Abstract
Cadherins and neuroligins (NLs) represent two families of cell adhesion proteins that are essential for the establishment of synaptic connections in vitro; however, it remains unclear whether these proteins act in concert to regulate synapse density. Using a combination of overexpression and knockdown analyses in primary hippocampal neurons, we demonstrate that NL1 and N-cadherin promote the formation of glutamatergic synapses through a common functional pathway. Analysis of the spatial relationship between N-cadherin and NL1 indicates that in 14-day in vitro cultures, almost half of glutamatergic synapses are associated with both proteins, whereas only a subset of these synapses are associated with N-cadherin or NL1 alone. This suggests that NL1 and N-cadherin are spatially distributed in a manner that enables cooperation at synapses. In young cultures, N-cadherin clustering and its association with synaptic markers precede the clustering of NL1. Overexpression of N-cadherin at this time point enhances NL1 clustering and increases synapse density. Although N-cadherin is not sufficient to enhance NL1 clustering and synapse density in more mature cultures, knockdown of N-cadherin at later time points significantly attenuates the density of NL1 clusters and synapses. N-cadherin overexpression can partially rescue synapse loss in NL1 knockdown cells, possibly due to the ability of N-cadherin to recruit NL2 to glutamatergic synapses in these cells. We demonstrate that cadherins and NLs can act in concert to regulate synapse formation.
Keywords: Adhesion, Development, Membrane, Neuron, Synapses, Cadherins, Neuroligins
Introduction
Synapse formation begins with the recognition of appropriate targets and formation of incipient contacts and is followed by the recruitment of pre- and postsynaptic proteins to exquisitely localized microdomains at points of cell-cell contact (1, 2). Transsynaptic cell adhesion complexes have come to the forefront as key players in the formation and maturation of synaptic connections (3, 4). Among these adhesion complexes, the homophilic cadherin complex and the heterophilic neurexin-neuroligin complex have been extensively studied and are known to exert key roles in synapse development (5–7).
The distributions of cadherins and neuroligins (NLs)2 at synaptic compartments have previously been analyzed; however, their spatial distribution with respect to one another is still unclear. This information is essential for understanding the functional interplay between these two adhesion systems. Cadherins and their associated catenins have been observed in both pre- and postsynaptic compartments in many neuronal populations in the CNS (8, 9). Previous reports demonstrate that in the brain, two of these, N- and E-cadherin, are localized to synaptic complexes in mutually exclusive distributions (8). As synapses mature, N-cadherin is excluded from inhibitory synapses, becoming primarily localized at glutamatergic synapses (10).
Evidence that cadherins play an important role in establishing synaptic junctions includes observations that cadherins rapidly accumulate at points of cell-cell contact prior to synaptic differentiation (11, 12) and that disruption of cadherin-based contact inhibits the formation of synapses in primary hippocampal cultures (12–14) and our own work showing that loss of interaction between cadherin and β-catenin inhibits the appropriate localization of synaptic vesicles to presynaptic compartments (15). Despite the fact that disrupting cadherin adhesion complexes abrogates the formation of nascent synapses, de novo synapse formation has not been observed in mammalian neurons upon cadherin overexpression (16–19). Overexpressing N-cadherin in zebrafish can enhance synapse density in young but not mature neurons (17).
Four NL subtypes exist in the CNS, all of which have been shown to localize to synapses. Whereas NL2 is enriched at GABAergic synapses, NL1, NL3, and NL4 have previously been shown to localize mainly at glutamatergic synapses (20–23). In stark contrast to what has been shown for cadherins, overexpression of NLs in cultured hippocampal neurons dramatically enhances the formation of glutamatergic and GABAergic synapses and also increases spine number (20, 24, 25). NLs are not only sufficient to induce synapse formation but are also required for the development of synapses. Indeed, disrupting neurexin-neuroligin complexes (26) or knocking down NL protein expression in cultured neurons results in a strong reduction in the number of excitatory and inhibitory contacts (24).
Recent work has shown that N-cadherin and NL1 can cooperate to control synaptic vesicle clustering at nascent synapses (27). Here, Stan et al. demonstrate that N-cadherin can cluster NL1 at synapses via interaction with the scaffolding molecule S-SCAM. Previous studies have shown that the cadherin-binding protein β-catenin interacts with S-SCAM and NL1 (28, 29).
Our study examines the spatial relationship between NL1 and N-cadherin and whether these adhesion systems cooperate to control synapse density. We demonstrate that approximately half of glutamatergic synapses express both adhesion proteins, indicating that these molecules are spatially distributed in such a way as to enable functional cooperation. Using knockdown and overexpression analyses, we demonstrate that NL1 and N-cadherin mediate synapse formation via a common pathway.
