Abstract
Background
In anticipation of vaccine introduction, we assessed epidemiology of rotavirus disease among children visiting medical centre due to acute diarrhoea in Ouagadougou, Burkina Faso.
Methods
Between November 2008 and February 2010, stool specimens from 447 children less than 5 years of age suffering from diarrhoea were tested for the presence of rotavirus by antigen detection using an immunochromatographic test. Sociodemographic, environmental and clinical factors were assessed during the study.
Results
Rotavirus antigen was detected in 151 (33.8%) of the patients. Most of the cases (94.2%) were in children < 24 months of age. Fever and vomiting were the symptoms most commonly reported in association with rotavirus diarrhoea and the patients were often hospitalized. Rotavirus-associated diarrhoea occurred mostly during the season from December to April (dry season). Rotavirus infection was significantly less frequent in breast-fed than among bottle-fed babies.
Conclusions
The results of this study underscore the need to control rotavirus infections among young children in Burkina Faso and may argue a decision on the introduction of rotavirus vaccine in Burkina Faso.
Background
Rotavirus is a major cause of acute gastroenteritis in infants and young children worldwide [1]. It has been estimated that about 39% of childhood diarrhoea hospitalizations are caused by rotaviruses and nearly half a million children die from rotavirus infections each year [2]. Furthermore, rotavirus mortality is concentrated in the developing countries on the Asian subcontinent, Africa, and Latin America where access to health care facilities is limited [3]. This may result in a significant disease burden and economic effect of direct medical costs, loss of work, quality of life and mortality. In other diarrhoeal diseases, improvement of hygiene and sanitation may reduce the incidence, but these measures are unlikely sufficient for rotavirus control. Vaccination is the only control measure likely to have a significant impact on the incidence of severely dehydrating rotavirus disease [4]. Two new live-attenuated rotavirus vaccines (Rotarix® and RotaTeq®) have demonstrated very good safety and efficacy profiles in large clinical trials in the Western industrialized countries and in Latin America [5-9]. Trials on these vaccines are now in progress in sub-Saharan Africa to assess their effectiveness and efficacy.
In Burkina Faso, very few data on illness caused by rotavirus have been published and these studies indicated that 14% of acute diarrhoea in children under the age of 5 years is due to rotavirus infection but epidemiological data are still incomplete [10].
The objectives of this study were to describe, for the first time, epidemiology of rotavirus disease among children visiting a local health centre because of acute gastroenteritis in Ouagadougou in Burkina Faso to provide background knowledge on the disease before vaccine introduction and to inform the policy makers on the need for the introduction of new rotavirus vaccines.
Methods
Study population and specimens
The study was conducted at Centre Médical avec Antenne Chirugicale (CMA) du Secteur 30 in the capital city of Ouagadougou, Burkina Faso. CMA du secteur 30, located in the Bogodogo district is one of the four secondary health care centers in Ouagadougou and its paediatric ward has a capacity of 30 beds and admits over 2260 children each year. Ouagadougou has a population of nearly two millions, whereas the Bogodogo district has a population of about 548000 with 81000 (15%) children < 5 years of age http://www.sante.gov.bf. The study protocol was approved by the Ethics Committee of Burkina Faso. Parents of all the paediatric patients were informed on the study details and their oral consent was obtained before stool specimen and epidemiological data collection during the course of treatment. Written consent was obtained from parents of the control group. All children under the age of 5 year visiting the paediatric service for treatment of gastroenteritis from November 2008 to February 2010 were included in the study. Diarrhoea was defined as the passage of three or more loose or watery stools in the preceding 24 h. During this study, 471 patients were included as soon as they were seen by a physician and fresh stool samples were collected and transferred to the Microbiology Laboratory at the National Public Health Laboratory, Ouagadougou, for rotavirus and adenovirus detection. Control stool samples were collected from 60 randomly selected children coming to the same health centre for routine immunization and not presenting gastroenteritis symptoms. The age of the control was paired with the patient's age. Information regarding the age, sex, type of nutrition (breast-fed and/or bottle-fed), hospitalization and clinical symptoms such as fever, vomiting and dehydration, and the characteristics of stool were recorded for each child. Also hygiene factors such as source of drinking water of the child were collected during the study.
Detection of rotavirus in stool samples
All stool samples were analyzed for group A rotavirus using one step rotavirus and adenovirus serotypes 40/41 test for determination of rotavirus and adenovirus in human feces (SD Bioline Rota/Adeno®; Standard diagnostics, Inc., Korea) following the manufacturer's instructions.
