Abstract
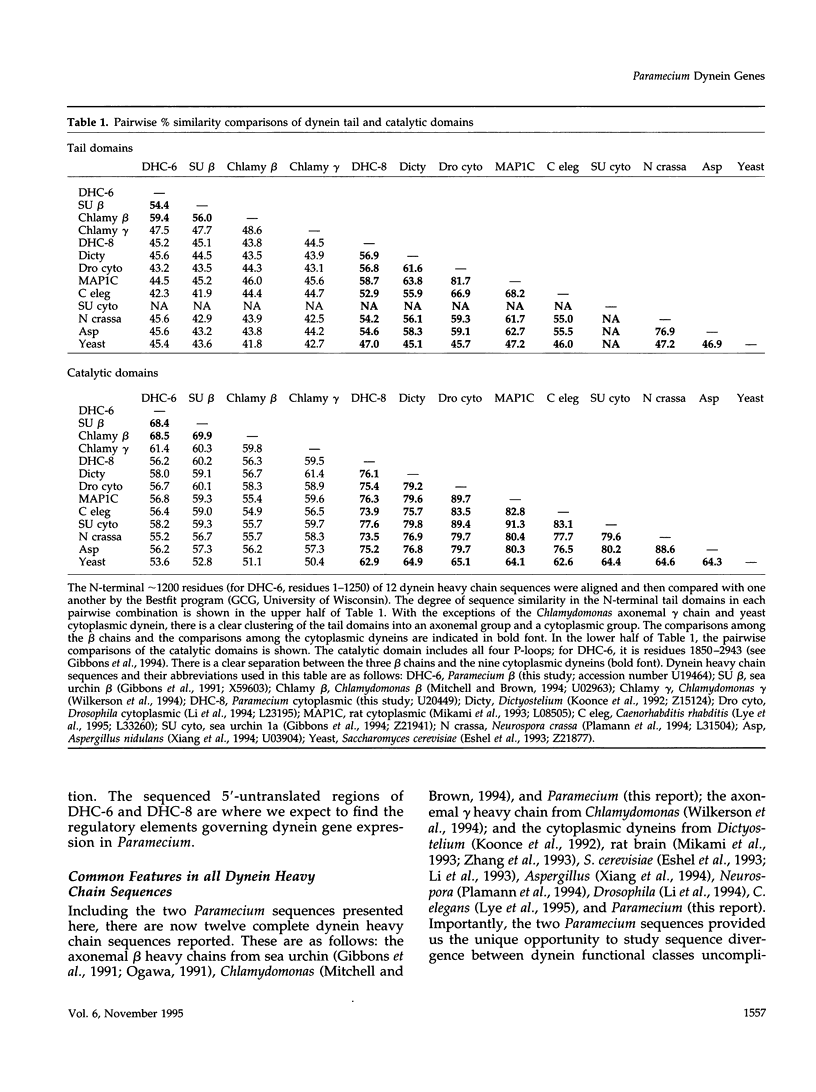
The genes encoding two Paramecium dynein heavy chains, DHC-6 and DHC-8, have been cloned and sequenced. Sequence-specific antibodies demonstrate that DHC-6 encodes ciliary outer arm beta-chain and DHC-8 encodes a cytoplasmic dynein heavy chain. Therefore, this study is the first opportunity to compare the primary structures and expression of two heavy chains representing the two functional classes of dynein expressed in the same cell. Deciliation of paramecia results in the accumulation of mRNA from DHC-6, but not DHC-8. Nuclear run-on transcription experiments demonstrate that this increase in the steady state concentration of DHC-6 mRNA is a consequence of a rapid induction of transcription in response to deciliation. This is the first demonstration that dynein, like other axonemal components, is transcriptionally regulated during reciliation. Analyses of the sequences of the two Paramecium dyneins and the dynein heavy chains from other organisms indicate that the heavy chain can be divided into three regions: 1) the sequence of the central catalytic domain is conserved among all dyneins; 2) the tail domain sequence, consisting of the N-terminal 1200 residues, differentiates between axonemal and cytoplasmic dyneins; and 3) the N-terminal 200 residues are the most divergent and appear to classify the isoforms. The organization of the heavy chain predicts that the variable tail domain may be sufficient to target the dynein to the appropriate place in the cell.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aniento F., Emans N., Griffiths G., Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993 Dec;123(6 Pt 1):1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
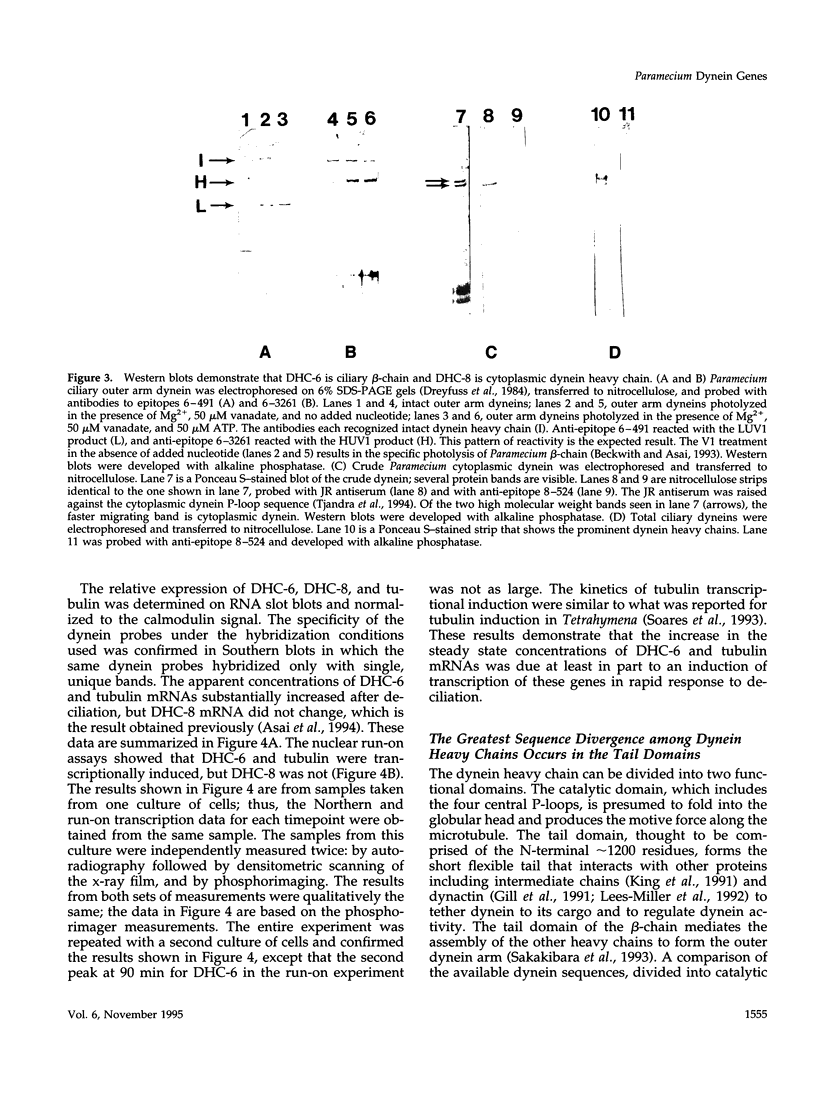
- Asai D. J., Beckwith S. M., Kandl K. A., Keating H. H., Tjandra H., Forney J. D. The dynein genes of Paramecium tetraurelia. Sequences adjacent to the catalytic P-loop identify cytoplasmic and axonemal heavy chain isoforms. J Cell Sci. 1994 Apr;107(Pt 4):839–847. doi: 10.1242/jcs.107.4.839. [DOI] [PubMed] [Google Scholar]
- Asai D. J., Brokaw C. J. Dynein heavy chain isoforms and axonemal motility. Trends Cell Biol. 1993 Nov;3(11):398–402. doi: 10.1016/0962-8924(93)90090-n. [DOI] [PubMed] [Google Scholar]
- Asai D. J., Criswell P. G. Identification of new dynein heavy-chain genes by RNA-directed polymerase chain reaction. Methods Cell Biol. 1995;47:579–585. doi: 10.1016/s0091-679x(08)60863-8. [DOI] [PubMed] [Google Scholar]