EXPERIMENTAL PROCEDURES
siRNA Constructs and Recombinant DNAs
N-cadherin siRNA (Dharmacon Inc., J-091851-09-0019) and a previously used NL1 siRNA (24) were transfected into rat hippocampal neurons to suppress expression of endogenous N-cadherin and NL1, respectively. The siRNA-resistant N-cadherin-CFP construct was made using site-directed mutagenesis (Stratagene) to introduce five silent point mutations into the N-cadherin coding sequence. The following primer was used: gctggtctggaccgagagaaaGTCCAGCAATACACCTTAAtaattcaagccactgacatg. The siRNA-resistant HA-NL1 construct was made in a similar way, using primer ccatggcggctcttacatGGAGGGAACAGGTAATCTGTatgatgggagtgtc. The siRNA-resistant N-cadherin and NL1 constructs were used in experiments involving N-cadherin or NL1 overexpression. ON-TARGET plus (nontargeting siRNA that is designed not to target any known gene in the cell) was used as a control (Dharmacon Inc., J-091851-09-0019). GFP-NL1 and N-cadherin-CFP were kind gifts from Alaa El-Husseini and Ann-Marie Craig, respectively.
Neuron Cultures
Hippocampi from embryonic day 18 rats were prepared as described previously (30) and plated at a density of 130 cells/mm2. To determine the effects of knockdown and overexpression, neurons were transfected using Lipofectamine 2000 (Invitrogen) following the manufacturer's recommendations.
Immunohistochemistry
Cells transfected at 2 or 10 days in vitro (DIV) were fixed at 6 or 14 DIV and immunolabeled as described previously (31). The primary antibodies used were guinea pig anti-VGlut-1 (Synaptic Systems), mouse anti-PSD-95 (Affinity BioReagents), rabbit anti-N-cadherin (generous gift from Dr. David Colman), and rabbit anti-NL2 (generous gift from Dr. Ann-Marie Craig). The secondary antibodies used were Alexa 488-, Cy5-, and Texas Red-conjugated goat anti-mouse, anti-rabbit, or anti-guinea pig (Molecular Probes).
Immunoblot Analysis
HEK293 cells were transfected with Lipofectamine 2000 and lysed 24 h later as described previously (32). Proteins were separated and visualized as described previously (31). The primary antibodies used were anti-HA (1:1000, Babco) and rabbit anti-GFP (1:1000, Synaptic Systems). HRP-conjugated goat anti-mouse or anti-rabbit secondary antibodies (1:3000, Bio-Rad) were used. Blots were visualized with SuperSignal West Pico chemiluminescent substrate (Thermo Scientific).
Confocal Imaging
Neurons were imaged using an Olympus Fluoview 1000 confocal microscope (10×/0.30 UPlan FL N, 20×/0.75 UPlan SApo, 60×/1.4 Oil Plan-Apochromat). All images in a given experiment were captured and analyzed with the same exposure time and conditions.
Neuronal Masks
To examine protein localization along a single transfected neuron, cells were imaged at ×60, and a “mask” was made of the GFP fluorescence using ImageJ. GFP cells were thresholded until all neurites were solidly highlighted. Using the “Selection” application, an outline of the highlighted neurites was selected. Cell bodies were eliminated, leaving a mask outlining all neurites of the transfected cell.
Colocalization Analyses
Images were analyzed using ImageJ with a colocalization plug-in downloaded from the program's web site. Briefly, thresholded puncta were obtained by subtracting the background immunofluorescence signal of each analyzed image. Colocalization of immunopositive puncta was done by measuring the frequency of overlapping signals of puncta between imaging channels. Points of colocalization were defined as regions greater than 3 pixels in size where the intensity ratio of the two channels was greater than 50. All the puncta were examined in a field.
GFP-NL1 Thresholding
GFP-NL1-expressing cells were manually thresholded using ImageJ so that only a few individual pixels outside the clusters were above the threshold. All images were thresholded using the same values. All GFP-NL1 puncta from 0.5 to 5 μm were included in the analysis.
RESULTS
Although the distributions of cadherins and NLs at synaptic compartments have been analyzed separately, their relative spatial distributions have not been studied. To examine the temporal relationship of N-cadherin and NL1 clustering at synapses, cells were immunolabeled with anti-VGlut-1 and anti-N-cadherin. As antibodies specific to NL1 were unavailable, the localization of NL1 at glutamatergic synapses was determined by expressing a GFP-tagged version of this protein (Fig. 1A). As demonstrated previously (24) and as shown below, transfecting cells with NL1 dramatically enhances the density of synapses. To circumvent the confounding problem of enhanced synapse density when analyzing the distribution of NL1 and N-cadherin at synapses, only cells expressing low levels of GFP-NL1 were analyzed. First, to minimize NL1 overexpression, cells were transfected using 2-fold less DNA than used in our overexpression experiments (see below). In addition, we immunolabeled 14-DIV cultures with the excitatory presynaptic marker VGlut-1 and confirmed that the density of VGlut-1 clusters in GFP-NL1-expressing cells was similar to that of control cells (GFP, 67.8 ± 4 puncta/100 μm of neurite; GFP-NL1, 72.8 ± 4 puncta/100 μm; p = 0.34 (Student's t test); n = 23 cells from three cultures). We therefore concluded that GFP-NL1 would be a faithful marker that we could use to examine the relationship between the distribution of NL1 and N-cadherin at glutamatergic synapses.