Statistical analyses
χ2 test was used to analyze the data and the p value less than 0.05 was considered statistically significant.
Results
Rotavirus prevalence
Out of the 471 children with acute diarrhea initially included in the study, 24 were subsequently excluded because for 19 of them no sample was collected and for 5 of them no epidemiological data were available. Of the 447 stool specimens analysed, 151 (33.8%) were found to contain rotavirus. Only one (1.7%) out of the 60 stool specimens collected from healthy children was positive by immunochromatographic test (ICG) (p < 0.0001). Adenovirus was detected in 17 of 447 (3.8%) stool samples and mixed infections with both rotavirus and adenovirus were observed in 11 (2.5%) stool samples.
Age and sex distribution of patients with rotavirus infection
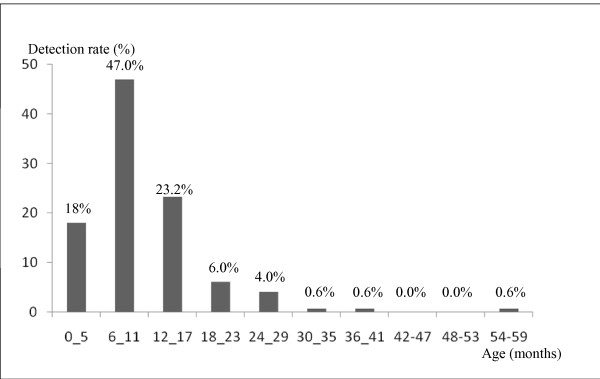
The age distribution of children with rotavirus is shown in Figure 1. Most cases of rotavirus infection (94.2%) occurred among children less than 2 years of age. The highest incidence was observed in children between 6 and 11 months of age. The median age for rotavirus infection was 8 months. There were more males (52.8%) than females, but the sex ratio among the rotavirus diarrhea patients was not significant (p = 0.1).
Figure 1.
Proportion of rotavirus infections by age groups among the 447 children suffering of gastroenteritis, between November 2008 to February 2010.
Seasonal distribution of rotavirus infections
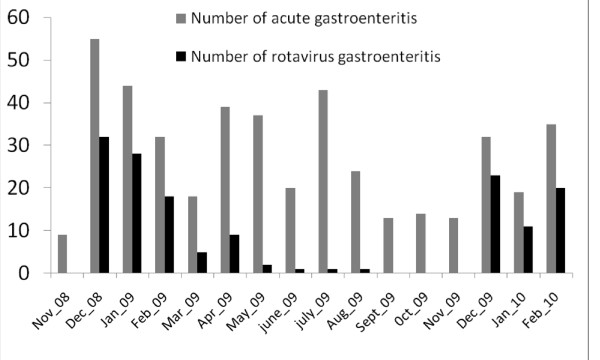
During the whole period of surveillance, the incidence of rotavirus infections varied significantly according to seasons (warm and cold) (p = 0.0001). Rotavirus infections occurred mostly during the season from December to April, corresponding to the dry season and relatively cold period (Figure 2).
Figure 2.
Seasonality of the acute gastroenteritis and the rotavirus infections among the 447 children suffering of gastroenteritis, between November 2008 to February 2010.
Hospitalization and clinical presentation of rotavirus infection
Of the 217 outpatient children, 48 (22.1%) were infected and of the 230 inpatient children, 103 (44.8%) were infected with rotavirus. The rotavirus infection prevalence was significantly higher among hospitalized children (p = 0.0001) illustrating a significant relationship between rate of hospitalization - severity of illness and diarrhoea associated with rotavirus infection. In addition, fever was the symptom most commonly reported in association with rotavirus diarrhoea (82.1%), followed by vomiting (72.8%) and dehydration (48.3%) (Table 1).
Table 1.
