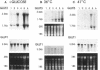
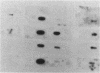
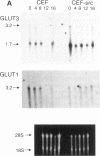
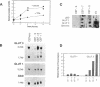
Abstract
Vertebrate cells that are transformed by oncogenes such as v-src or are stimulated by mitogens have increased rates of glucose uptake. In rodent cells, the mechanisms whereby glucose transport is up-regulated are well understood. Stimulation of glucose transport involves an elevation in mRNA encoding the GLUT1 glucose transporter that is controlled at the levels of both transcription and mRNA stability. Cloning and sequencing of chicken GLUT1 cDNA showed that it shares 95% amino acid sequence similarity to mammalian GLUT1s. Nevertheless, unlike mammalian GLUT1 mRNA, it was not induced by v-src, serum addition, or treatment with the tumor promoter 12-O-tetradecanoylphorbol 13-acetate in chicken embryo fibroblasts. Rather, the induction of glucose transport in chicken embryo fibroblasts by v-src, serum, and 12-O-tetradecanoylphorbol 13-acetate was associated with induction of GLUT3 mRNA level and GLUT3 transcription. Rat fibroblasts were also found to express both GLUT1 and GLUT3 isoforms, but v-src induced GLUT1 and not GLUT3. This suggests that animal cells require both a basal and an upregulatable glucose transporter and that these functions have been subsumed by different GLUT isoforms in avian and mammalian cells.
