
For immunhistochemistry, brains of six wild-type mice (P7, P14, and P21) and of two shiverer mice (P21) were used. The animals were deeply anesthetized by intraperitoneal application of Avertin (1.25 mg/dl 2,2,2-tribromoethanol, 2.5 ml/d,l-2-methyl-2-butanol in aqua distilled, 0.2 ml at 10 g weight). Next, the right atrium of the heart was opened by a small cut, and the vascular system was perfused via a cannula in the left ventricle, first with Hanks' buffered salt solution for 5 min, then with 4% PFA in PBS for 15 min. The isolated brain tissue was fixed overnight in 4% PFA in PBS and then frozen at −80 °C. Preparation of brain sections, all in a transversal orientation, was performed with a cryostat. The 30-μm-thick sections were put in a 24-hole plate with a buffer of PBS with 25% ethylene glycol and 25% glycerin and then frozen at −20 °C.
Cell Culture
Primary cultures of rat oligodendrocytes were prepared as described previously (17). Rat neonatal hemispheres were stripped free of meninges and then digested and cultured in Eagle's basal medium including 10% horse serum on poly-l-Lysine-coated flasks at 37 °C in 7% CO2. After 10–14 days, oligodendrocytes were isolated from mixed glial cultures using mechanical dissociation. Purified cells then were plated in DMEM containing B27 supplement, 1% exosome-free horse serum, l-thyroxine, triiodothyronine, glutamine, pyruvate, and penicillin/streptomycin on poly-l-lysine-coated dishes or glass coverslips.
Primary cultures enriched in neurons were obtained by preparing a mixed brain culture from 16-day-old fetal mice (23). The cells were plated and grown for 2 weeks in Sato-B27/1% exosome-free horse serum. The neuronal conditioned medium was collected after culturing for 2 weeks, centrifuged for 10 min at 1500 rpm, and used immediately. Coculture of neurons and oligodendrocytes were prepared by adding the oligodendrocytes to a 2-week-old neuronal culture.
Exosome Preparation
Exosomes were prepared by performing sequential centrifugation steps as described (22). Culture medium was collected from primary oligodendrocytes cultured for 6–8 days, the media was centrifuged for 10 min at 3000 × g and two times for 10 min at 4000 × g before it was subjected for ultracentrifugation at 10,000 × g for 30 min and at 100,000 × g for 1 h. The collected pellets were finally resuspended in PBS, medium, or sample buffer.
Proliferation Assay
Proliferation of oligodendrocytes was performed using a thymidine analog bromodeoxyuridine (BrdU) in situ detection kit (BD Pharmingen). Briefly, 1 day after shaking, oligodendrocytes were incubated with 10 μm BrdU, and the proliferation assay was assessed after 24 h. Cells were washed and fixed, then immunofluorescence was performed with antibodies against A2B5 to visualize oligodendrocyte progenitors and with biotinylated anti-BrdU antibody and streptavidin-HRP to detect proliferating cells. BrdU incorporation was defined as the percentage of A2B5-positive cells that were positive for BrdU.
RhoA Activity Measurements
RhoA activity was measured by affinity precipitation of active Rho (GTP-bound). Oligodendrocytes were cultured for 4 days, and RhoA activity was evaluated using Rho assay reagent (Rhotekin RBD, agarose) from Millipore Corporation. Cells were lysed (Mg2+ lysis/wash buffer with “Complete” protease inhibitor cocktails) according to the manufacturer's instructions. After removing cell debris, the lysates were incubated with Rho assay reagent, which specifically binds to the active form of Rho (Rho-GTP), for 45 min at 4 °C. Beads were then washed three times with Mg2+ lysis/wash buffer and once with PBS. The final pellets were then resuspended in 30 μl sample buffer, boiled for 10 min, and subjected to 12% SDS-PAGE for Western blot analysis using anti-RhoA antibody.
Immunoblot Analysis
Cells were washed with PBS and incubated for 10 min on ice with lysis buffer (2% Nonidet P-40, 0.2% SDS, 1 mm EGTA in PBS supplied with Complete protease and phosphatase inhibitor mixture from Roche Diagnostics) and then scraped and centrifuged at 10,000 × g for 10 min. A fraction of the supernatant was mixed with sample buffer (20% glycerol, 4 mm EDTA, 4% SDS, 4% 2-mercaptoethanol, and 100 mm Tris-HCl, pH 6.8). The samples were subjected to 8, 10, or 12% SDS-PAGE electrophoresis and transferred to nitrocellulose membranes using standard protocols. Western blots were revealed by enhanced chemiluminescence (Pierce/Thermo Scientific) and bands were quantified using ImageJ software. For analysis of p-Src family kinase, Fyn, and FAK, cells were lysed using radioimmune precipitation assay buffer (10 mm Tris-HCl, pH 7.4, 100 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm NaF, 20 mm Na4P2O4, 2 mm Na3VO4, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100 supplied with Complete protease, and phosphatase inhibitor cocktails).