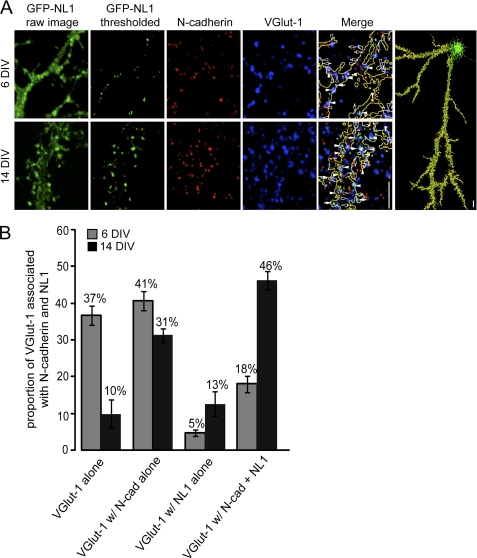
FIGURE 1.
Spatial distribution of N-cadherin and NL1 at glutamatergic synapses. A, confocal images of 6- and 14-DIV neurons transfected at 2 and 10 DIV with GFP-NL1 and immunolabeled with VGlut-1 and N-cadherin. The low magnification image (far right) illustrates a neuron transfected with GFP-NL1 with a mask outlining the area of the cell chosen for analysis. Higher magnification images depict representative raw images of GFP-NL1 clusters and GFP-NL1 clusters following thresholding, as well as immunostaining for VGlut-1 and N-cadherin. Open arrows denote VGlut-1 clusters with no colocalization; closed arrows show colocalization between VGlut-1 and GFP-NL1; open arrowheads show colocalization between VGlut-1 and N-cadherin; closed arrowheads show triple colocalization between VGlut-1, GFP-NL1, and N-cadherin. B, quantification of the proportion of VGlut-1 puncta within the mask that is associated with GFP-NL1, N-cadherin (N-cad), or both. n = an average of 857.6 μm of neurite/cell from 23–27 cells per condition from three separate cultures. Scale bars = 10 μm.
In 6-DIV cultures, N-cadherin was distributed in a punctate pattern and was partly colocalized with VGlut-1. In contrast, NL1 exhibited a more diffuse pattern of localization (Fig. 1A). By 14 DIV, both N-cadherin and NL1 were well clustered (Fig. 1A). Comparing the clustering of N-cadherin and NL1 in young and mature neurons suggests that N-cadherin clustering precedes that of NL1.
To further quantify the localization of N-cadherin and NL1 at nascent synapses, we examined the proportion of glutamatergic synapses that contained N-cadherin or GFP-NL1. Only VGlut-1 puncta opposed to transfected cells were quantified. This was done by creating a mask of a GFP- or GFP-NL1-transfected cell (Fig. 1A (right); (see “Experimental Procedures”). As GFP-NL1 puncta were not as pronounced at 6 DIV as at later time points, image thresholding was required to more clearly observe GFP-NL1 clusters (Fig. 1A). All image acquisition and thresholding were done identically for cells at 6 DIV and for cells at 14 DIV. At 6 DIV, 37% of the VGlut-1 puncta were not associated with N-cadherin or NL1 (Fig. 1B). These puncta may represent mobile VGlut-1 clusters that have not yet become associated with synapses or VGlut-1-positive synapses that are independent of N-cadherin and NL1 function. 41 ± 2.5% of the VGlut-1 clusters associated with N-cadherin alone, 5 ± 0.9% with NL1 alone, and only 18 ± 2.2% of VGlut-1 clusters associated with both adhesion molecules (Fig. 1B). At 14 DIV the majority of VGlut-1 clusters localized with N-cadherin, with 46 ± 2.5% of VGlut-1 clusters associating with both N-cadherin and NL1 (Fig. 1B). This developmental profile of the localization of VGlut-1 with N-cadherin and NL1 suggests that N-cadherin is localized first to nascent synapses and that NL1 becomes subsequently localized to these sites.
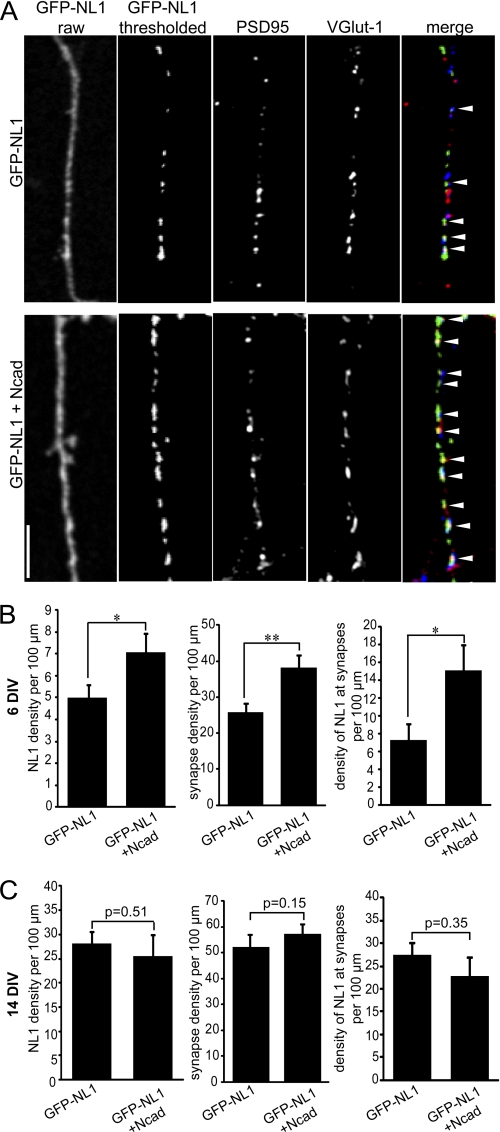
To assess the possibility of a functional interaction between N-cadherin and NL1, we overexpressed N-cadherin in immature neurons and observed the effects on GFP-NL1 clustering. Image thresholding was once again required to more clearly observe GFP-NL1 clusters at this time point. All image acquisition and thresholding were done identically for cells expressing GFP-NL1 alone or GFP-NL1 plus N-cadherin (Fig. 2A). Overexpression of N-cadherin enhanced the density of GFP-NL1 clusters in young cells (Fig. 2, A and B). To examine whether the increased density of GFP-NL1 clusters translates to an enhancement of synapse density, cells were immunolabeled with VGlut-1 and PSD-95, and synapses were defined as sites of overlap between these synaptic proteins. Furthermore, to ensure that only contacts along transfected cells were included in the analysis, masks were drawn to outline the dendrites of transfected cells. A significant increase in synapse density was observed in cells overexpressing N-cadherin, with a corresponding increase in the density of NL1 clusters associated with synapses. In more mature cultures, overexpression of N-cadherin was not sufficient to increase the density of NL1 clusters or synaptic sites (Fig. 2C).