- Beckwith S. M., Asai D. J. Ciliary dynein of Paramecium tetraurelia: photolytic maps of the three heavy chains. Cell Motil Cytoskeleton. 1993;24(1):29–38. doi: 10.1002/cm.970240104. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Control of flagellar bending: a new agenda based on dynein diversity. Cell Motil Cytoskeleton. 1994;28(3):199–204. doi: 10.1002/cm.970280303. [DOI] [PubMed] [Google Scholar]
- Corthésy-Theulaz I., Pauloin A., Pfeffer S. R. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992 Sep;118(6):1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel D., Urrestarazu L. A., Vissers S., Jauniaux J. C., van Vliet-Reedijk J. C., Planta R. J., Gibbons I. R. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fok A. K., Wang H., Katayama A., Aihara M. S., Allen R. D. 22S axonemal dynein is preassembled and functional prior to being transported to and attached on the axonemes. Cell Motil Cytoskeleton. 1994;29(3):215–224. doi: 10.1002/cm.970290304. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons B. H., Asai D. J., Tang W. J., Hays T. S., Gibbons I. R. Phylogeny and expression of axonemal and cytoplasmic dynein genes in sea urchins. Mol Biol Cell. 1994 Jan;5(1):57–70. doi: 10.1091/mbc.5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I. R., Asai D. J., Tang W. J., Gibbons B. H. A cytoplasmic dynein heavy chain in sea urchin embryos. Biol Cell. 1992;76(3):303–309. doi: 10.1016/0248-4900(92)90432-z. [DOI] [PubMed] [Google Scholar]
- Gibbons I. R., Gibbons B. H., Mocz G., Asai D. J. Multiple nucleotide-binding sites in the sequence of dynein beta heavy chain. Nature. 1991 Aug 15;352(6336):640–643. doi: 10.1038/352640a0. [DOI] [PubMed] [Google Scholar]
- Gill S. R., Schroer T. A., Szilak I., Steuer E. R., Sheetz M. P., Cleveland D. W. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991 Dec;115(6):1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley D., Rudman B. M., Preer J. R., Jr, Polisky B. Multilevel regulation of surface antigen gene expression in Paramecium tetraurelia. Mol Cell Biol. 1990 Apr;10(4):1538–1544. doi: 10.1128/mcb.10.4.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur E. L., Vallee R. B. DYNEINS: molecular structure and cellular function. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
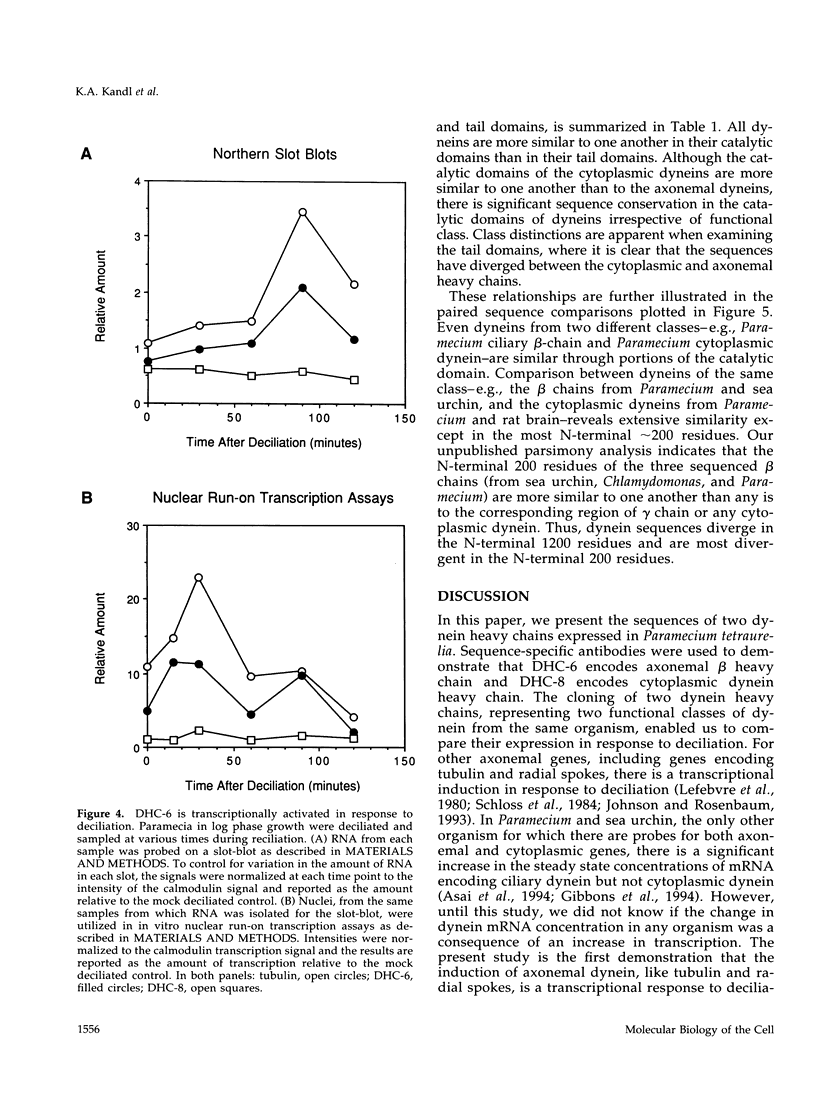
- Johnson K. A., Rosenbaum J. L. Flagellar regeneration in Chlamydomonas: a model system for studying organelle assembly. Trends Cell Biol. 1993 May;3(5):156–161. doi: 10.1016/0962-8924(93)90136-o. [DOI] [PubMed] [Google Scholar]
