Abstract
Background
Children undergoing congenital heart surgery often receive corticosteroids with the aim of reducing the inflammatory response following cardiopulmonary bypass; however the value of this approach is unclear.
Methods and Results
The Pediatric Health Information Systems Database was used to evaluate outcomes associated with corticosteroids in children (0-18y) undergoing congenital heart surgery at 38 US centers from 2003-2008. Propensity scores were constructed to account for potential confounders: age, sex, race, prematurity, genetic syndrome, type of surgery [Risk Adjustment in Congenital Heart Surgery (RACHS-1) category], center, and center volume. Multivariable analysis, adjusting for propensity score and individual covariates, was performed to evaluate in-hospital mortality, postoperative length of stay (LOS), duration of ventilation, infection, and use of insulin.
A total of 46,730 children were included; 54% received corticosteroids. In multivariable analysis, there was no difference in mortality among corticosteroid recipients and non-recipients (OR 1.13, 95% CI 0.98-1.30). Corticosteroids were associated with longer LOS [least square mean difference 2.18d, 95% CI 1.62-2.74d], greater infection (OR 1.27, 95% CI 1.10-1.46) and use of insulin (OR 2.45, 95% CI 2.24-2.67). There was no difference in duration of ventilation. In stratified analysis by RACHS-1 category, no significant benefit was seen in any group, and the association of corticosteroids with increased morbidity was most prominent in RACHS-1 categories 1-3.
Conclusions
In this observational analysis of children undergoing congenital heart surgery, we were unable to demonstrate a significant benefit associated with corticosteroids, and found that corticosteroids may be associated with increased morbidity, particularly in lower risk patients.
Keywords: pediatrics, surgery, drugs
Clinical Summary.
Children undergoing congenital heart surgery often receive corticosteroids with the aim of reducing the inflammatory response following cardiopulmonary bypass; however the value of this approach is unclear. Using the Pediatric Health Information Systems Database we evaluated outcomes associated with corticosteroids in a multi-center cohort of more than 40,000 children undergoing congenital heart surgery from 2003-2008. We were unable to demonstrate a significant benefit associated with corticosteroids, and found that corticosteroids may be associated with increased morbidity, particularly in lower risk patients. These data indicate the need for an adequately powered clinical trial in high risk patients to evaluate efficacy and safety of corticosteroids in this population. Further analysis focusing on the potential impact of different dosing regimens and timing of corticosteroid administration relative to surgery may help to inform the design of a future trial.
Introduction
Many children undergoing congenital heart surgery receive peri-operative corticosteroids with the aim of reducing post-operative inflammation and capillary leak following cardiopulmonary bypass (1). Numerous studies have shown a reduction in various inflammatory markers associated with administration of corticosteroids before or during cardiopulmonary bypass (2,3). However, the impact of corticosteroids on clinical outcomes is less clear, with some studies suggesting improvement in outcomes such as post-operative length of stay and duration of ventilation, while others have not shown a significant difference (2-5). In addition, there are limited data regarding the impact of corticosteroids on peri-operative mortality, as well as potential risks of corticosteroids in this setting, which include hyperglycemia and infection. A recent meta-analysis did not show a significant reduction in duration of ventilation or intensive care unit length of stay associated with corticosteroids in children undergoing congenital heart surgery (6). There were too few deaths to assess mortality as data from only 127 children in 4 trials could be pooled for this analysis, and safety was not assessed. Thus the impact of corticosteroids in children undergoing congenital heart surgery remains unclear, and survey data suggest wide variation from center to center in use of corticosteroids in this population (1).
The purpose of this study was to evaluate efficacy and safety associated with corticosteroids in a large multi-institutional cohort of children undergoing congenital heart surgery. Specifically, we examined the impact of corticosteroids on post-operative mortality, length of stay, and duration of ventilation, as well as potential morbidities such as hyperglycemia and infection.
Methods
Data Source
Data for this multi-center retrospective cohort study were obtained from the Pediatric Health Information System (PHIS) Database, which contains resource utilization data from 39 freestanding, tertiary care children’s hospitals affiliated with the Child Health Corporation of America (Shawnee Mission, KS). PHIS-participating hospitals, which account for 20% of all tertiary care children’s hospitals, provide discharge data including patient demographics, diagnoses, and procedures. Billing data are also available detailing medications, imaging studies, laboratory tests, and supplies charged to each patient. Data quality and reliability are assured through a joint effort between Child Health Corporation of America and participating hospitals. Systematic monitoring occurs to ensure data quality, including bimonthly coding consensus meetings, coding consistency reviews, and quarterly data quality reports. The Children’s Hospital of Philadelphia Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Population
Centers with an average annual congenital heart surgery volume of ≥ 50 cases per year were eligible for inclusion. Children ≤ 18 years of age discharged from these centers between January 1, 2003 and December 31, 2008 who underwent congenital heart surgery classified in any of the Risk Adjustment for Congenital Heart Surgery, Version 1 (RACHS-1) categories were included (7). The RACHS-1 method has been used extensively in risk stratification of patients undergoing congenital heart surgery (8,9). It consists of 6 categories of mortality risk with category 1 being lowest risk and category 6 being the highest risk (7). Cases where cardiopulmonary bypass was not utilized for surgery were excluded.