Immunostaining, Microscopy and Image Analysis
For immunohistochemistry, the already fixed brain sections were washed in PBS then incubated with blocking solution (5% horse serum and 0,5% Triton X-100 in PBS or with 1% BSA and 1% Tween in PBS) for 1 h at room temperature. Next, the sections were incubated with primary antibodies diluted in blocking solution overnight at 4 °C. After washing in PBS, sections were stained with the secondary antibody.
For immunofluorescence, cells were fixed with 4% PFA for 15 min and were permeabilized with 0.1% Triton X-100 in PBS. Fixed cells were then incubated with blocking solution (2% BSA, 0.2% Fish gelatin, and 2% fetal calf serum in PBS) for 30 min at room temperature. Next, cells were incubated with primary antibodies diluted in blocking solution, washed with PBS, and then incubated with secondary antibodies. Fluorescence images were acquired using a Leica (Nussloch, Germany) DMRXA microscope and a confocal laser scanning microscope (TCS SP equipped with AOBS, Leica) with a 40× oil plan-apochromat objective (Leica). Quantification of fluorescence intensities was performed as described previously (17). The cell surface area of individual cells was approximated by measuring the projected area (the area covered by the cell projected onto a single optical plane).
Cells in all immunofluorescence-stained cryosections were counted using a fluorescence microscope of the type Zeiss Axiophot (Carl Zeiss MicroImaging GmbH; Jena, Deutschland). Images were gained with a confocal laser scanning microscope of the type LSM 510 Meta (Carl Zeiss MicroImaging GmbH), using the objective plan-Neofluar 40×/1.3 oil differential interference contrast (Carl Zeiss MicroImaging GmbH). An argon laser with a wavelength of 488 nm and a filter of 500–530 nm as well as the 543-nm line of a HeNe laser with a filter of 685 nm were used. Electron microscopy was performed as described (22).
RESULTS
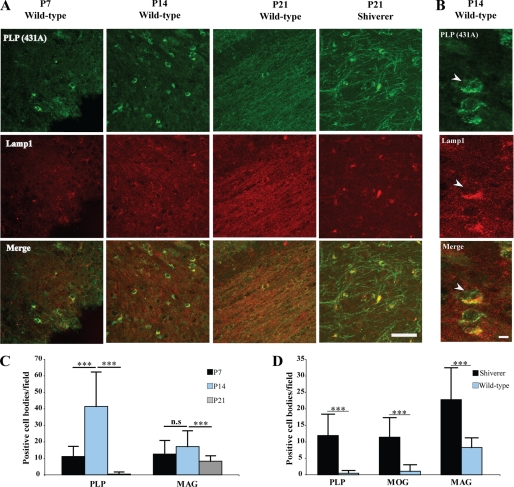
We have previously shown that a relatively large fraction of PLP is localized intracellularly in oligodendrocytes within Lamp-1 positive late endosomes/lysosomes (17). To characterize the localization of PLP in more detail in vivo, an immunohistochemical analysis of brain section of different ages was performed. PLP was detected in the cell body of oligodendrocytes colocalizing with Lamp-1 at P7 and to an even greater extent at P14 (Fig. 1A). At P21, there was a marked decrease of PLP within the cell body (Fig. 1A). MAG also showed a similar distribution as PLP (supplemental Fig. S1 and Fig. 1B). These data show that the extent of endosomal localization of these myelin membrane proteins is dependent on the developmental stage of oligodendrocytes. Oligodendrocytes that contain intracellular accumulations of PLP and MAG may represent premyelinating oligodendrocytes that have not yet elaborated fully developed myelin sheaths. To test whether these oligodendrocytes accumulate in conditions where myelination is impaired, we analyzed brain sections of shiverer mice that lack functional myelin basic protein. Indeed, we observed a much higher number of oligodendrocytes containing PLP, MAG, and MOG within the cell body at P21 of shiverer mice than sections prepared from wild-type mice (Fig. 1, A and C). Olig2 staining confirmed that the cell bodies belonged to oligodendrocytes (supplemental Fig. S2). Moreover, using Iba-1 as a marker, we verified that the cells were not microglia-accumulating PLP, MAG, and MOG after phagocytosis (data not shown).
FIGURE 1.
Subcellular localization of PLP in the developing brain of wild-type and shiverer mice. A, immunohistochemistry of brain sections of P7, P14, and P21 wild-type mice for PLP (green) and Lamp-1 (red) as well as of P21 shiverer mice is shown. Colocalization was observed in sections from P7 and P14 but not P21 wild-type mice. Colocalization is found in brain sections from P21 shiverer mice. Bars, 50 μm. B, immunohistochemistry of brain sections of P14 wild-type mice for PLP (green) and Lamp-1 (red) in higher magnification. Bars, 5 μm. C, quantification of PLP and MAG in the cell body of brain sections from P7, P14, and P21 wild-type mice is displayed (n = 30 fields from different animals). D, the comparison of cell body localization of PLP, MOG, and MAG in P21 brain sections of wild-type and shiverer mice is shown (n = 60 fields from different animals). ***, p < 0.001; t test. n.s., not significant.