FIGURE 2.
N-cadherin overexpression enhances the density of NL1 clusters in young but not mature neurons. A, confocal images of 6-DIV neurons transfected at 2 DIV with GFP-NL1 or GFP-NL1 + N-cadherin (Ncad)-CFP and immunolabeled with VGlut-1 and PSD-95. Raw images of GFP-NL1 are shown on the far left, and thresholded images are shown in the second column. B, quantification of 6-DIV neurons demonstrates an increase in NL1 density, synapse density, and density of synaptically localized NL1 in N-cadherin-overexpressing cells. C, quantification of 14-DIV neurons demonstrates no change in NL1 density, synapse density, or density of synaptically localized NL1 in N-cadherin-overexpressing cells. n = 20 cells per condition from three separate cultures. *, p < 0.05; **, p < 0.01 (Student's t test). Scale bar = 10 μm.
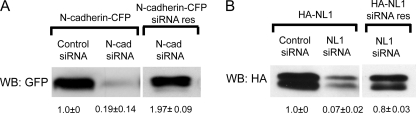
To test the functional relationship between these two adhesion systems in mature neurons, we used a combination of overexpression and siRNA knockdown analyses. To test the efficacy of siRNAs, HEK293 cells were transfected with control siRNA, N-cadherin siRNA, or a previously described NL1 siRNA construct (24), together with either wild-type or point-mutated, siRNA-resistant forms of N-cadherin and NL1 (Fig. 3). NL1 and N-cadherin siRNA decreased NL1 and N-cadherin levels by 93 ± 2 and 81 ± 14%, respectively, demonstrating that these siRNAs efficiently knock down their respective target proteins. Coexpression of these siRNAs with the siRNA-resistant forms of NL1 or N-cadherin failed to knock down these proteins, indicating that they were appropriate siRNA rescue constructs.
FIGURE 3.
siRNA-mediated knockdown of N-cadherin and NL1. A, HEK293 cells transfected with N-cadherin siRNA plus wild-type N-cadherin-CFP display a significant decrease in N-cadherin-CFP levels compared with cells transfected with control nonspecific siRNA plus wild-type N-cadherin-CFP. N-cadherin siRNA did not attenuate levels of siRNA-resistant (res) N-cadherin-CFP. B, HEK293 cells transfected with NL1 siRNA plus wild-type HA-NL1 exhibit a significant decrease in HA-NL1 levels compared with cells transfected with control nonspecific siRNA plus wild-type HA-NL1. Conversely, NL1 siRNA did not attenuate levels of siRNA-resistant HA-NL1. n = three blots with three independent cultures. WB, Western blot.
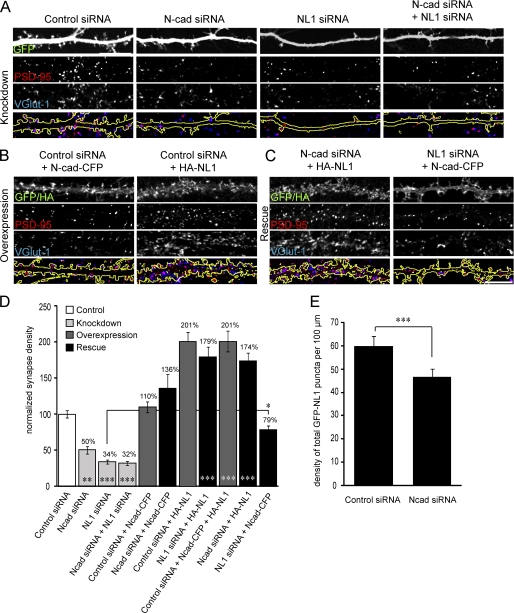
To determine the effects of NL1 and N-cadherin knockdown and overexpression, neurons were transfected at 10 DIV with the specified construct, fixed, and immunolabeled at 14 DIV for VGlut-1 and PSD-95 (Fig. 4, A–C). The density of synapses along the transfected cell (density of VGlut-1/PSD-95 puncta along the GFP mask) was then determined for each condition and normalized to cells expressing control siRNA (Fig. 4D). A 50% reduction in the density of synapses being formed onto cells expressing N-cadherin siRNA was observed, whereas a 66% reduction in the density of synapses on cells expressing NL1 siRNA was seen (Fig. 4, A and D). These decreases are comparable to the reported ∼45% decrease in PSD-95 punctum density in N-cadherin knock-out mice (27) and the ∼60% decrease in synapse density in NL1 knockdown cells (24). Coexpression of N-cadherin and NL1 siRNA resulted in a similar deficit in synapse density as seen in cells expressing NL1 siRNA alone (Fig. 4, A and D). This suggests that NL1 and N-cadherin promote the formation of synapses through a similar pathway. Indeed, if these proteins were mediating synapse formation through parallel pathways or if different populations of synapses were being affected, one would expect an additive effect of the double knockdown.