Data Collection and Study Definitions
Corticosteroid recipients were defined as those who received any type of corticosteroid (methylprednisolone, prednisolone, dexamethasone, or hydrocortisone) on the day before or day of surgery. Medication data in the PHIS Database are derived from the hospital bill and reflect those medications charged to the patient. Corticosteroids administered intravenously or directly into the cardiopulmonary bypass circuit were included in the analysis. A query was performed at an institution known to administer corticosteroids directly in the cardiopulmonary bypass circuit to confirm that this mode of administration was captured in the database. Other variables collected included patient age, sex, race/ethnicity, weight (for neonates), prematurity (ICD-9 codes 765.0x–765.3x), any genetic syndrome/chromosomal abnormality (ICD-9 diagnosis codes 758.0-758.9, 553.3, 756.6–756.7, 759.7–759.9), and primary payer (private insurance, government insurance, other). Center characteristics collected included region and average annual congenital heart surgery volume (for surgeries included in the RACHS-1 categories) during the study period.
Outcome Measures
The primary outcome was in-hospital mortality. Secondary outcomes included total and intensive care unit length of stay, duration of post-operative mechanical ventilation, post-operative infection, and post-operative insulin use (which was used as a surrogate for hyperglycemia). Post-operative infection was defined as a diagnosis code for mediastinitis (519.2), wound infection (998.3 or 998.5), or sepsis (038.xx, or 995.91, or 995.92) and 7 or more consecutive days of intravenous antibiotics administered in the post-operative period within 30 days of surgery. This time-frame was chosen as it is the standard used in the definition of post-operative infection and is generally accepted as the period of impaired immune response following systemic corticosteroid administration (10). Post-operative insulin use was defined as the administration of intravenous insulin in the post-operative period within 30 days of surgery, excluding those with a diagnosis code for diabetes (250.xx).
Statistical Analysis
Study variables were summarized using frequencies and percentages for categorical variables, and median and interquartile range (IQR) or means and standard deviations for continuous variables. Patient and center characteristics were compared between corticosteroid recipients and non-recipients using Chi-square and Wilcoxon Rank-Sum tests. The distribution of corticosteroid use by center was evaluated, and compared with center average annual volume of RACHS-1 classified surgeries, using Spearman’s correlation. Outcomes in corticosteroid recipients and non-recipients were compared in unadjusted analyses in both the overall cohort and stratified by RACHS-1 category using Chi-square and Wilcoxon Rank-Sum tests. Similar to previous studies using the RACHS-1 classification, we combined RACHS-1 categories 5 and 6 for analysis due to the small number of patients in RACHS-1 category 5 (8). Stratified analysis was performed to evaluate whether the impact of corticosteroids on outcome varied by RACHS-1 risk category.
Propensity scores were then constructed using multivariable logistic regression to estimate the likelihood of receiving corticosteroids given an observed set of baseline confounders (11-13). Variables entered into the model included age, sex, race/ethnicity, prematurity, genetic syndrome, RACHS-1 category, center, and mean annual center volume. Patient weight (for neonates) was initially considered, but not included in the final model as it was highly collinear with prematurity. All 2-term interactions were tested and none were significant. The propensity model’s c-statistic was 0.86. Propensity score distributions overlapped between the corticosteroid and no corticosteroid groups (Data Supplement, Figure 1).
Multivariable analysis was performed to evaluate outcomes independently associated with corticosteroids, adjusting for propensity score and each individual covariate described above (14,15). Models with and without propensity score in addition to the individual covariates were compared using Akaike’s information criterion (AIC), with a smaller AIC representing a better model (16). The AIC was smaller for models of all outcome measures incorporating the propensity score in addition to the individual covariates (with the exception of one outcome where AIC was similar), indicating better model performance with the propensity score included. Thus, both propensity score and individual covariates were included in all models. For dichotomous outcome variables, modeling consisted of logistic regression using the method of generalized estimating equations (GEE). For continuous outcome variables, mixed effect models were used treating center as a random effect. This analytic strategy accounts for unexplained between hospital variation by treating observations within a hospital as clustered (correlated). Odds ratios and 95% confidence intervals are reported for the GEE models, and difference in least square means and 95% confidence intervals comparing corticosteroid recipients and non-recipients from the mixed effects models. Analyses were performed both for the overall cohort and stratified by RACHS-1 category. A subanalysis was also performed in neonates (age ≤ 28 days at surgery), as previous studies have suggested that this subgroup may receive the most benefit from corticosteroids (4,17,18).
Finally a sensitivity analysis was performed to compare results using two different propensity score methodologies: 1. the methodology outlined above (which incorporated non-transformed propensity scores into the models for all outcome measures, along with individual covariates) vs. 2. methodology using inverse probability weighted estimators incorporating propensity score entered into the models, along with individual covariates.
All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). The conservative Bonferroni correction was used to account for multiple comparisons (19). Thus, a p-value <0.008 was considered statistically significant.
Results
Patient Characteristics and Corticosteroid Use
A total of 46,730 patients from 38 centers were included (Table 1). Overall, 55% were male and median age was 8 months (IQR 2 months–4 years). The study population was comprised of patients from a diverse ethnic/racial background, included those with government and private insurance, and represented a wide range of hospital size and geographic location.
Table 1.