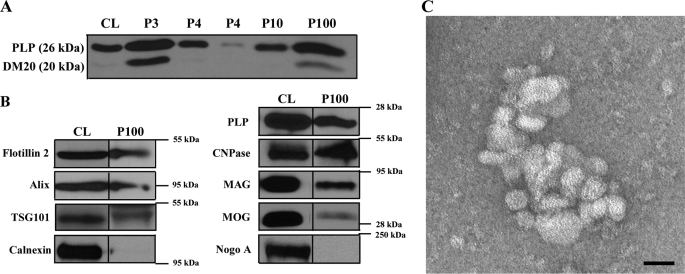
It is not clear why oligodendrocytes transiently accumulate myelin membrane proteins in late endosomes/lysosomes prior to myelination. One possibility is that oligodendrocytes produce myelin in excess, which is subsequently routed to lysosomes for degradation. Another possibility is that late endosomes or MVBs are part of a signaling system that coordinates the process of myelin membrane generation. In fact, we and others (21, 24) have shown that oligodendroglial cells secrete exosomes that are thought to be generated within MVBs and released after the fusion of the MVBs with the plasma membrane, a process regulated by intracellular Ca2+ levels. Previous studies have shown that oligodendrocytes respond to neuronal action potential by raising intracellular Ca2+ levels (25). These exosomes are therefore excellent candidate signaling vehicles with a possible function in initiating myelin membrane formation. To determine the possible function of exosomes in myelination, we established an exosome purification protocol from conditioned medium of primary cultures of rat oligodendrocytes. Sequential centrifugation steps with increasing centrifugal forces up to 100,000 × g yielded a pellet containing small membrane vesicles with a diameter of ∼30 to 100 nm (Fig. 2C).
FIGURE 2.
Identification and characterization of exosome-like vesicles secreted from primary cultures of oligodendrocytes. A, the medium of primary oliogodendrocytes cultured for 6–8 days was collected and submitted to sequential centrifugation steps (3000 × g pellet (P3); 4000 × g pellet (P4); 10,000 × g (P10), and 100,000 × g (P100)) as indicated. The resulting pellets of each centrifugation step were analyzed by Western blotting for PLP. B, cell lysates (CL) and 100,000 × g pellets (P100) were analyzed by Western blotting for the indicated proteins. C, the 100,000 × g pellet was prepared and negatively stained with 1% uranyl acetate. Scale bar, 50 nm.
The enrichment of PLP in the 100,000 × g pellet confirmed the biochemical purification procedure (Fig. 2A). Moreover, the exosomal membrane fraction contained significant amounts of flotillin-2, Alix, TSG101, CNPase, MAG, and MOG, whereas calnexin and Nogo A were absent (Fig. 2B).
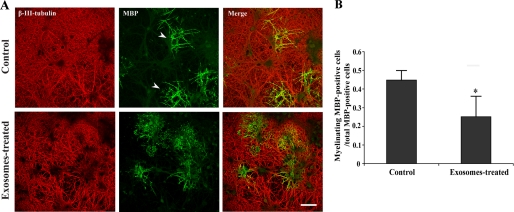
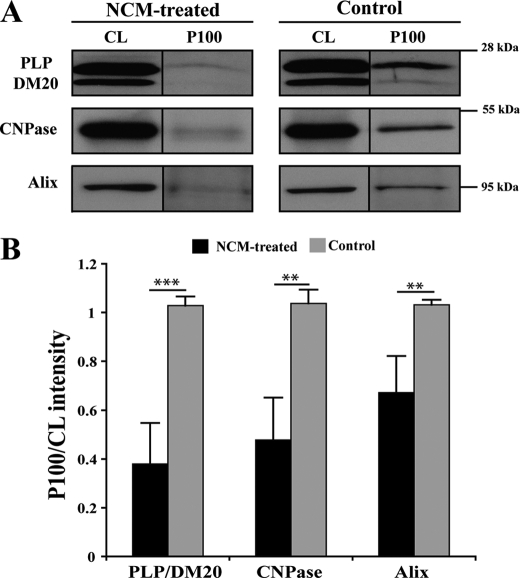
A primary oligodendrocyte-mixed brain coculture system was used to assess the possible role of exosomes in CNS myelination. Exosomes were isolated from ∼106 oligodendrocytes and added to a coculture at day 1 in vitro (∼0.1 μg/ml). Myelination was monitored 4 days later. MBP and β-III-tubulin were used to identify myelinated internodes as described previously (Fig. 3A) (25, 26). Surprisingly, we found that exosome-like vesicles inhibited myelination (Fig. 3B). Because these results pointed to a regulatory role of these vesicles in myelination, we tested whether their secretion was influenced by neuronal signals. We have previously shown that the treatment of oligodendroglial cells with conditioned medium from mixed brain cultures (neuronal conditioned medium (NCM)) reduces the late endosomal/lyososomal pool of PLP (17). To assess the effect of NCM on exosome secretion, the medium of oligodendrocytes was replaced by exosome-depleted NCM after 6 days in culture, and vesicle secretion was monitored 24 h later by determining the amount of PLP per 100,000 × g pellet. Treatment of oligodendrocytes with NCM led to a dramatic inhibition of PLP release (Fig. 4A). Neuronal conditioned medium also reduced the secretion of CNPase and Alix (Fig. 4B). Surprisingly, flotillin-2 release was unaffected (data not shown). At present, we do not know whether this is due to distinct sorting processes into the same or separate population of exosomes.