FIGURE 4.
N-cadherin and NL1 cooperate to regulate synapse formation. A–C, confocal images of 14-DIV neurons transfected at 10 DIV with the indicated constructs and immunolabeled with VGlut-1 and PSD-95. D, quantification of the density of synapses on transfected neurons normalized to cells transfected with control siRNA. Asterisks along the x axis denote significant difference from cells transfected with control siRNA. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (one-way analysis of variance with Tukey's post hoc test). n = an average of 640 μm of neurite/cell from 21–50 cells per condition from four separate cultures. E, the density of NL1 clusters is decreased in N-cadherin (N-cad) siRNA-expressing cells. n = 28–29 cells/condition from three separate cultures. ***, p < 0.001 (Student's t test). Scale bar = 10 μm.
Overexpression of N-cadherin-CFP did not enhance the density of synapses at 14 DIV (Figs. 2C and 4, B and D), whereas overexpression of HA-NL1 enhanced synapse density to 201% of control (Fig. 4, B and D). Simultaneous overexpression of N-cadherin and NL1 resulted in a synapse density similar to that seen with NL1 alone, further demonstrating that overexpression of N-cadherin is not sufficient to promote the formation of supernumerary synapses (Fig. 4D). We define supernumerary synapses here as those that are in excess of the number of synapses typically observed in control cells.
One of the known caveats of siRNA is its potential for producing “off-target” effects, whereby proteins other than the specified target are knocked down. As a further control to confirm that the phenotypes observed in cells expressing N-cadherin and/or NL1 siRNAs were specifically due to knockdown of these proteins, N-cadherin or NL1 knockdown cells were cotransfected with siRNA-insensitive versions of either N-cadherin or NL1, respectively (Fig. 4D). Synapse density in cells coexpressing N-cadherin siRNA and siRNA-resistant N-cadherin was not statistically different from that in those expressing control siRNA or N-cadherin alone. Similarly, cells coexpressing NL1 siRNA and NL1 had statistically similar synaptic densities compared with cells expressing NL1 alone (p > 0.05; one-way analysis of variance with Tukey's post hoc test). Thus, overexpression of siRNA-resistant N-cadherin-CFP in N-cadherin knockdown cells or siRNA-resistant HA-NL1 in NL1 knockdown cells completely rescued the deficits in synapse density.
Because double knockdown of N-cadherin and NL1 indicated that these proteins promote synapse formation through a similar pathway, we further tested the functional interaction between these proteins. To determine whether NL1 could rescue the deficit in synapse density observed following N-cadherin knockdown, cells were cotransfected with N-cadherin siRNA and HA-NL1 (Fig. 4C). This produced a significant increase in the number of synapses compared with cells expressing N-cadherin siRNA alone; however, this was most likely due to the ability of NL1 to induce supernumerary synapses (Fig. 4, A–D). As compared with cells overexpressing HA-NL1 alone, coexpression of N-cadherin siRNA did not significantly reduce synapse density (N-cadherin siRNA + NL1 = 174 ± 11%; NL1 = 201 ± 13%; p > 0.05; one-way analysis of variance with Tukey's post hoc test).
We have shown that N-cadherin overexpression enhances the clustering of NL1 in young (6 DIV) but not older (14 DIV) cultures (Fig. 2). To examine whether N-cadherin knockdown perturbs the proper localization of NL1, cells were cotransfected with GFP-NL1 (at low levels) and N-cadherin siRNA at 10 DIV, and the density of GFP-NL1 was determined at 14 DIV. N-cadherin knockdown was found to significantly reduce the density of GFP-NL1 clusters (Fig. 4E), further demonstrating that N-cadherin is involved in the clustering of NL1. As the density of NL1 clusters is decreased in N-cadherin knockdown cells, it may initially appear inconsistent that N-cadherin knockdown does not attenuate NL1-mediated increases in synapse density. However, localization was determined using the weakly expressing GFP-NL1 used in Figs. 1–3, as GFP-NL1 expression is sufficiently weak to abrogate any increases in synapse density normally observed following high levels of NL1 expression. We therefore believe that expressing low levels of GFP-NL1 is reasonably representative of the endogenous situation. In contrast, the vector expressing HA-NL1 is highly expressed in cells and can significantly enhance synapse formation. We propose that flooding the neuron with NL1 enables sufficient amounts to reach the membrane to induce synapse formation, regardless of the mechanisms normally responsible for its localization. Thus, the effects of expression of HA-NL1 at high levels cannot be attenuated by N-cadherin knockdown (compare HA-NL1 in Fig. 4, B and C).