Patient and Center Characteristics
| Overall | No Steroids | Steroids | p | |
|---|---|---|---|---|
| n=46730 | n=21617 | n=25113 | ||
| Sex | ||||
| Male | 25884 (55.4) | 11928 (55.2) | 13956 (55.6) | 0.38 |
| Age | ||||
| ≤ 30d | 10115 (21.7) | 3799 (17.6) | 6316 (25.2) | <0.001 |
| 31d-1y | 16126 (34.5) | 7446 (34.5) | 8680 (34.6) | |
| 2y-5y | 11538 (24.7) | 5656 (26.2) | 5882 (23.4) | |
| 6y-12y | 4083 (8.7) | 2063 (9.5) | 2020 (8.0) | |
| >12 y | 4868 (10.4) | 2653 (12.3) | 2215 (8.8) | |
| Median [IQR] | 8m [2m, 4y] | 11m [3m, 5y] | 6m [1m, 3y] | <0.001 |
| Race | ||||
| Non-Hispanic White | 24527 (54.6) | 10661 (51.1) | 13866 (57.7) | <0.001 |
| Non-Hispanic Black | 5765 (12.8) | 2611 (12.5) | 3154 (13.1) | |
| Hispanic | 8439 (18.8) | 4281 (20.5) | 4158 (17.3) | |
| Asian | 1386 (3.1) | 791 (3.8) | 595 (2.5) | |
| Other | 4785 (10.7) | 2515 (12.1) | 2270 (9.4) | |
| RACHS-1 Level | ||||
| 1 | 3883 (8.3) | 1876 (8.7) | 2007 (8.0) | <0.001 |
| 2 | 16802 (36.0) | 7942 (36.7) | 8860 (35.3) | |
| 3 | 18777 (40.2) | 9144 (42.3) | 9633 (38.34 | |
| 4 | 4923 (10.5) | 2017 (9.3) | 2906 (11.6) | |
| 5/6 | 2345 (5.0) | 638 (3.0) | 1707 (6.8) | |
| Genetic abnormality | 7086 (15.2) | 3174 (14.7) | 3912 (15.6) | 0.007 |
| Prematurity* | 2081 (4.5) | 768 (3.6) | 1313 (5.2) | <0.001 |
| Payor | ||||
| Government | 18057 (38.7) | 7731 (35.8) | 10326 (41.2) | <0.001 |
| Private | 16498 (35.4) | 7656 (35.5) | 8842 (35.2) | |
| Other | 12119 (26.0) | 6195 (28.7) | 5924 (23.6) | |
| Center Region | ||||
| Northeast | 7247 (15.5) | 4165 (19.3) | 3082 (12.3) | <0.001 |
| South | 16773 (35.9) | 6950 (32.2) | 9823 (39.1) | |
| North Central | 12135 (26.0) | 4087 (18.9) | 8048 (32.1) | |
| West | 10575 (22.6) | 6415 (29.7) | 4160 (16.6) | |
| Center Annual Volume** | ||||
| <150 | 5464 (11.7) | 2661 (12.3) | 2803 (11.2) | <0.001 |
| 150-250 | 22878 (49.0) | 8598 (39.8) | 14280 (56.9) | |
| 251-350 | 4004 (8.6) | 2228 (10.3) | 1776 (7.1) | |
| >350 | 14384 (30.8) | 8130 (37.6) | 6254 (24.9) | |
neonates only,
center mean annual congenital heart surgery volume (only surgeries included in the RACHS-1 categories) during the study period.
Fifty-four percent (n=25,113) of patients received peri-operative corticosteroids. The use of corticosteroids was associated with younger age, white race, prematurity, genetic abnormality, higher RACHS-1 category, government insurance, and being treated at a center in the South or North Central US (Table 1). Corticosteroid use by center varied with a range of 1-96% of patients per center receiving corticosteroids (Figure 1). There was no significant linear correlation seen between center volume and use of corticosteroids (p= 0.62).
Figure 1.
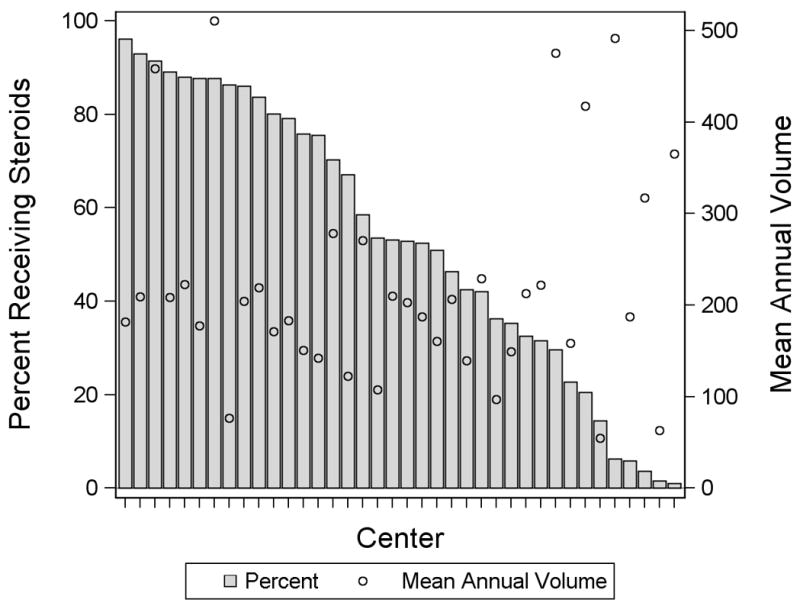
Distribution of Corticosteroid Use by Center (bar). The mean annual congenital heart surgery volume is also shown (circle).