FIGURE 3.
Exosome-like vesicles inhibit myelination. A, oligodendrocytes and neurons were treated 1 day after coculture with exosomes for 4 days. The cells were immunolabeled for β-III-tubulin (red) and MBP (green). Scale bar, 100 μm. B, myelinating oligodendrocytes were quantified by determining the number of MBP-positive cells with multiple parallel processes (arrows) and expressed as the ratio of total MBP-positive cells (n ∼ 500 cells from three independent experiments. *, p < 0.05; t test.
FIGURE 4.
Neuronal conditioned medium reduces PLP, CNPase, and Alix release from oligodendrocytes. A, primary oligodendrocytes were cultured for 6 days, switched to exosome-depleted NCM and exosome-like vesicles prepared 24 h later from the medium by sequential centrifugation steps. B, the amount of PLP, CNPase, and Alix was determined in the cell lysate (CL) and in the exosome fraction (P100). **, p < 0.01; ***, p < 0.001; t test.
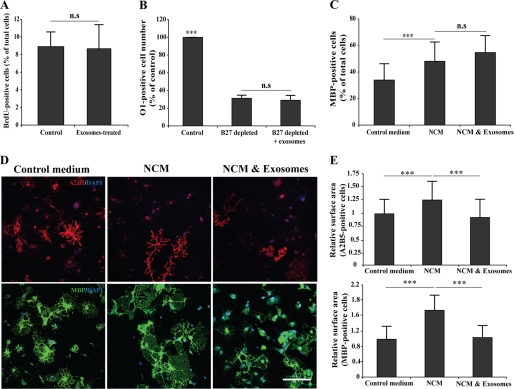
To elucidate the mechanisms involved in exosomes inhibiting myelination, we studied the effect of exosome-like vesicles on cultured oligodendrocytes. First, we tested whether exosomes modulate oligodendrocyte proliferation by performing a BrdU assay on freshly plated cells. No significant changes in BruU incorporation was observed in exosome-treated oligodendrocytes when compared with the controls (Fig. 5A), and the cell numbers were not significantly changed (data not shown). Next, we assessed the possible effects of exosome-like vesicles on oligodendrocytes survival by applying a growth factor deprivation assay (27). Depletion of B27 supplement induced cell death, which was not affected by the treatment with exosome-like vesicles (Fig. 5B). We also tested the role of exosomes in modulating oligodendrocyte differentiation. When oligodendrocytes were cultured in normal medium or in NCM and analyzed 2–3 days later, we observed that NCM promoted oligodendrocyte differentiation as determined by an increase in the fraction of MBP-positive cells. Treatment of oligodendrocytes with exosome-like vesicles did not affect this change (Fig. 5C). However, we did observe in these experiments an effect of exosome-like vesicles on cell surface area. NCM treatment resulted in a relatively robust increase in the size of oligodendrocytes as compared with cells kept in unconditioned control medium. This effect was observed in both undifferentiated (A2B5-positive) and differentiated (MBP-positive) cells (Fig. 5D). Incubation of cells with exosome-like vesicles reversed the effect of NCM on cell surface extension (Fig. 5E).
FIGURE 5.
Exosome-like vesicles inhibits cell surface expansion of oligodendrocytes. A, oligodendrocytes were treated with 10 μm BrdU after 1 day in culture, and the proliferation assay was performed after 24 h (n = 3). B, primary cultures of oligodendrocytes were grown in presence (control) or absence of B27 supplement. Treatment of oligodendrocytes with exosome-like vesicles did not prevent oligodendrocyte cell death (n = 50 confocal images from two independent experiments). C, treatment of oligodendrocytes with NCM for 3 days increased the number of MBP-positive cells. Cotreatment of NCM with exosome-like vesicles did not change the number of MBP-positive cells (n = 50 confocal images from three independent experiments). D and E, primary oligodendrocytes were treated for 3 days with NCM and incubated with exosome-like vesicles during the last 2 days as indicated. Cell surface area of A2B5- and MBP-positive cells was determined as described under “Experimental Procedures.” Treatment with NCM resulted in an increase in cell surface area. Addition of exosome-like vesicles completely prevented the increase in cell surface area (n = 50 confocal images from three independent experiments). ***, p < 0.001; analysis of variance. Scale bar, 100 μm. n.s., not significant.
Having identified a role of exosome-like vesicles in regulating cell surface size, we studied the potential signal transduction pathways involved in this process. The mTOR pathway is known to regulate the myelin membrane formation in oligodendrocytes (28–30). Activation of mTOR signaling results in the phosphorylation of Akt and the ribosomal subunit 6; however, exosome-like vesicles did not change the phosphorylation status of these proteins when compared with control conditions, which argues against this pathway playing a role in mediating the changes in cell surface area (supplemental Fig. S3).