To determine whether N-cadherin could rescue the phenotype observed following NL1 knockdown, cells were cotransfected with NL1 siRNA and N-cadherin-CFP (Fig. 4C). There was a significant increase in the density of synapses in cells coexpressing NL1 siRNA and N-cadherin (79 ± 5.3%) compared with cells expressing NL1 siRNA alone (34.1 ± 2.8%; p > 0.001; one-way analysis of variance with Tukey's post hoc test), suggesting that overexpression of N-cadherin can at least partially rescue the synaptic deficits observed in NL1 knockdown cells (Fig. 4, A, C, and D). This suggests that although overexpression of N-cadherin is not sufficient to promote the formation of supernumerary synapses in wild-type cells, it is able to at least partially rescue synapse loss observed following NL1 knockdown.
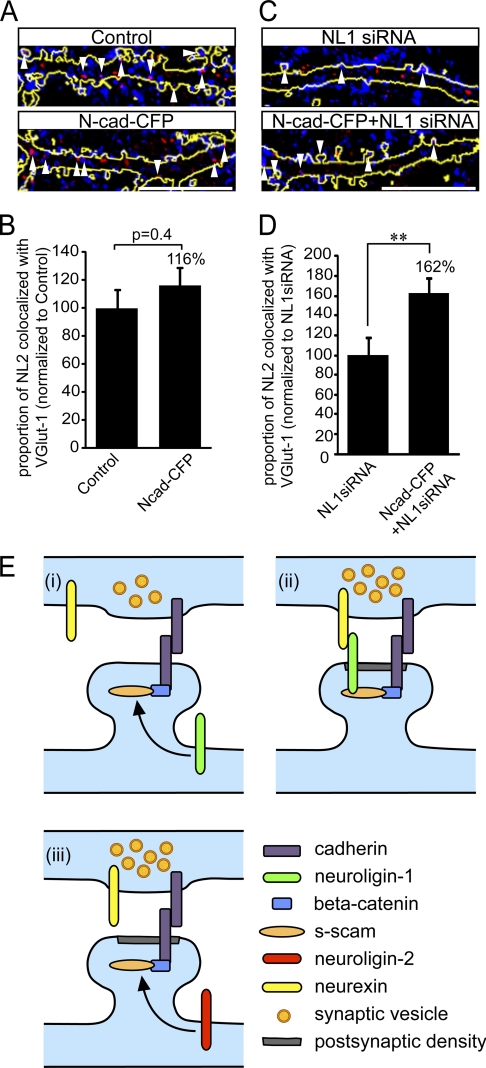
This work, as well as that recently reported by Stan et al. (27), indicates that N-cadherin is important for the clustering of NL1. Because NL2 has also been implicated in the formation of glutamatergic synapses (32), we next determined whether N-cadherin overexpression can rescue the NL1 knockdown phenotype by recruiting NL2, which in turn induces synapse formation. Previous work has shown that NL2 can shift from GABAergic to glutamatergic synapses following knockdown of the postsynaptic inhibitory scaffold protein gephyrin (32) or overexpression of the excitatory postsynaptic scaffold molecule PSD-95 (20). Cells were transfected with control siRNA, NL1 siRNA, or N-cadherin-CFP alone or cotransfected with NL1 siRNA and N-cadherin-CFP and then immunolabeled for NL2 and VGlut-1 (Fig. 5, A and C). Overexpression of N-cadherin alone did not significantly alter the proportion of NL2 puncta that colocalized with VGlut-1 clusters compared with the control (Fig. 5, A and B). However, there was a significant increase in the proportion of NL2 that localized with VGlut-1 clusters in NL1 knockdown cells overexpressing N-cadherin compared with NL1 knockdown cells alone (Fig. 5, C and D). Although N-cadherin overexpression does increase the density of VGlut-1 clusters in NL1 knockdown cells (NL1 siRNA, 23.4 ± 2.3 puncta/100 μm; NL1 siRNA + N-cadherin, 31.6 ± 2.3 puncta/100 μm; p = 0.02 (Student's t test)) the density of NL2 clusters is unchanged (NL1 siRNA, 12.8 ± 1.1 puncta/100 μm; NL1 siRNA + N-cadherin, 10.6 ± 0.8 puncta/100 μm; p = 0.13 (Student's t test)). This indicates that our observations are not due to alterations in NL2 density but are due to the relocalization of NL2 to glutamatergic synapses. These findings suggest that NL2 is only recruited to glutamatergic synapses when cells are depleted of NL1.
FIGURE 5.
The proportion of NL2 at glutamatergic synapses is increased in NL1 knockdown cells overexpressing N-cadherin. A–C, confocal images of 14-DIV neurons transfected at 10 DIV with the indicated constructs and immunolabeled with VGlut-1 (blue) and NL2 (red). There is no change in the proportion of NL2 associated with VGlut-1 in N-cadherin-overexpressing cells (A, arrowheads; C). There is a significant increase in the proportion of NL2 associated with VGlut-1 in NL1 knockdown cells overexpressing N-cadherin (N-cad) (B, arrowheads; D). n = 9–15 cells/condition from two separate cultures. **, p < 0.01 (Student's t test). Scale bars = 10 μm. E, model for the functional interaction between N-cadherin and NLs. N-cadherin colocalizes with VGlut-1 clusters prior to the recruitment of NL1, and overexpression of N-cadherin enhances the recruitment of NL1 to these sites (i). Recruitment of NL1 to nascent synapses enhances the further development of synapses through NL1 interaction with neurexin (ii). In the absence of NL1, N-cadherin recruits NL2 to nascent synapses partially rescuing the effect of NL1 knockdown on synapse density (iii).