- Kanabrocki J. A., Saimi Y., Preston R. R., Haynes W. J., Kung C. Efficient transformation of cam2, a behavioral mutant of Paramecium tetraurelia, with the calmodulin gene. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10845–10849. doi: 10.1073/pnas.88.23.10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. M., Wilkerson C. G., Witman G. B. The Mr 78,000 intermediate chain of Chlamydomonas outer arm dynein interacts with alpha-tubulin in situ. J Biol Chem. 1991 May 5;266(13):8401–8407. [PubMed] [Google Scholar]
- Koonce M. P., Grissom P. M., McIntosh J. R. Dynein from Dictyostelium: primary structure comparisons between a cytoplasmic motor enzyme and flagellar dynein. J Cell Biol. 1992 Dec;119(6):1597–1604. doi: 10.1083/jcb.119.6.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J., Barkalow K., Hamasaki T., Satir P. Structural and functional characterization of paramecium dynein: initial studies. J Protozool. 1991 Jan-Feb;38(1):55–61. doi: 10.1111/j.1550-7408.1991.tb04801.x. [DOI] [PubMed] [Google Scholar]
- Lee-Eiford A., Ow R. A., Gibbons I. R. Specific cleavage of dynein heavy chains by ultraviolet irradiation in the presence of ATP and vanadate. J Biol Chem. 1986 Feb 15;261(5):2337–2342. [PubMed] [Google Scholar]
- Leeck C. L., Forney J. D. The upstream region is required but not sufficient to control mutually exclusive expression of Paramecium surface antigen genes. J Biol Chem. 1994 Dec 9;269(49):31283–31288. [PubMed] [Google Scholar]
- Lees-Miller J. P., Helfman D. M., Schroer T. A. A vertebrate actin-related protein is a component of a multisubunit complex involved in microtubule-based vesicle motility. Nature. 1992 Sep 17;359(6392):244–246. doi: 10.1038/359244a0. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. A., Silflow C. D., Wieben E. D., Rosenbaum J. L. Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell. 1980 Jun;20(2):469–477. doi: 10.1016/0092-8674(80)90633-9. [DOI] [PubMed] [Google Scholar]
- Li M., McGrail M., Serr M., Hays T. S. Drosophila cytoplasmic dynein, a microtubule motor that is asymmetrically localized in the oocyte. J Cell Biol. 1994 Sep;126(6):1475–1494. doi: 10.1083/jcb.126.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Y., Yeh E., Hays T., Bloom K. Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10096–10100. doi: 10.1073/pnas.90.21.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. X., Collins C. A. Immunolocalization of cytoplasmic dynein to lysosomes in cultured cells. J Cell Sci. 1992 Jan;101(Pt 1):125–137. doi: 10.1242/jcs.101.1.125. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Machemer H., Ogura A. Ionic conductances of membranes in ciliated and deciliated Paramecium. J Physiol. 1979 Nov;296:49–60. doi: 10.1113/jphysiol.1979.sp012990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde D. N., O'Toole E. T., McDonald K. L., McIntosh J. R., Porter M. E. Arrangement of inner dynein arms in wild-type and mutant flagella of Chlamydomonas. J Cell Biol. 1992 Sep;118(5):1145–1162. doi: 10.1083/jcb.118.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A., Paschal B. M., Mazumdar M., Vallee R. B. Molecular cloning of the retrograde transport motor cytoplasmic dynein (MAP 1C). Neuron. 1993 May;10(5):787–796. doi: 10.1016/0896-6273(93)90195-w. [DOI] [PubMed] [Google Scholar]
- Mitchell D. R., Brown K. S. Sequence analysis of the Chlamydomonas alpha and beta dynein heavy chain genes. J Cell Sci. 1994 Mar;107(Pt 3):635–644. doi: 10.1242/jcs.107.3.635. [DOI] [PubMed] [Google Scholar]
- Mitchell D. R. Cell and molecular biology of flagellar dyneins. Int Rev Cytol. 1994;155:141–180. doi: 10.1016/s0074-7696(08)62098-7. [DOI] [PubMed] [Google Scholar]
- Mocz G., Gibbons I. R. ATP-insensitive interaction of the amino-terminal region of the beta heavy chain of dynein with microtubules. Biochemistry. 1993 Apr 6;32(13):3456–3460. doi: 10.1021/bi00064a032. [DOI] [PubMed] [Google Scholar]
- Moss A. G., Gatti J. L., Witman G. B. The motile beta/IC1 subunit of sea urchin sperm outer arm dynein does not form a rigor bond. J Cell Biol. 1992 Sep;118(5):1177–1188. doi: 10.1083/jcb.118.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely M. D., Erickson H. P., Boekelheide K. HMW-2, the Sertoli cell cytoplasmic dynein from rat testis, is a dimer composed of nearly identical subunits. J Biol Chem. 1990 May 25;265(15):8691–8698. [PubMed] [Google Scholar]
- Ogawa K. Four ATP-binding sites in the midregion of the beta heavy chain of dynein. Nature. 1991 Aug 15;352(6336):643–645. doi: 10.1038/352643a0. [DOI] [PubMed] [Google Scholar]
- Pallini V., Mencarelli C., Bracci L., Contorni M., Ruggiero P., Tiezzi A., Manetti R. Cytoplasmic nucleoside-triphosphatase similar to axonemal dynein occur widely in different cell types. J Submicrosc Cytol. 1983 Jan;15(1):229–235. [PubMed] [Google Scholar]