Clinical and epidemiological features of children with and without rotavirus detected in the diarrheal stool sample.
| Epidemiological and clinical characteristics | Rotavirus Diarrhea | Non Rotavirus Diarrhea | p | |
|---|---|---|---|---|
| (N = 151) | (N = 296) | |||
| Sex | ||||
| Male | 71 (47.0%) | 165 (55.7%) | p = 0.1 | |
| Female | 80 (53.0%) | 131 (44.8%) | ||
| Mean age (months) | 10.9 | 16.6 | ||
| Patient status | ||||
| Inpatient | 103 (44.8%) | 127 (55.2%) | p = 0.0001 | |
| Outpatient | 48 (22.1%) | 169 (77.9%) | ||
| Symptom | ||||
| Fever | 124 (82.1%) | 161 (54.4%) | p = 0.0001 | |
| Vomiting | 110 (72.8%) | 80 (27.0%) | p = 0.0001 | |
| Dehydration | 73 (48.3%) | 54 (18.2%) | p = 0.0001 | |
| Season* | ||||
| Warm | 5 (2.9%) | 168 (97.1%) | p = 0,0001 | |
| Cold | 146 (53.3%) | 128 (46.7%) | ||
| Breast feeding** | ||||
| Yes | 59 (59.0%) | 105 (75.5%) | p = 0.010 | |
| No | 41 (41.0%) | 34 (24.5%) | ||
| Drinking water source | ||||
| Municipal supply | 108 (71.5%) | 142 (48.0%) | p = 0.0001 | |
| Bottled water | 43 (28.5%) | 154 (52.0%) | ||
*Percentage of rotavirus diarrhea versus non-rotavirus diarrhea for both seasons.
**Percentage of rotavirus diarrhea versus non-rotavirus diarrhea according to method of feeding among infants ≤ 9 months of age.
Nutrition and drinking water
Among the infants ≤ 9 months of age, who had rotavirus diarrhoea and for whom the method of feeding was recorded, 55 out of 154 were breast-fed (35.7%) and 32 out of 61 were bottle-fed (52.5%) (p = 0.01). Analysis of hygiene factors such as the source of drinking water showed that children drinking municipal water were more affected by rotavirus diarrhoea than children drinking mineral water sold in bottles (p = 0.0001) (Table 1).
Discussion
This was the first study in Burkina Faso to investigate the prevalence, clinical characteristic and risk factors of rotavirus gastroenteritis among children. Regardless our results, rotavirus diarrhoea appears to be a major public health problem for children in Burkina Faso, as in the other developing countries. Our results show that a significant proportion of acute diarrhoea is due to rotavirus (33.8%) and rotavirus may be responsible for almost one-half (44.8%) of all hospitalizations for diarrhea in children < 5 years of age in Burkina Faso. The detected prevalence appears to be similar to those reported from other West African countries, which ranged from 33% to 39% [11-13]. In addition, a cumulative experience from 15 African countries suggested that rotavirus is the most important cause of severe diarrhoea in African children [14].
As observed in the other parts of the world, the burden of rotavirus disease is predominantly borne by children less than 2 years of age [15] with a high incidence among children 6-11 months of age. This can be explained by the protective effect of maternal antibodies in < 6 months old, and the development of natural immunity after repeated infections in children over 2 years of age [16,17].
Our results showed that rotavirus occurred mostly during the season from December to April, corresponding to the dry season and relatively cold period, as has been reported from Northern Ghana near Burkina Faso [13] and Guinea-Bissau [11]. Some studies conducted in other African countries indicated that rotavirus infections are present throughout the year, but with much higher prevalence in a certain period of a year [14].
Comparison of the clinical characteristics and severity of the acute gastroenteritis among the rotavirus-positive and rotavirus-negative patients indicated that vomiting, fever and dehydration were more frequently observed among diarrheal children with rotavirus than among those without rotavirus infection, as reported in the other countries [11,18].
Our confirmation of a previous observation made in the other parts of the world that during the first year of life breastfeeding is associated with a lower incidence of rotavirus diarrhoeal episodes adds to the multitude of benefits that have been associated with breastfeeding [19,20]. In addition, it has been shown that even if the breast-fed infants get infected with rotavirus, a milder disease occurs and hospitalization rate is significantly lower [21].
Another important issue, which was shown in this study, is a significant association between rotavirus diarrhea and municipal drinking water. This may be due to the possible contamination of municipal water for human consumption or in inter-human contamination which drinking water may be a potential risk of rotavirus transmission. Rotavirus has been described as a causative agent in several waterborne outbreaks in the industrialized countries [22-25], indicating good survival of rotavirus in water. In Burkina Faso, the evaluation of drinking water quality does not require testing for rotavirus but our results show the importance of including routine virological analysis of drinking water during rotavirus season.