DISCUSSION
Disruption of either cadherin or neurexin-neurolignin adhesion complexes in cultured hippocampal neurons has been shown to drastically reduce synapse density, suggesting key roles for these adhesive systems in synapse formation (5–7). Although both N-cadherin and NL1 have been shown to localize primarily to glutamatergic synapses, it was previously unclear whether they colocalize at individual synapses or whether they localize to different synaptic subtypes and if these adhesion systems function in concert to regulate synapse formation. Here we propose that N-cadherin plays an important role in synaptogenesis and that this is mediated in part by the recruitment and clustering of NL1.
We demonstrate that N-cadherin is localized in a punctate pattern early on in developing neurons, at a time when NL1 is still diffusely localized along neurites (Fig. 1A). Our data also suggest that N-cadherin localizes to nascent synapses prior to recruitment of NL1 and that overexpressing N-cadherin at 6 DIV increases the density of GFP-NL1 clusters. We suggest that at this time point, N-cadherin can induce the formation of synapses by enhancing the clustering of NL1. By 14 DIV, almost half of glutamatergic synapses are associated with both N-cadherin and NL1. At this time point, N-cadherin overexpression is not able to further enhance NL1 cluster density, nor does it enhance the density of synapses. Despite this, N-cadherin knockdown at this time point significantly attenuates the density of NL1 clusters. A similar reduction of NL1 clustering in N-cadherin knock-out cells has recently been reported (27).
It is unlikely that the involvement of N-cadherin in synapse formation is entirely due to its ability to regulate the density of NL1 clusters. Indeed, the density of synapses is reduced by 50% in N-cadherin knock-out cells, whereas the density of NL1 clusters is reduced by only 28%. Moreover, we have previously shown an important role for presynaptic cadherin-β-catenin-Scribble complexes in the clustering of synaptic vesicles (31), suggesting that N-cadherin can regulate presynaptic development using mechanisms independent of its ability to cluster neurexin-NL complexes (27).
Whereas knockdown of N-cadherin reduces synapse density by 50%, NL1 knockdown decreases synapse density by 65%. Although this may result from disruption of two separate populations of synapses (ones that are N-cadherin-dependent and ones that are NL1-dependent), this is unlikely, as knockdown of N-cadherin and NL1 together results in a similar decrease in synapse density as NL1 knockdown alone. Moreover, the majority of glutamatergic synapses express both N-cadherin and NL1, with only a subset expressing these molecules separately. The observed difference in synapse density between N-cadherin knockdown and NL1 knockdown may also be due to the fact that NL1 siRNA is more efficient than N-cadherin siRNA at knocking down protein levels.
Recent reports have suggested that cadherin and β-catenin can recruit NLs through the scaffold protein S-SCAM (29). Also, the intracellular scaffold proteins PSD-95 and gephyrin have been shown to modulate the clustering of NLs at excitatory and inhibitory synapses. These studies demonstrate that scaffold molecules play an important role in regulating localization and maintaining proper distribution of adhesion molecules at synapses.
Overexpression of N-cadherin in NL1 knockdown cells partially rescued the effects of NL1 knockdown on synapse density. We suggest this is due to the recruitment of NL2 to N-cadherin clusters and the subsequent induction of synapse formation (Fig. 5E). Indeed, overexpression of N-cadherin enhanced the proportion of NL2 associated with VGlut-1 clusters and increased synapse density in NL1 knockdown cells. Although NL2 is preferentially localized to inhibitory synapses, NL2 expression has also been shown to induce the formation of glutamatergic synapses (24, 27, 28). In addition, NL2 distribution shifts from inhibitory to excitatory contacts upon altered expression of postsynaptic scaffolding proteins (20, 32, 33). Overexpression of N-cadherin in wild-type cells was insufficient to enhance NL2/VGlut-1 colocalization, nor did overexpression of N-cadherin alone enhance synapse density. Although it remains unclear exactly how N-cadherin recruits NL2 to VGlut-1 clusters, S-SCAM has also been shown to associate with NL2 at inhibitory synapses and may be involved in this process (34).
Previous work has demonstrated that cadherin plays a larger role in regulating synapse density in younger neurons. Indeed, disruption of cadherin-cadherin interactions in older hippocampal neurons results in a significantly milder loss of synapses in comparison with young neurons (13). Furthermore, overexpression of N-cadherin enhances synapse formation in young but not mature Rohan-Beard cells in zebrafish (17).
Footnotes
- NL
- neuroligin
- DIV
- days in vitro
- CFP
- cyan fluorescent protein.