- Piperno G., Ramanis Z., Smith E. F., Sale W. S. Three distinct inner dynein arms in Chlamydomonas flagella: molecular composition and location in the axoneme. J Cell Biol. 1990 Feb;110(2):379–389. doi: 10.1083/jcb.110.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamann M., Minke P. F., Tinsley J. H., Bruno K. S. Cytoplasmic dynein and actin-related protein Arp1 are required for normal nuclear distribution in filamentous fungi. J Cell Biol. 1994 Oct;127(1):139–149. doi: 10.1083/jcb.127.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M. E., Knott J. A., Gardner L. C., Mitchell D. R., Dutcher S. K. Mutations in the SUP-PF-1 locus of Chlamydomonas reinhardtii identify a regulatory domain in the beta-dynein heavy chain. J Cell Biol. 1994 Sep;126(6):1495–1507. doi: 10.1083/jcb.126.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson K., Serr M., Gepner J., Gibbons I., Hays T. S. A family of dynein genes in Drosophila melanogaster. Mol Biol Cell. 1994 Jan;5(1):45–55. doi: 10.1091/mbc.5.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C. B., Fraga D., Hinrichsen R. D. Extremely short 20-33 nucleotide introns are the standard length in Paramecium tetraurelia. Nucleic Acids Res. 1994 Apr 11;22(7):1221–1225. doi: 10.1093/nar/22.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H., Takada S., King S. M., Witman G. B., Kamiya R. A Chlamydomonas outer arm dynein mutant with a truncated beta heavy chain. J Cell Biol. 1993 Aug;122(3):653–661. doi: 10.1083/jcb.122.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale W. S., Fox L. A. Isolated beta-heavy chain subunit of dynein translocates microtubules in vitro. J Cell Biol. 1988 Nov;107(5):1793–1797. doi: 10.1083/jcb.107.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders W. S., Koshland D., Eshel D., Gibbons I. R., Hoyt M. A. Saccharomyces cerevisiae kinesin- and dynein-related proteins required for anaphase chromosome segregation. J Cell Biol. 1995 Feb;128(4):617–624. doi: 10.1083/jcb.128.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. A., Silflow C. D., Rosenbaum J. L. mRNA abundance changes during flagellar regeneration in Chlamydomonas reinhardtii. Mol Cell Biol. 1984 Mar;4(3):424–434. doi: 10.1128/mcb.4.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder C. C., Fok A. K., Allen R. D. Vesicle transport along microtubular ribbons and isolation of cytoplasmic dynein from Paramecium. J Cell Biol. 1990 Dec;111(6 Pt 1):2553–2562. doi: 10.1083/jcb.111.6.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Leeck C., Forney J. Molecular and genetic analyses of the B type surface protein gene from Paramecium tetraurelia. Genetics. 1993 May;134(1):189–198. doi: 10.1093/genetics/134.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares H., Galego L., Cóias R., Rodrigues-Pousada C. The mechanisms of tubulin messenger regulation during Tetrahymena pyriformis reciliation. J Biol Chem. 1993 Aug 5;268(22):16623–16630. [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Tang W. Y., Gibbons I. R. Photosensitized cleavage of dynein heavy chains. Cleavage at the V2 site by irradiation at 365 NM in the presence of oligovanadate. J Biol Chem. 1987 Dec 25;262(36):17728–17734. [PubMed] [Google Scholar]