Conclusions
In conclusion, this study provides information on the epidemiology and the extent of rotavirus infections in Burkina Faso. Our results indicate that gastroenteritis caused by rotavirus in the country is an important health problem, particularly among children less than 2 years of age and during the cold season. These data will be useful for making an informed decision about the introduction of rotavirus vaccine in Burkina Faso and will provide a baseline against which the impact of the vaccine introduction can be measured in the future.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
IJOB, IS and NB conceived the study; BB and IJOB were in charge of recruitment, examination, treatment and follow-up of patients, controls and undertook laboratory analysis; IJOB, IS and NB analyzed the data and prepared the manuscript; NB, IS, FB, SOC, KH and AST secured the study execution and provided ideas and comments during manuscript preparation. All authors have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Isidore JO Bonkoungou, Email: ouindgueta@gmail.com.
Idrissa Sanou, Email: idrissasanou@yahoo.com.
Fabienne Bon, Email: fabon@u-bourgogne.fr.
Benoit Benon, Email: cliniquetisserins@yahoo.fr.
Sheick O Coulibaly, Email: sheickoumar2@yahoo.fr.
Kaisa Haukka, Email: kaisa.haukka@thl.fi.
Alfred S Traoré, Email: astraore@univ-ouaga.bf.
Nicolas Barro, Email: nicolas_barro@univ-ouaga.bf.
Acknowledgements
This study was supported by the Laboratory of Biochemstry and Molecular Biology of CRSBAN/UFR-SVT (University of Ouagadougou) and the National Public Health Laboratory in Burkina Faso. We thank the staff of Centre Médical du Secteur 30 de Ouagadougou, all the children and their parents who participated in this research. We express our gratitude to Dr Fidèle Tiendrébéogo for the technical assistance in the statistical analysis.
References
- Desselberger U, Wolleswinkel-van den Bosch J, Mrukowicz J, Rodrigo C, Giaquinto C, Vesikari T. Rotavirus types in Europe and their significance for 638 vaccinations. Pediatr Infect Dis J. 2006;25(1):S30–41. doi: 10.1097/01.inf.0000195787.99199.4a. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–5772. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua KB, Emmanuel SC, Goh P, Quak SH, Lee BW, Han HH, Ward RL, Bernstein DI, De Vos B, Bock HL. A rotavirus vaccine for infants: the Asian experience. Ann Acad Med Singapore. 2006;35(1):38–47. [PubMed] [Google Scholar]
- Dennehy PH. Rotavirus vaccines: an overview. Clin Microbiol Rev. 2008;21:198–208. doi: 10.1128/CMR.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, López P, Macías-Parra M, Ortega-Barría E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavía-Ruz N, Salmerón J, Rüttimann R, Tinoco JC, Rubio P, Nuñez E, Guerrero ML, Yarzábal JP, Damaso S, Tornieporth N, Sáez-Llorens X, Vergara RF, Vesikari T, Bouckenooghe A, Clemens R, De Vos B, O'Ryan M. Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CD, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O'Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA, Heaton PM . Safety and efficacy of pentavalent human-bovine (WC3) reassortant rotavirus vaccine in preventing rotavirus gastroenteritis and reducing associated health care resource utilization. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370(9601):1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- Block SL, Vesikari T, Goveia MG, Rivers SB, Adeyi BA, Dallas MJ, Bauder J, Boslego JW, Heaton PM. Efficacy: immunogenicity, and safety of the pentavalent human-bovine (WC3) reassortant Rotavirus Vaccine at the end of shelf life. Pediatrics. 2007;119(1):11–18. doi: 10.1542/peds.2006-2058. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12(2):304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkoungou OJI, Sanou I, Barro N, Toé L, Sanfo MS, Ouédraogo/Traoré R, Traore AS. Electrophoretypes characterization of human Rotavirus in two pediatrics services, Ouagadougou, Burkina Faso. J Med Sci. 2008;8:371–377. doi: 10.3923/jms.2008.371.377. [DOI] [Google Scholar]