Methylprednisolone was the most common peri-operative corticosteroid used (69.8%), followed by dexamethasone (26.6%) and hydrocortisone (3.6%). Corticosteroids were most often given on the day of surgery only (79.1%), followed by the day prior to and day of surgery (12.2%), and the day prior to surgery only (8.9%). Of note, 35.4% of patients in the peri-operative corticosteroid recipient group, and 27.8% of patients on the non-recipient group received at least one dose of a corticosteroid at some point in the post-operative period.
Outcomes
Unadjusted outcomes are displayed in Table 2 (overall) and the Data Supplement Table 1 (stratified by RACHS-1 category). Adjusted outcomes for the overall cohort are displayed in Table 3a. In multivariable analysis, there was no difference in mortality. Corticosteroid recipients had longer total and ICU length of stay, and greater post-operative infection and use of insulin. Of note, in the group who received post-operative insulin, this was initiated at median post-operative day 0 (IQR 0-3 days) following surgery. There was no difference in post-operative duration of mechanical ventilation. In multivariable analysis stratified by RACHS-1 category, no significant benefit was seen in any group, and the association of corticosteroids with increased morbidities was most prominent in RACHS-1 categories 1 – 3 (Table 3b). The absolute number of deaths in RACHS-1 category 1 was small [steroid recipients (n=26) 1.3% vs. non-recipients (n=6) 0.3%].
Table 2.
Unadjusted Post-operative Outcomes
| Overall | No Steroids | Steroids | p | |
|---|---|---|---|---|
| Mortality | 1632 (3.5) | 578 (2.7) | 1054 (4.2) | <0.001 |
| Total length of stay, days | 9.9 (9.3) | 8.8 (7.5) | 11.1 (11.0) | <0.001 |
| ICU length of stay, days | 4.1 (4.6) | 3.3 (3.5) | 5.0 (5.7) | <0.001 |
| Infection | 1366 (2.9) | 550 (2.5) | 816 (3.3) | <0.001 |
| Duration of ventilation, days | 4.3 (3.6) | 3.9 (3.0) | 4.7 (4.1) | <0.001 |
| Post-operative insulin | 4710 (10.1) | 1316 (6.1) | 3394 (13.5) | <0.001 |
Data are displayed as n (%) for dichotomous variables and mean (standard deviation) for continuous variables. ICU = intensive care unit
Table 3.
a. Adjusted Post-operative Outcomes
| OR (95% CI) | p | |
|---|---|---|
| Mortality | 1.13 (0.98, 1.30) | 0.07 |
| Infection | 1.27 (1.10, 1.46) | 0.001 |
| Post-operative insulin | 2.45 (2.24, 2.67) | <0.001 |
| LSM Difference Steroids − No Steroids | p | |
| Total length of stay, days | +2.18 (+1.62, +2.74) | <0.001 |
| ICU length of stay, days | +1.90 (+1.56, +2.23) | <0.001 |
| Duration of ventilation, days | +0.22 (-0.17, +0.61) | 0.28 |
| b. Adjusted Post-operative Outcomes Stratified by RACHS-1 Category | |||||
| OR (95% CI) | p-value | LSM Difference Steroids – No Steroids | p-value | ||
| RACHS-1 Category 1 | |||||
| Mortality | 9.87(3.07, 31.72) | <0.001 | Total length of stay, days | +3.79 (+2.14, +5.44) | <0.001 |
| Infection | 1.68 (0.80, 3.48) | 0.17 | ICU length of stay, days | +3.45 (+2.57, +4.32) | <0.001 |
| Post-operative insulin | 2.61 (1.80, 3.79) | <0.001 | Duration of ventilation, days | +0.68 (-0.40, +1.77) | 0.22 |
| RACHS-1 Category 2 | |||||
| Mortality | 1.39 (0.96, 2.01) | 0.08 | Total length of stay, days | +2.03 (+1.29, +2.76) | <0.001 |
| Infection | 1.42 (1.02, 1.96) | 0.04 | ICU length of stay, days | +1.34 (+0.97, +1.71) | <0.001 |
| Post-operative insulin | 2.55 (2.17, 2.99) | <0.001 | Duration of ventilation, days | +0.14 (-0.35, +0.65) | 0.58 |
| RACHS-1 Category 3 | |||||
| Mortality | 1.20 (0.96, 1.50) | 0.1 | Total length of stay, days | +2.42 (+1.54, +3.30) | <0.001 |
| Infection | 1.57 (1.27, 1.94) | <0.001 | ICU length of stay, days | +2.32 (+1.78, +2.86) | <0.001 |
| Post-operative insulin | 2.67 (2.33, 3.04) | <0.001 | Duration of ventilation, days | +0.42 (-0.18, +1.04) | 0.17 |
| RACHS-1 Category 4 | |||||
| Mortality | 0.87 (0.66, 1.13) | 0.29 | Total length of stay, days | +0.16(-2.28, +2.60) | 0.90 |
| Infection | 0.88 (0.63, 1.20) | 0.43 | ICU length of stay, days | +1.57 (+0.01, +3.12) | 0.047 |
| Post-operative insulin | 2.09 (1.68, 2.59) | <0.001 | Duration of ventilation, days | -1.56 (-3.25, +0.12) | 0.07 |
| RACHS-1 Category 5/6 | |||||
| Mortality | 1.02 (0.75, 1.40) | 0.87 | Total length of stay, days | +3.00 (-0.30, +6.29) | 0.08 |
| Infection | 0.82 (0.55, 1.22) | 0.33 | ICU length of stay, days | +2.41 (-0.07, +4.90) | 0.06 |
| Post-operative insulin | 2.49 (1.70, 3.64) | <0.001 | Duration of ventilation, days | +2.28 (-0.14, +4.71) | 0.07 |
LSM = least square means
ICU = intensive care unit
In sub-analysis in 10,018 neonates, corticosteroids were associated with significantly longer post-operative ICU length of stay and greater use of post-operative insulin; there were no other significant differences in outcomes (Table 4).