- Travis S. M., Nelson D. L. Purification and properties of dyneins from Paramecium cilia. Biochim Biophys Acta. 1988 Jul 14;966(1):73–83. doi: 10.1016/0304-4165(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Vaisberg E. A., Koonce M. P., McIntosh J. R. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J Cell Biol. 1993 Nov;123(4):849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Wall J. S., Paschal B. M., Shpetner H. S. Microtubule-associated protein 1C from brain is a two-headed cytosolic dynein. Nature. 1988 Apr 7;332(6164):561–563. doi: 10.1038/332561a0. [DOI] [PubMed] [Google Scholar]
- Vallee R. Molecular analysis of the microtubule motor dynein. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8769–8772. doi: 10.1073/pnas.90.19.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Berrez J. M., Antony C., Karsenti E. Taxol-induced microtubule asters in mitotic extracts of Xenopus eggs: requirement for phosphorylated factors and cytoplasmic dynein. J Cell Biol. 1991 Mar;112(6):1177–1187. doi: 10.1083/jcb.112.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Asai D. J., Robinson K. R. Retrograde but not anterograde bead movement in intact axons requires dynein. J Neurobiol. 1995 Jun;27(2):216–226. doi: 10.1002/neu.480270208. [DOI] [PubMed] [Google Scholar]
- Wilkerson C. G., King S. M., Witman G. B. Molecular analysis of the gamma heavy chain of Chlamydomonas flagellar outer-arm dynein. J Cell Sci. 1994 Mar;107(Pt 3):497–506. doi: 10.1242/jcs.107.3.497. [DOI] [PubMed] [Google Scholar]
- Williams B. D., Mitchell D. R., Rosenbaum J. L. Molecular cloning and expression of flagellar radial spoke and dynein genes of Chlamydomonas. J Cell Biol. 1986 Jul;103(1):1–11. doi: 10.1083/jcb.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X., Beckwith S. M., Morris N. R. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2100–2104. doi: 10.1073/pnas.91.6.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Tanaka Y., Nonaka S., Aizawa H., Kawasaki H., Nakata T., Hirokawa N. The primary structure of rat brain (cytoplasmic) dynein heavy chain, a cytoplasmic motor enzyme. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7928–7932. doi: 10.1073/pnas.90.17.7928. [DOI] [PMC free article] [PubMed] [Google Scholar]