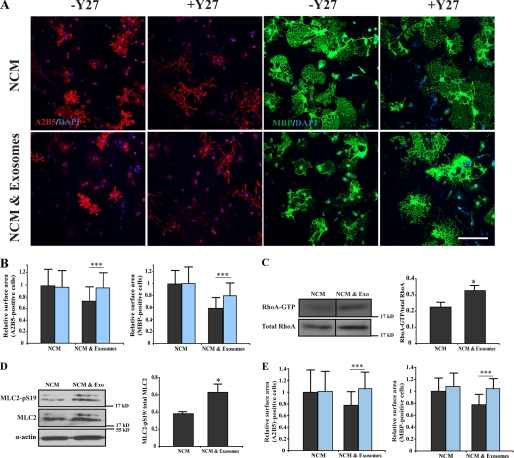
Several previous studies have established a critical role for the RhoA-ROCK (Rho-associated kinase) pathway in oligodendrocyte branching and cell surface expansion (11, 16, 31, 32). Thus, we tested the possibility that exosome-like vesicles act by modulating RhoA-ROCK. Indeed, when we used ROCK inhibitor Y-27632, the inhibitory effect of exosome-like vesicles on cell surface expansion was prevented. Similar results were obtained when the myosin II inhibitor, blebbistatin, was used in the experiments (Fig. 6E). Importantly, we found that both blebbistatin and Y-27632 did not affect cell surface size of oligodendrocytes cultured in NCM in the absence of exosome-like vesicles (Fig. 6, A and B). Together, these data indicate that exosome-like vesicles may activate the Rho-ROCK-myosin signaling axis to prevent cell surface expansion of oligodendrocytes. To confirm the contribution of RhoA pathway its activity was measured by affinity precipitation of active (GTP-bound) Rho from cell lysates using agarose beads containing the Rho-binding domain of rhotekin. A pulldown assay showed that the active form of RhoA (RhoA-GTP) is increased upon treatment of oligodendrocytes with exosome-like vesicles (Fig. 6C). To obtain further support for this pathway, the phosphorylation level of MLC2 (myosin light chain 2), a downstream molecule of RhoA-ROCK, was studied. We found that MLC2 phosphorylation increased when cells were treated with exosome-like vesicles (Fig. 6D). Together, these data indicate that exosome-like vesicles act by influencing actomyosin contractility in oligodendrocytes.
FIGURE 6.
Inhibition of actomyosin contractility prevents the effects of exosome-like vesicles on cell surface area. A, primary oligodendrocytes were treated for 3 days with NCM and incubated with exosome-like vesicles during the last 2 days in the absence or presence of 10 μm Y27632 (Y27). The cells were immunolabled for A2B5 (red) and MBP (green). Scale bar, 100 μm. B, Y27632 did not change cell surface area of A2B5- and MBP-positive cells treated with only NCM, but the inhibitory effect of exosomes on cell surface expansion was reduced by Y27632. Black column, without Y27632; blue column, with Y27632 (n = 42 confocal images from three independent experiments). C, oligodendrocytes were cultured in NCM for 4 days with or without exosome-like vesicles. Using a pulldown assay, the amount of active RhoA (RhoA-GTP) compared with total RhoA was measured. Treatment with exosome like-vesicles leads to an increase in RhoA activity as compared with the control (n = 3). D, the phosphorylation of MLC2 (Ser-19) was evaluated after treatment of oligodendrocytes with exosome-like vesicles (n = 3). E, blebbistatin (50 μm) did not change cell surface area of A2B5- and MBP-positive cells treated with only NCM, but the inhibitory effect of exosomes on cell surface expansion was reduced by blebbistatin (n = 30 confocal images from two independent experiments). Black column, without blebbistatin; blue column, with blebbistatin (*, p < 0.05; ***, p < 0.001; t test). exo, exosomes.
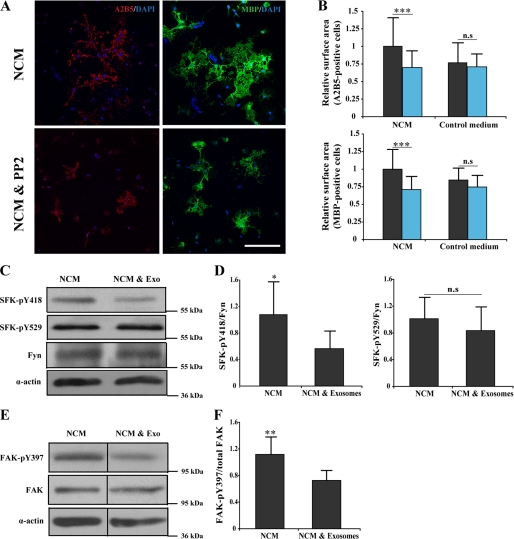
Fyn activation is known to inactivate RhoA in oligodendrocytes (31). Previous studies have shown that Fyn, a member of SFKs, plays an important role in CNS myelination by regulating morphological differentiation of oligodendrocytes (26, 33–38). We used the small molecular inhibitor PP2 to find out whether NCM increase oligodendrocytes cell surface area in a Src kinase-dependent manner. Treatment of cultured oligodendrocytes with PP2 blocked the cell surface expansion induced by NCM (Fig. 7, A and B). Because SFKs are regulated by the phosphorylation of an activating tyrosine (Tyr-418 in Src and Tyr-420 in Fyn) and by an inhibitory tyrosine (Tyr-529 in Src and Tyr-531 in Fyn) in the C-terminal tail, we examined the phosphorylation status of Tyr-420 and Tyr-531 after exosome treatment. Western blot analysis revealed a significant decrease in the activating phosphate (Tyr-420), but not the inactivating phosphate (Tyr-531) in cells that had been incubated with exosomes (Fig. 7, C and D). Fyn signaling is connected to another kinase, the FAK, which has been reported to regulate CNS myelination (39, 40). Integrins and various extracellular signals activate FAK, which in turn leads to FAK autophosphorylated at Tyr-397, generating a binding site for the SH2 (Src homology 2) domain of SFKs such as Fyn. We examined the autophosphorylation status of tyrosine 397 residue in FAK and observed a significant decrease in Tyr-397 phosphorylation after exosome treatment (Fig. 7, E and F).