Table 4.
Adjusted Post-operative Outcomes in Neonate Subgroup
| OR (95% CI) | p | |
|---|---|---|
| Mortality | 1.07 (0.89, 1.27) | 0.48 |
| Infection | 0.85 (0.68, 1.05) | 0.14 |
| Post-operative insulin | 2.32 (1.97, 2.73) | <0.001 |
| LSM Difference Steroids – No Steroids | p | |
| Total length of stay, days | 0.48 (-1.52, 2.47) | 0.64 |
| ICU length of stay, days | 2.5 (1.34, 3.66) | <0.001 |
| Duration of ventilation, days | -1.11 (-2.47, 0.25) | 0.11 |
LSM = least square means
ICU = intensive care unit
In sensitivity analysis performed to compare the results from propensity score methodology reported above (use of non-transformed propensity score and individual covariates in the models) vs. methodology using inverse probability weighted estimators incorporating propensity score entered into the models, along with individual covariates, results were unchanged (data not shown).
Discussion
In this large multi-center observational analysis, we were unable to demonstrate a significant benefit associated with corticosteroids, and found that corticosteroids may be associated with increased morbidity particularly in lower risk patients. Cardiopulmonary bypass triggers the release of various pro-inflammatory and vasoactive agents leading to a post-operative systemic inflammatory response and capillary leak syndrome (20-22). This can lead to multi-system dysfunction, contributing to substantial post-operative morbidity and mortality (6,20-22). Thus, the aim of corticosteroid administration is to attenuate this inflammatory response and associated adverse outcomes.
Results from our study confirm that corticosteroids are used commonly, with over half (n=25,113) of children undergoing congenital heart surgery at 38 US hospitals receiving corticosteroids over a 5 year period from 2003-2008 (1). However, we also found wide variation in measured practice patterns with some centers using corticosteroids in nearly all patients and some using corticosteroids very infrequently. This variation in practice may reflect the uncertainty regarding the benefits of corticosteroids in this setting and conflicting results demonstrated by previous studies. While numerous adult and pediatric studies have demonstrated that corticosteroids are effective in reducing post-operative levels of inflammatory markers such as interleukins, tumor necrosis factor, and C-reactive protein, the impact of corticosteroids on clinical outcomes is less clear (2,3,23). In adults, a meta-analysis combining results from 44 randomized trials of corticosteroid use in patients undergoing cardiac surgery and cardiopulmonary bypass found that corticosteroids were associated with a significant reduction in post-operative atrial fibrillation, and a small but statistically significant reduction in length of stay (24). There was no significant impact on mortality. Other safety outcomes were not assessed. There are currently two ongoing large clinical trials in the adult population examining the efficacy and safety of methylprednisolone and dexamethasone in patients undergoing cardiac surgery (25,26).
A meta-analysis has also been performed in the pediatric population (6). This analysis did not show any significant difference in outcomes, including duration of ventilation or intensive care unit length of stay, associated with corticosteroids in children undergoing congenital heart surgery (6). Data from only 127 children in 4 trials could be pooled for this analysis due to poor study design, varying inclusion and exclusion criteria between studies, and heterogeneity in outcomes reported. There were too few deaths in these small trials to assess the impact of corticosteroids on mortality. Our multi-center study evaluating more than 40,000 children supports the results from these small trials, in that we did not find a benefit regarding length of stay or duration of mechanical ventilation associated with corticosteroid administration in this setting. Our large sample size also allowed assessment of mortality, and a benefit was not seen.
A possible explanation for why the attenuation of inflammation associated with corticosteroid administration in this setting is not translated to a clinical benefit is that corticosteroids alone may not address the complex, multi-faceted nature of the inflammatory response following cardiopulmonary bypass. Several studies have shown that while corticosteroids can reduce levels of certain inflammatory markers, this effect is not universal. Lindberg et al. found that administration of dexamathasone to children undergoing congenital heart surgery did not decrease von Willebrand factor antigen (a marker of endothelial activation), or S100B (a marker of blood/brain barrier dysfunction) (27). Jansen et al. found that complement activation was not diminished with multiple different regimens of corticosteroids (28). Alternatively, it is possible that only certain regimens of corticosteroids are effective in this setting. For example, it has been suggested that a regimen combining pre-operative and intra-operative administration of corticosteroids may be more effective at attenuating the inflammatory response than the use of only intra-operative administration in both animal and human models (4,29). In this study, we were not able to assess the dose of corticosteroids given or exact timing in relation to surgery.
In our stratified analysis by RACHS-1 category, no significant benefit was seen in any group, and the association of corticosteroids with increased morbidity was most prominent in the lower risk groups (RACHS-1 categories 1 – 3). We also found an increased mortality risk associated with corticosteroids in RACHS-1 category 1. While statistically significant, the absolute number of deaths in this category was small. It is possible that in the lower risk categories the balance between potential risks and benefits of corticosteroid therapy is shifted toward favoring relatively greater risk. The lower risk surgeries are generally associated with shorter cardiopulmonary bypass times and attenuated post-operative inflammatory response compared with surgeries with longer cardiopulmonary bypass times (30). Thus, not much benefit from the anti-inflammatory properties of corticosteroids would be expected, and the potential risks of corticosteroid therapy may have a relatively greater impact on outcome.