- Rodrigues A, de Carvalho M, Monteiro S, Mikkelson SC, Aaby P, Molbakk K, Fischer TK. Hospital surveillance of rotavirus infection and nosocomial transmission of rotavirus disease among children in Guinea-Bissau. Pediatr Infect Dis J. 2007;26(3):233–237. doi: 10.1097/01.inf.0000254389.65667.8b. [DOI] [PubMed] [Google Scholar]
- de Villiers FPR, Sawyerr TN, de Villiers GK. The incidence and clinical presentation of infantile rotavirus diarrhoea in Sierra Leone. S Afr Med J. 2009;99(4):249–252. [PubMed] [Google Scholar]
- Binka FN, Anto FK, Oduro AR, Awini EA, Nazzar AK, Armah GE, Asmah RH, Hall AJ, Cutts F, Alexander N, Brown D, Green J, Gray J, Iturriza-Gómara M. Navrongo Rotavirus Research Group. Incidence and risk factors of paediatric rotavirus diarrhoea in Northern Ghana. Trop Med Int Health. 2003;8(9):840–846. doi: 10.1046/j.1365-3156.2003.01097.x. [DOI] [PubMed] [Google Scholar]
- Cunliffe NA, Kilgore PE, Bresee JS, Steele AD, Luo N, Hart CA, Glass RI. Epidemiology of rotavirus diarrhea in Africa: a review to assess the need for rotavirus immunization. Bull WHO. 1998;76(5):525–37. [PMC free article] [PubMed] [Google Scholar]
- De Zoysa I, Feachem RG. Interventions for the control of diarrhoeal disease among young children: rotavirus and cholera immunization. Bull WHO. 1985;63(3):569–583. [PMC free article] [PubMed] [Google Scholar]
- Offit PA. Host factors associated with protection against rotavirus disease: the skies are clearing. J Infect Dis. 1996;174(Suppl 1):S59–S64. doi: 10.1093/infdis/174.supplement_1.s59. [DOI] [PubMed] [Google Scholar]
- Jiang B, Gentsch JR, Glass RI. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin Infect Dis. 2002;34(10):1351–1361. doi: 10.1086/340103. [DOI] [PubMed] [Google Scholar]
- Zarnani AH, Modarres S, Jadali F, Sabahi F, Moazzeni SM, Vazirian F. Role of rotaviruses in children with acute diarrhea in Tehran, Iran. J Clin Virology. 2004;29(3):189–193. doi: 10.1016/S1386-6532(03)00123-9. [DOI] [PubMed] [Google Scholar]
- Naficy AB, Abu-Elyazeed R, Holmes JL, Rao RM, Savarino JS, Kim Y , Wierzba TF, Peruski L, Lee YJ, Gentsch JR, Glass RI, Clemens JD. Epidemiology of Rotavirus Diarrhea in Egyptian Children and Implications for Disease Control. Am J Epidemiol. 1999;150(7):770–777. doi: 10.1093/oxfordjournals.aje.a010080. [DOI] [PubMed] [Google Scholar]
- Clemens J, Rao M, Ahmed F, Ward R, Huda S, Chakraborty J, Yunus M, Khan M R, Ali M, Kay B, van Loon F, Sack D. Breast-feeding and the risk of life-threatening rotavirus diarrhea: prevention or postponement? Pediatrics. 1993;92(5):680–685. [PubMed] [Google Scholar]
- Kapikian AZ, Yasutaka H, Chanok RM. Field's Virology. 4. Philadelphia: Lippincott Williams and Wilkins Co; 2001. Rotaviruses; pp. 1787–834. [Google Scholar]
- Kukkula M, Arstila P, Klossner ML, Maunula L, Bonsdorff CH, Jaatinen P. Waterborne outbreak of viral gastroenteritis. Scand J Infect Dis. 1997;29(4):415–418. doi: 10.3109/00365549709011840. [DOI] [PubMed] [Google Scholar]
- Villena C, Gabrieli R, Pinto RM, Guix S, Donia D, Buonomo E, Palombi L, Cenko F, Bino S, Bosch A, Divizia M. A large infantile gastroenteritis outbreak in Albania caused by multiple emerging rotavirus genotypes. Epidemiol Infect. 2003;131(3):1105–1110. doi: 10.1017/S0950268803001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt MA, Haas NL, Hunt RJ. Vulnerability of drinking-water wells in La Crosse, Wisconsin, to enteric-virus contamination from surface water contributions. Appl Environ Microbiol. 2004;70(10):5937–5946. doi: 10.1128/AEM.70.10.5937-5946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli D, Prato R, Chironna M, Sallustio A, Caputi G, Conversano M, Ciofi Degli Atti M, D'Ancona FP, Germinario CA, Quarto M. Large outbreak of viral gastroenteritis caused by contaminated drinking water in Apulia, Italy, May- October 2006. Euro Surveill. 2007;12(16):Article 1. doi: 10.2807/esw.12.16.03176-en. [DOI] [PubMed] [Google Scholar]