FIGURE 7.
Exosome-like vesicles affect SFK phosphorylation. A, primary oligodendrocytes were cultured for 3 days in NCM in the absence or presence of 1 μm SFK inhibitor, PP2, for the last 2 days. The cells were immunolabeled for A2B5 (red) and MBP (green). Scale bar, 100 μm. B. Treatment of cells with PP2 leads to a significant reduction in cell surface area of A2B5- and MBP-positive cells. PP2 did not affect cell surface area of oligodendrocytes cultured in the absence of NCM (n = 40 confocal images from three different experiments); black column, without PP2; blue column, with PP2. C, primary oligodendrocytes were treated for 3 days with NCM and either incubated with exosome-like vesicles (Exo) during the last 2 days or not. Cell lysates were analyzed by Western blotting using antibody specific for phosphorylated SFK (Tyr-418, Tyr-529), for Fyn (the major SFK in oligodendrocytes) and for actin as a loading control. D, the quantification of four independent experiments is shown. E, the autophosphorylation of FAK was evaluated using antibody specific for phosphorylated FAK (Tyr-397). F, phosphorylation of FAK is shown as the mean from three independent experiments *, p < 0.05; **, p < 0.01; ***, p < 0.001; t test). n.s., not significant.
DISCUSSION
In summary, we have shown that oligodendrocyte secrete exosome-like vesicles that seem to have an autoinhibitory effect by reducing oligodendrocyte surface expansion. Interestingly, we find that one or more factors in neuronal conditioned medium inhibit the release of exosome-like vesicles from oligodendrocytes. Based on these observations, we propose a model in which neurons control terminal differentiation of oligodendrocytes by regulating the secretion of autoinhibitory exosomes from oligodendrocytes.
Exosomes do not seem to influence the differentiation of oligodendrocytes from OPCs, but rather appear to control the growth properties of the cells. Among the numerous factors that have been shown to influence the life cycle of oligodendrocytes, many of them influence the conversion of proliferating, immature OPCs into postmitotic oligodendrocytes (1). Intriguingly, many of the factors are expressed on axons and seem to inhibit the differentiation of OPCs rather than promoting it. For example, polysialylated neural cell adhesion molecule expressed on axons during development or the Notch receptor Jagged1 prevents OPC differentiation (9, 10, 12). Other systems that repress OPC differentiation are the Wnt signaling pathway, LINGO-1 activation, and connective tissue growth factor secretion (11, 12, 14, 41). In chronic multiple sclerosis lesions, the activation of these and other pathways might be responsible for the differentiation block that results in inefficient remyelination (42).
In addition, premyelinating oligodendrocytes have been observed in chronic MS lesions that extend multiple processes positive for myelin proteins (43). These cells have succeeded in differentiating from OPCs but have failed to remyelinate lesion, possibly because they are not receiving the appropriate signals from the chronically demyelinated axons. Premyelinating oligodendrocytes are usually not found in the healthy adult brain but appear for a relatively short time during the development of the CNS (15).
It is likely that the oligodendrocytes with PLP, MAG, and MOG within their cell bodies described in our study represent premyelinating oligodendrocytes. We find that localization of PLP to late endosomes/lysosomes peaks at P14 and is almost absent at P21. It is tempting to speculate that these endosomes represent the compartment in which exosomes are generated. It is possible that at later stages of oligodendrocyte development, neuronal signals reduce the generation of MVBs and thereby the biogenesis of new exosomes. Nonetheless, it is also feasible that exosomes are also formed directly at the plasma membrane by outward pinching of vesicles into the extracellular space.
Interestingly, the premyelinating oligodendrocytes that have been observed in chronic MS lesions contain large perikarya positive for the proteolipid protein (43). It will be interesting to find out whether PLP accumulates in late endosomes/lysosomes and is released in association with exosomes in these premyelinating oligodendrocytes in MS lesions.