In evaluation of specific morbidities, we found that corticosteroid administration was associated with more frequent infection, more frequent use of post-operative insulin, and prolonged length of stay. While we can not know for certain that the use of postoperative insulin was related to hyperglycemia, this is likely the most common reason for its use in the post-operative period. It is also likely that center practices regarding insulin administration for hyperglycemia differ; however, we attempted to account for this and other center related differences in our analytic strategy by adjusting for center level factors and accounting for between hospital variation through treating observations within a hospital as clustered (correlated) observations. Previous analyses have demonstrated that hyperglycemia can predispose to poor outcomes including infection, prolonged length of stay, and increased mortality in critically ill children (31-33). The rate of post-operative infection identified in our analysis (2.9%) is similar to that reported in clinical registry data (2.8%) in the congenital heart surgery population (34). Our results regarding infection differ from a recent meta-analysis in the adult population which showed that the administration of peri-operative corticosteroids did not increase the risk of infection (35). Previous analyses in the pediatric population have shown that post-operative infection in children undergoing congenital heart surgery has been associated with prolonged length of stay and increased mortality (34,36). Peri-operative morbidities such as prolonged length of stay may also have an important impact on longer term outcomes as well, as prior studies have shown prolonged hospital stay to be an independent predictor of future neurodevelopmental status in patients undergoing congenital heart surgery (37).
Limitations
This study is subject to the limitations of any observational investigation including selection bias and the potential impact of confounders. While we attempted to account for this in our analytic strategy through the use of propensity scores and adjustment for know patient and center confounders, there may be other unmeasured patient or center factors which may impact the receipt of corticosteroids and/or outcome. These may include, among other factors, those relating to cardiopulmonary bypass protocols which may vary from center to center, such as the use of modified ultrafiltration (38,39). As the purpose of our study was to assess the relationship between pre-operative corticosteroid administration and outcome, we did not adjust for post-operative factors or complications, including the administration of post-operative corticosteroids, as it is possible that these factors may be in the causal pathway between our predictor variable of interest and outcome. Conversely, it is possible that some of these may also be confounding factors not accounted for in the analysis.
Our study is also subject to the limitations of the database. We were unable to evaluate the impact of different dosing regimens or exact timing of corticosteroid administration in relation to surgery as this information is not collected in the database. Regarding our evaluation of potential morbidities, we were unable to assess laboratory values such as glucose levels. Instead, we evaluated the use of post-operative insulin. However, it is likely that there are patients with hyperglycemia who do not receive insulin, and that practices regarding insulin administration differ across centers. Our analytic strategy attempts to account for this and other factors which may vary by center by adjusting for center-level variables and treating observations within a hospital as correlated observations. However, this strategy may not account for all potential differences between centers. In addition, there may be other unmeasured factors impacting glucose levels and in turn, use of insulin, in the post-operative period. Among these factors are duration of cardiopulmonary bypass and the use of deep hypothermic circulatory arrest (40,41). Finally, the accuracy of coding of congenital heart surgery in administrative datasets is not known. Random coding errors may be mitigated by the use of large datasets and groupings of procedures, as in this study. The similarities between overall outcomes of patients undergoing congenital heart surgery in our dataset compared with other large clinical datasets support the validity of our data (34,42).
Conclusions
In this multi-center observational analysis of children undergoing congenital heart surgery, we were unable to demonstrate a significant benefit associated with corticosteroids, and found that corticosteroids may be associated with increased morbidity, particularly in lower risk patients. These data indicate the need for an adequately powered clinical trial in high risk patients to evaluate efficacy and safety of corticosteroids in this population. Further analysis focusing on the potential impact of different dosing regimens and timing of corticosteroid administration relative to surgery may help to inform the design of a future trial.
Supplementary Material
Acknowledgments
Funding Sources Dr. Pasquali receives grant support (KL2 RR024127-02) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and from the American Heart Association (AHA) Mid-Atlantic Affiliate Clinical Research Program. Dr. Shah receives support from the National Institute of Allergy and Infectious Diseases (K01 AI73729) and the Robert Wood Johnson (RWJ) Foundation under its Physician Faculty Scholar Program. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NIH, AHA, or RWJ Foundation.
Footnotes
Presented in part at the 2009 American Heart Association Scientific Sessions: William WL Glenn Lecture and Pediatric Surgery Abstracts.