REFERENCES
- 1. Ziv N. E., Garner C. C. (2004) Nat. Rev. Neurosci. 5, 385–399 [DOI] [PubMed] [Google Scholar]
- 2. Gerrow K., El-Husseini A. (2006) Front. Biosci. 11, 2400–2419 [DOI] [PubMed] [Google Scholar]
- 3. Dalva M. B., McClelland A. C., Kayser M. S. (2007) Nat. Rev. Neurosci. 8, 206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biederer T., Stagi M. (2008) Curr. Opin. Neurobiol. 18, 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brusés J. L. (2006) Mol. Neurobiol. 33, 237–252 [DOI] [PubMed] [Google Scholar]
- 6. Craig A. M., Kang Y. (2007) Curr. Opin. Neurobiol. 17, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arikkath J., Reichardt L. F. (2008) Trends Neurosci. 31, 487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fannon A. M., Colman D. R. (1996) Neuron 17, 423–434 [DOI] [PubMed] [Google Scholar]
- 9. Uchida N., Honjo Y., Johnson K. R., Wheelock M. J., Takeichi M. (1996) J. Cell Biol. 135, 767–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benson D. L., Tanaka H. (1998) J. Neurosci. 18, 6892–6904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jontes J. D., Emond M. R., Smith S. J. (2004) J. Neurosci. 24, 9027–9034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Togashi H., Abe K., Mizoguchi A., Takaoka K., Chisaka O., Takeichi M. (2002) Neuron 35, 77–89 [DOI] [PubMed] [Google Scholar]
- 13. Bozdagi O., Valcin M., Poskanzer K., Tanaka H., Benson D. L. (2004) Mol. Cell. Neurosci. 27, 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saglietti L., Dequidt C., Kamieniarz K., Rousset M. C., Valnegri P., Thoumine O., Beretta F., Fagni L., Choquet D., Sala C., Sheng M., Passafaro M. (2007) Neuron 54, 461–477 [DOI] [PubMed] [Google Scholar]
- 15. Bamji S. X., Shimazu K., Kimes N., Huelsken J., Birchmeier W., Lu B., Reichardt L. F. (2003) Neuron 40, 719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scheiffele P., Fan J., Choih J., Fetter R., Serafini T. (2000) Cell 101, 657–669 [DOI] [PubMed] [Google Scholar]
- 17. Latefi N. S., Pedraza L., Schohl A., Li Z., Ruthazer E. S. (2009) Dev. Neurobiol. 69, 518–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sara Y., Biederer T., Atasoy D., Chubykin A., Mozhayeva M. G., Südhof T. C., Kavalali E. T. (2005) J. Neurosci. 25, 260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Linhoff M. W., Laurén J., Cassidy R. M., Dobie F. A., Takahashi H., Nygaard H. B., Airaksinen M. S., Strittmatter S. M., Craig A. M. (2009) Neuron 61, 734–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levinson J. N., Chéry N., Huang K., Wong T. P., Gerrow K., Kang R., Prange O., Wang Y. T., El-Husseini A. (2005) J. Biol. Chem. 280, 17312–17319 [DOI] [PubMed] [Google Scholar]
- 21. Song J. Y., Ichtchenko K., Südhof T. C., Brose N. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 1100–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Varoqueaux F., Aramuni G., Rawson R. L., Mohrmann R., Missler M., Gottmann K., Zhang W., Südhof T. C., Brose N. (2006) Neuron 51, 741–754 [DOI] [PubMed] [Google Scholar]
- 23. Varoqueaux F., Jamain S., Brose N. (2004) Eur. J. Cell Biol. 83, 449–456 [DOI] [PubMed] [Google Scholar]
- 24. Chih B., Engelman H., Scheiffele P. (2005) Science 307, 1324–1328 [DOI] [PubMed] [Google Scholar]
- 25. Prange O., Wong T. P., Gerrow K., Wang Y. T., El-Husseini A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 13915–13920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chubykin A. A., Liu X., Comoletti D., Tsigelny I., Taylor P., Südhof T. C. (2005) J. Biol. Chem. 280, 22365–22374 [DOI] [PubMed] [Google Scholar]
- 27. Stan A., Pielarski K. N., Brigadski T., Wittenmayer N., Fedorchenko O., Gohla A., Lessmann V., Dresbach T., Gottmann K. Proc. Natl. Acad. Sci. U.S.A. 107, 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishimura W., Yao I., Iida J., Tanaka N., Hata Y. (2002) J. Neurosci. 22, 757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iida J., Hirabayashi S., Sato Y., Hata Y. (2004) Mol. Cell. Neurosci. 27, 497–508 [DOI] [PubMed] [Google Scholar]
- 30. Xie C., Markesbery W. R., Lovell M. A. (2000) Free Radic. Biol. Med. 28, 665–672 [DOI] [PubMed] [Google Scholar]
- 31. Sun Y., Aiga M., Yoshida E., Humbert P. O., Bamji S. X. (2009) Mol. Biol. Cell 20, 3390–3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levinson J. N., Li R., Kang R., Moukhles H., El-Husseini A., Bamji S. X. Neuroscience 165, 782–793 [DOI] [PubMed] [Google Scholar]
- 33. Graf E. R., Zhang X., Jin S. X., Linhoff M. W., Craig A. M. (2004) Cell 119, 1013–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sumita K., Sato Y., Iida J., Kawata A., Hamano M., Hirabayashi S., Ohno K., Peles E., Hata Y. (2007) J. Neurochem. 100, 154–166 [DOI] [PubMed] [Google Scholar]