The signals responsible for the regulation of exosome secretion are unknown, but they may lead to changes in intracellular Ca2+ levels that are known to stimulate exosome release (21). It is interesting to compare the effects of exosomes to myelin debris generated by demyelinating events. Myelin debris exert a powerful inhibitory effect on OPC differentiation unless they are cleared from the extracellular space by phagocytosis (44–46). Injection of purified myelin in areas of experimentally induced focal demyelination results in inhibition of remyelination by arresting oligodendrocyte differentiation in a premyelinating stage (44). Recently, evidence has been provided that the inhibitory effect on OPC differentiation by myelin is mediated by Fyn-RhoA-ROCK signaling (47). These results show that so far, unknown myelin membrane components present within myelin debris and in exosomes regulate strikingly similar processes by a common signaling pathway. An alternative conclusion is that the myelin membrane proteins are merely cargo, and another unidentified exosome-associated protein mediates this effect. LINGO-1 is another molecule that activates the RhoA-ROCK pathway and decreases Fyn expression and activation (11). In addition, Netrin 1 has been identified to regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA (48).
It is not clear why there are so many inhibitory cues to regulate myelin membrane formation in the CNS, whereas many inductive factors have been identified to control myelination by Schwann cells in the peripheral nervous system. The complex task of coordinating the biogenesis of up to 50 different myelin segments on separate axons by one oligodendrocyte is likely to be one reason. The removal of inhibitory cues from the microenvironment of oligodendrocytes may initiate myelination formation of all process at the same time.
In conclusion, our results provide new evidence for small and diffusible oligodendroglial-derived vesicular carriers within the extracellular space that have inhibitory properties on cellular growth. We propose that secretion of these exosomes coordinate myelin membrane biogenesis.
Supplementary Material
This work was supported by an European Research Council starting grant, the EMBO Young Investigator Program YIP and by the Deutsche Forschungsgemeinshaft through the DFG-Research Center for Molecular Physiology of the Brain, CMPB (FZT 103) (to M. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- OPC
- oligodendrocyte precursor cell
- PLP
- proteolipid protein
- P
- postnatal day
- MBP
- myelin basic protein
- MVB
- multivesicular body
- NCM
- neuronal conditioned medium
- CNPase
- 2′,3′-cyclic nucleotide 3′-phosphodiesterase
- MAG
- myelin-associated glycoprotein
- MOG
- myelin oligodendrocyte glycoprotein.
REFERENCES
- 1. Chong S. Y., Chan J. R. (2010) J. Cell Biol. 188, 305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson W. D., Kessaris N., Pringle N. (2006) Nat. Rev. Neurosci. 7, 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baumann N., Pham-Dinh D. (2001) Physiol. Rev. 81, 871–927 [DOI] [PubMed] [Google Scholar]
- 4. Simons M., Trotter J. (2007) Curr. Opin Neurobiol. 17, 533–540 [DOI] [PubMed] [Google Scholar]
- 5. Sherman D. L., Brophy P. J. (2005) Nat. Rev. Neurosci. 6, 683–690 [DOI] [PubMed] [Google Scholar]
- 6. Temple S., Raff M. C. (1986) Cell 44, 773–779 [DOI] [PubMed] [Google Scholar]
- 7. Barres B. A., Lazar M. A., Raff M. C. (1994) Development 120, 1097–1108 [DOI] [PubMed] [Google Scholar]
- 8. Dugas J. C., Ibrahim A., Barres B. A. (2007) J. Neurosci. 27, 6185–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang S., Sdrulla A. D., diSibio G., Bush G., Nofziger D., Hicks C., Weinmaster G., Barres B. A. (1998) Neuron 21, 63–75 [DOI] [PubMed] [Google Scholar]
- 10. Charles P., Hernandez M. P., Stankoff B., Aigrot M. S., Colin C., Rougon G., Zalc B., Lubetzki C. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 7585–7590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mi S., Miller R. H., Lee X., Scott M. L., Shulag-Morskaya S., Shao Z., Chang J., Thill G., Levesque M., Zhang M., Hession C., Sah D., Trapp B., He Z., Jung V., McCoy J. M., Pepinsky R. B. (2005) Nat. Neurosci. 8, 745–751 [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y., Argaw A. T., Gurfein B. T., Zameer A., Snyder B. J., Ge C., Lu Q. R., Rowitch D. H., Raine C. S., Brosnan C. F., John G. R. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 19162–19167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fancy S. P., Baranzini S. E., Zhao C., Yuk D. I., Irvine K. A., Kaing S., Sanai N., Franklin R. J., Rowitch D. H. (2009) Genes Dev. 23, 1571–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye F., Chen Y., Hoang T., Montgomery R. L., Zhao X. H., Bu H., Hu T., Taketo M. M., van Es J. H., Clevers H., Hsieh J., Bassel-Duby R., Olson E. N., Lu Q. R. (2009) Nat. Neurosci. 12, 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trapp B. D., Nishiyama A., Cheng D., Macklin W. (1997) J. Cell Biol. 137, 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kippert A., Trajkovic K., Rajendran L., Ries J., Simons M. (2007) J. Neurosci. 27, 3560–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trajkovic K., Dhaunchak A. S., Goncalves J. T., Wenzel D., Schneider A., Bunt G., Nave K. A., Simons M. (2006) J. Cell Biol. 172, 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simons M., Raposo G. (2009) Curr. Opin Cell Biol. 21, 575–581 [DOI] [PubMed] [Google Scholar]