Disclosures None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Checchia PA, Bronicki RA, Costello JM, Nelson DP. Steroid use before pediatric cardiac operations using cardiopulmonary bypass: An international survey of 36 centers. Pediatr Crit Care Med. 2005;6:441–4. doi: 10.1097/01.PCC.0000163678.20704.C5. [DOI] [PubMed] [Google Scholar]
- 2.Lindberg L, Forsell C, Jogi P, Olsson K. Effects of dexamethasone on clinical course, C-reactive protein, S100B protein and von Willebrand factor antigen after pediatric cardiac surgery. Br J Anaesth. 2003;90:728–732. doi: 10.1093/bja/aeg125. [DOI] [PubMed] [Google Scholar]
- 3.Bronicki R, Backer C, Baden H, Mavroudis C, Crawford S, Green T. Dexamethasone reduces the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. 2000;69:1490–1495. doi: 10.1016/s0003-4975(00)01082-1. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder V, Pearl JM, Schwartz SM, Shanley TP, Manning PB, Nelson DP. Combined steroid treatment for congenital heart surgery improves oxygen delivery and reduces postbypass inflammatory mediator expression. Circulation. 2003;107:2823–2828. doi: 10.1161/01.CIR.0000070955.55636.25. [DOI] [PubMed] [Google Scholar]
- 5.Varan B, Tokel K, Mercan S, Donmez A, Aslamaci S. Systemic inflammatory response related to cardiopulmonary bypass and its modification by methyl prednisolone: High dose versus low dose. Pediatr Cardiol. 2002;23:437–41. doi: 10.1007/s00246-002-0118-3. [DOI] [PubMed] [Google Scholar]
- 6.Robertson-Malt S, Afrane B, Elbarbary M. Prophylactic steroids for pediatric open heart surgery. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD005550.pub2. Art. No.: CD005550. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 8.Welke KF, Diggs BS, Karamlou T, Ungerleider RM. Comparison of pediatric cardiac surgical mortality rates from national administrative data to contemporary clinical standards. Ann Thorac Surg. 2009;87:216–223. doi: 10.1016/j.athoracsur.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Larsen SH, Pedersen J, Jacobsen J, Johnsen SP, Hansen OK, Hjortdal V. The RACHS-1 risk categories reflect mortality and length of stay in a Danish population of children operated for congenital heart disease. Eur J Cardiothorac Surg. 2005;28:877–881. doi: 10.1016/j.ejcts.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Checchia PA, Karamlou T, Maruszewski B, Ohye RG, Bronicki R, Dodge-Khatami A. Haematological and infectious complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the Multi-societal Database Committee for Pediatric and Congenital Heart Disease. Cardiol Young. 2008;18:226–233. doi: 10.1017/S1047951108002965. [DOI] [PubMed] [Google Scholar]
- 11.Sturmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59:437–447. [Google Scholar]
- 12.Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 13.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. 2004;13:841–853. doi: 10.1002/pds.969. [DOI] [PubMed] [Google Scholar]
- 14.Imbens GW. Nonparametric estimation of average treatment effects under erogeneity: a review. Rev Econ Stat. 2004;86:4–29. [Google Scholar]
- 15.Greenland S. Introduction to regression modeling. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3. Philadelphia PA: Lippincott Williams & Wilkins; 2008. pp. 446–451. [Google Scholar]
- 16.Akaike, Hirotugu A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 17.Duval E, Kavelaars A, Veenhuizen L, van Vught AJ, van de Wal HJ, Heijnen CJ. Pro- and anti-inflammatory cytokine patterns during and after cardiac surgery in young children. Eur J Pediatr. 1999;158:387–393. doi: 10.1007/s004310051098. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich B, Schmidt R, Reiss I, Gunther A, Seeger W, Muller M, Thul J, Schranz D, Gorter L. Changes in biochemical and biophysical surfactant properties with cardiopulmonary bypass in children. Crit Care Med. 2003;31:284–290. doi: 10.1097/00003246-200301000-00045. [DOI] [PubMed] [Google Scholar]
- 19.Bonferroni CE. Studi in Onore del Professore Salvatore Ortu Carboni. Rome: 1935. Il calcolo delle assicurazioni su gruppi di teste; pp. 13–60. [Google Scholar]
- 20.Casey LC. Roles of cytokines in the pathogenesis of cardiopulmonary-induced multisystem organ failure. Ann Thorac Surg. 1993;56:S92–S96. doi: 10.1016/0003-4975(93)91143-b. [DOI] [PubMed] [Google Scholar]
- 21.Boyle EM, Pohlman TH, Johnson MC, Verrier ED. Endothelial cell injury in cardiovascular surgery: the systemic inflammatory response. Ann Thorac Surg. 1997;63:277–284. doi: 10.1016/s0003-4975(96)01061-2. [DOI] [PubMed] [Google Scholar]
- 22.Brix-Christensen V. The systemic inflammatory response after cardiac surgery with cardiopulmonary bypass in children. Acta Anaestesiol Scand. 2001;45:671–679. doi: 10.1034/j.1399-6576.2001.045006671.x. [DOI] [PubMed] [Google Scholar]
- 23.Teoh KH, Bradley CA, Gauldie J, Burrows H. Steroid inhibition of cytokine mediated vasodilation after warm heart surgery. Circulation. 1995;92:II347–II353. doi: 10.1161/01.cir.92.9.347. [DOI] [PubMed] [Google Scholar]
- 24.Whitlock RP, Chan S, Devereaux PJ, Sun J, Rubens FD, Thorlund K, Teoh KH. Clinical benefit of steroid use in patients undergoing cardiopulmonary bypass: a meta-analysis of randomized trials. Eur Heart J. 2008;29:2592–2600. doi: 10.1093/eurheartj/ehn333. [DOI] [PubMed] [Google Scholar]
- 25.Steroids In caRdiac Surgery Trial (SIRS Trial) [November 24, 2009]; http://www.clinicaltrials.gov/ct2/show/NCT00427388?term=sirs+trial&rank=1.
- 26.Dexamethasone for Cardiac Surgery Trial. [November 24, 2009]; http://www.clinicaltrials.gov/ct2/show/NCT00293592?term=nct00293592&rank=1.
- 27.Lindberg L, Forsell C, Jogi P, Olsson AK. Effects of dexamethasone on clinical course, C-reactive protein, S100B protein and von Willebrand factor antigen after paediatric cardiac surgery. Br J Anaesth. 2003;90:728–732. doi: 10.1093/bja/aeg125. [DOI] [PubMed] [Google Scholar]
- 28.Jansen NJG, van Oeveren W, van Vilet M, Stoutenbeek CP, Eysman L, Wildevuur CRH. The role of different types of corticosteroids on the inflammatory mediators in cardiopulmonary bypass. Eur J Cardio-thorac Surg. 1991;5:211–217. doi: 10.1016/1010-7940(91)90032-f. [DOI] [PubMed] [Google Scholar]
- 29.Lodge AJ, Chai PJ, Daggett CW, Ungerleider RM, Jaggers J. Methylprednisolone reduces the inflammatory response to cardiopulmonary bypass in neonatal piglets: Timing of dose is important. J Thorac Cardiovasc Surg. 1999;117:515–522. doi: 10.1016/s0022-5223(99)70331-4. [DOI] [PubMed] [Google Scholar]
- 30.Jensen E, Andreasson S, Bengtsson A, Berggren H, Ekroth R, Lindholm L, Ouchterlong J. Influence of two difference perfusion systems on inflammatory response in pediatric heart surgery. Ann Thorac Surg. 2003;75:919–925. doi: 10.1016/s0003-4975(02)04501-0. [DOI] [PubMed] [Google Scholar]
- 31.Ghafoori AF, Twite MD, Friesen RH. Postoperative hyperglycemia is associated with mediastinitis following pediatric cardiac surgery. Pediatr Anesth. 2008;18:1202–1207. doi: 10.1111/j.1460-9592.2008.02808.x. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5:329–336. doi: 10.1097/01.pcc.0000128607.68261.7c. [DOI] [PubMed] [Google Scholar]
- 33.Yates AR, Dyke PC, Taeed R, Hoffman TM, Hayes J, Feltes TF, Cua CL. Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patients. Pediatr Crit Care Med. 2006;7:351–355. doi: 10.1097/01.PCC.0000227755.96700.98. [DOI] [PubMed] [Google Scholar]
- 34.Barker GM, O’Brien SM, Welke KF, Jacobs ML, Jacobs JP, Benjamin DK, Jr, Peterson ED, Jaggers J, Li JS. Major infection after pediatric cardiac surgery: a risk estimation model. Ann Thorac Surg. 2010;89:843–850. doi: 10.1016/j.athoracsur.2009.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho KM, Tan JA. Benefits and risks of corticosteroid prophylaxis in adult cardiac surgery: a dose-response meta-analysis. Circulation. 2009;119(14):1853–66. doi: 10.1161/CIRCULATIONAHA.108.848218. [DOI] [PubMed] [Google Scholar]
- 36.Holzmann-Pazgal G, Hopkins-Broyles D, Recktenwald A, Hohrein M, Kieffer P, Huddleston C, Anshuman S, Fraser V. Case-control study of pediatric cardiothoracic surgical site infections. Infect Control Hosp Epidemiol. 2008;29:76–9. doi: 10.1086/524323. [DOI] [PubMed] [Google Scholar]
- 37.Mahle WT, Visconti KJ, Freier C, Kanne SM, Hamilton WG, Sharkey AM, Chinnock RE, Jenkins KJ, Isquith PK, Burns TG, Jenkins PC. Relationship of surgical approach to neurodevelopmental outcomes in hypoplastic left heart syndrome. Pediatrics. 2006;117:e90–e97. doi: 10.1542/peds.2005-0575. [DOI] [PubMed] [Google Scholar]
- 38.Elliott MJ. Ultrafiltration and modified ultrafiltration in pediatric open-heart operations. Ann Thorac Surg. 1993;56:1518–1522. doi: 10.1016/0003-4975(93)90744-3. [DOI] [PubMed] [Google Scholar]
- 39.Andreasson S, Gothberg S, Berggren H, Bengtsson A, Eriksson E, Risberg B. Hemofiltration modifies complement activation after extracorporeal circulation in infants. Ann Thorac Surg. 1993;56:1515–1517. doi: 10.1016/0003-4975(93)90743-2. [DOI] [PubMed] [Google Scholar]
- 40.Benzing G, Fancis PD, Kaplan S, Helmsworth JA, Sperling MA. Glucose and insulin changes in infants and children undergoing hypothermic open-heart surgery. Am J Cardiol. 1983;52:133–136. doi: 10.1016/0002-9149(83)90083-8. [DOI] [PubMed] [Google Scholar]
- 41.Doenst T, Wijevsundera D, Karkouti K, Zechner C, Maganti M, Rao V, Borger MA. Hyperglycemia during cardiopulmonary bypass is and independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2005;130:1144.e1–1144.e8. doi: 10.1016/j.jtcvs.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs JP, Jacobs ML, Lacour-Gayet FG, Jenkins KJ, Gauvreau K, Bacha E, Maruszewski B, Clarke DR, Tchervenkov CI, Gaynor JW, Spray TL, Stellin G, O’Bien SM, Elliott MJ, Mavroudis C. Stratification of complexity improves the utility and accuracy of outcomes analysis in a multi-institutional congenital heart surgery database: Application of the Risk Adjustment in Congenital Heart Surgery (RACHS-1) and Aristotle Systems in the Society of Thoracic Surgeons Congenital Heart Surgery Database. Pediatr Cardiol. 2009;30:1117–1130. doi: 10.1007/s00246-009-9496-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