- 19. Thery C., Ostrowski M., Segura E. (2009) Nat. Rev. Immunol. [DOI] [PubMed] [Google Scholar]
- 20. Lakkaraju A., Rodriguez-Boulan E. (2008) Trends Cell Biol. 18, 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krämer-Albers E. M., Bretz N., Tenzer S., Winterstein C., Möbius W., Berger H., Nave K. A., Schild H., Trotter J. (2007) Proteomics Clinical Appl. 18, 1446–1461 [DOI] [PubMed] [Google Scholar]
- 22. Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. (2008) Science 319, 1244–1247 [DOI] [PubMed] [Google Scholar]
- 23. Fitzner D., Schneider A., Kippert A., Möbius W., Willig K. I., Hell S. W., Bunt G., Gaus K., Simons M. (2006) EMBO J. 25, 5037–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hsu C., Morohashi Y., Yoshimura S., Manrique-Hoyos N., Jung S., Lauterbach M. A., Bakhti M., Grønborg M., Möbius W., Rhee J., Barr F. A., Simons M. (2010) J. Cell Biol. 189, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stevens B., Porta S., Haak L. L., Gallo V., Fields R. D. (2002) Neuron 36, 855–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laursen L. S., Chan C. W., ffrench-Constant C. (2009) J. Neurosci. 29, 9174–9185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. (1992) Cell 70, 31–46 [DOI] [PubMed] [Google Scholar]
- 28. Tyler W. A., Gangoli N., Gokina P., Kim H. A., Covey M., Levison S. W., Wood T. L. (2009) J. Neurosci. 29, 6367–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Narayanan S. P., Flores A. I., Wang F., Macklin W. B. (2009) J. Neurosci. 29, 6860–6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flores A. I., Narayanan S. P., Morse E. N., Shick H. E., Yin X., Kidd G., Avila R. L., Kirschner D. A., Macklin W. B. (2008) J. Neurosci. 28, 7174–7183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang X., Draghi N. A., Resh M. D. (2004) J. Neurosci. 24, 7140–7149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kippert A., Fitzner D., Helenius J., Simons M. (2009) BMC Cell Biol. 10, 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang P. S., Wang J., Xiao Z. C., Pallen C. J. (2009) J. Biol. Chem. 284, 33692–33702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Relucio J., Tzvetanova I. D., Ao W., Lindquist S., Colognato H. (2009) J. Neurosci. 29, 11794–11806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. White R., Gonsior C., Krämer-Albers E. M., Stöhr N., Hüttelmaier S., Trotter J. (2008) J. Cell Biol. 181, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goto J., Tezuka T., Nakazawa T., Sagara H., Yamamoto T. (2008) Mol. Cell Neurosci. 38, 203–212 [DOI] [PubMed] [Google Scholar]
- 37. Osterhout D. J., Wolven A., Wolf R. M., Resh M. D., Chao M. V. (1999) J. Cell Biol. 145, 1209–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Umemori H., Sato S., Yagi T., Aizawa S., Yamamoto T. (1994) Nature 367, 572–576 [DOI] [PubMed] [Google Scholar]
- 39. Forrest A. D., Beggs H. E., Reichardt L. F., Dupree J. L., Colello R. J., Fuss B. (2009) J. Neurosci. Res. 87, 3456–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Câmara J., Wang Z., Nunes-Fonseca C., Friedman H. C., Grove M., Sherman D. L., Komiyama N. H., Grant S. G., Brophy P. J., Peterson A., ffrench-Constant C. (2009) J. Cell Biol. 185, 699–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stritt C., Stern S., Harting K., Manke T., Sinske D., Schwarz H., Vingron M., Nordheim A., Knöll B. (2009) Nat. Neurosci. 12, 418–427 [DOI] [PubMed] [Google Scholar]
- 42. Franklin R. J., Ffrench-Constant C. (2008) Nat. Rev. Neurosci. 9, 839–855 [DOI] [PubMed] [Google Scholar]
- 43. Chang A., Tourtellotte W. W., Rudick R., Trapp B. D. (2002) N. Engl. J. Med. 346, 165–173 [DOI] [PubMed] [Google Scholar]
- 44. Kotter M. R., Li W. W., Zhao C., Franklin R. J. (2006) J. Neurosci. 26, 328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kotter M. R., Setzu A., Sim F. J., Van Rooijen N., Franklin R. J. (2001) Glia 35, 204–212 [DOI] [PubMed] [Google Scholar]
- 46. Miller R. H. (1999) Dev. Biol. 216, 359–368 [DOI] [PubMed] [Google Scholar]
- 47. Baer A. S., Syed Y. A., Kang S. U., Mitteregger D., Vig R., Ffrench-Constant C., Franklin R. J., Altmann F., Lubec G., Kotter M. R. (2009) Brain 132, 465–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rajasekharan S., Baker K. A., Horn K. E., Jarjour A. A., Antel J. P., Kennedy T. E. (2009) Development 136, 415–426 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.