Full text
PDF


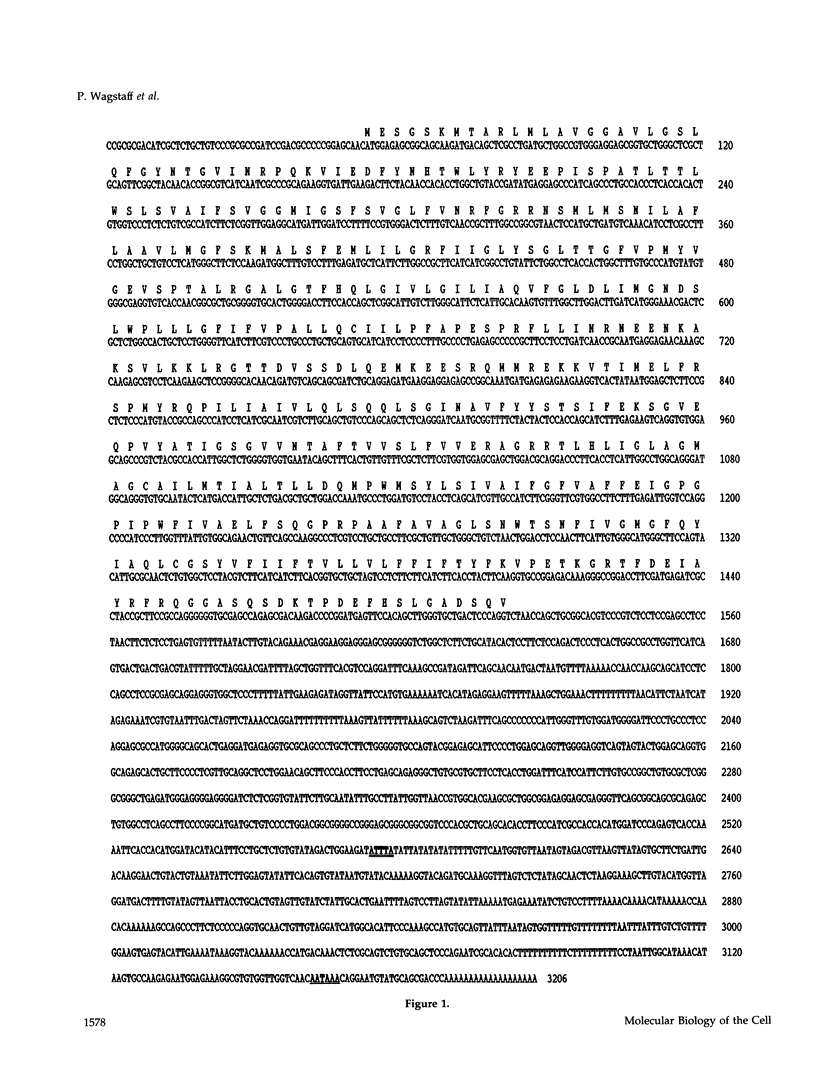



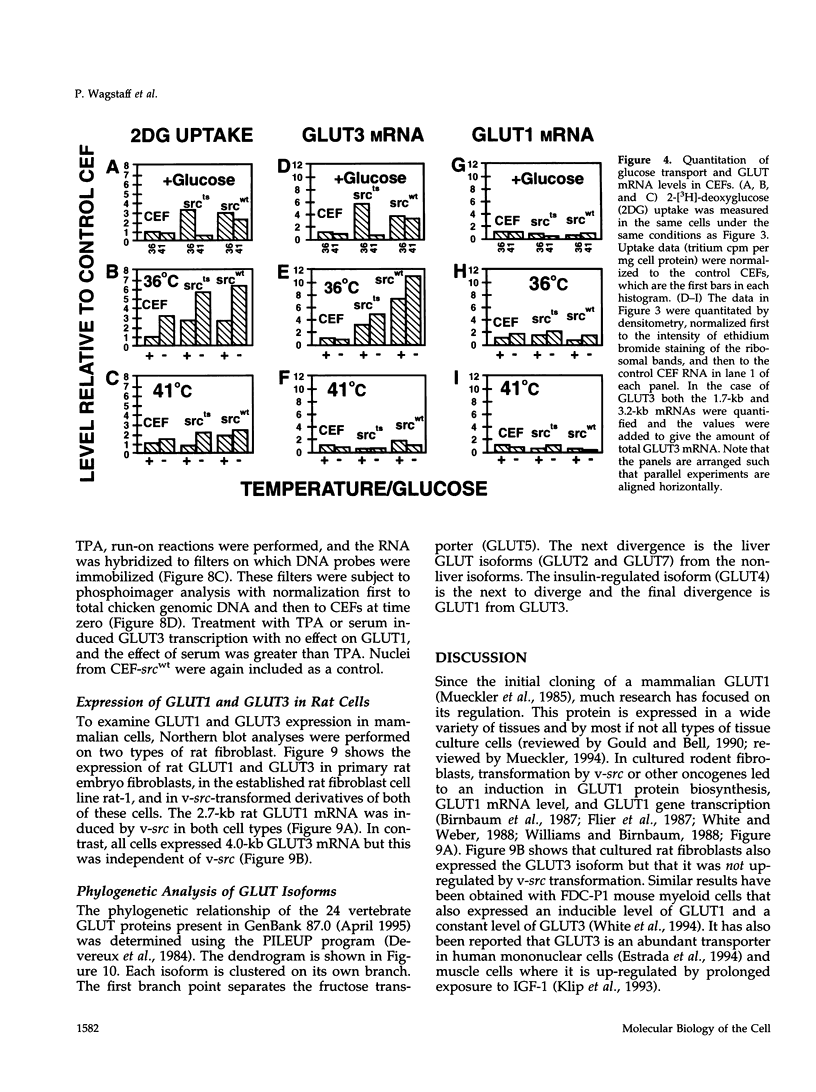
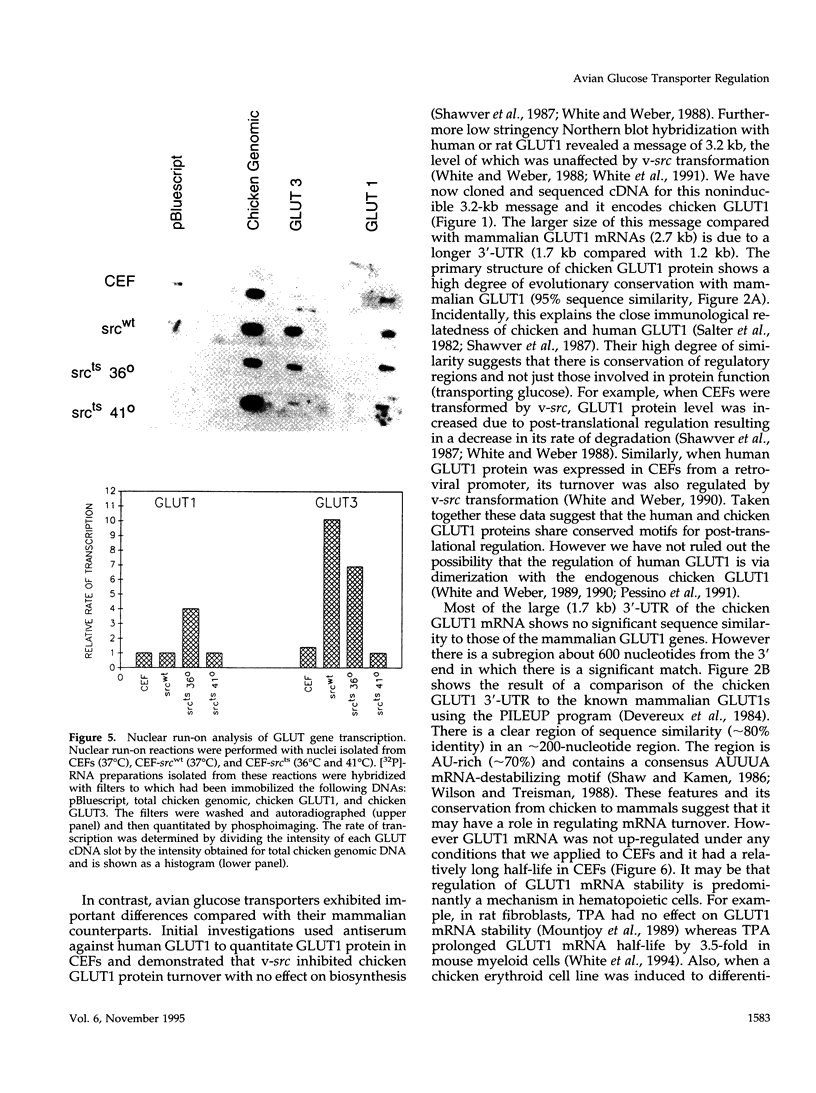



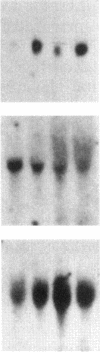
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. M., Scolnick E. M. Construction and isolation of a transforming murine retrovirus containing the src gene of Rous sarcoma virus. J Virol. 1983 May;46(2):594–605. doi: 10.1128/jvi.46.2.594-605.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilan P. J., Mitsumoto Y., Maher F., Simpson I. A., Klip A. Detection of the GLUT3 facilitative glucose transporter in rat L6 muscle cells: regulation by cellular differentiation, insulin and insulin-like growth factor-I. Biochem Biophys Res Commun. 1992 Jul 31;186(2):1129–1137. doi: 10.1016/0006-291x(92)90864-h. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987 Mar 20;235(4795):1495–1498. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Takeda J., Brot-Laroche E., Bell G. I., Davidson N. O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992 Jul 25;267(21):14523–14526. [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. The effect of phorbol diesters on chicken embryo fibroblasts. Cancer Res. 1977 Sep;37(9):3257–3265. [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J. A., Stone E. M., Dennison O. E., Rothblum K. N., Schwartz R. J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Estrada D. E., Elliott E., Zinman B., Poon I., Liu Z., Klip A., Daneman D. Regulation of glucose transport and expression of GLUT3 transporters in human circulating mononuclear cells: studies in cells from insulin-dependent diabetic and nondiabetic individuals. Metabolism. 1994 May;43(5):591–598. doi: 10.1016/0026-0495(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M. M., Usher P., Lodish H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987 Mar 20;235(4795):1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Fukumoto H., Seino S., Imura H., Seino Y., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Bell G. I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5434–5438. doi: 10.1073/pnas.85.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G. W., Bell G. I. Facilitative glucose transporters: an expanding family. Trends Biochem Sci. 1990 Jan;15(1):18–23. doi: 10.1016/0968-0004(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Hay A. J. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. doi: 10.1016/0042-6822(74)90335-3. [DOI] [PubMed] [Google Scholar]
- Hiraki Y., Rosen O. M., Birnbaum M. J. Growth factors rapidly induce expression of the glucose transporter gene. J Biol Chem. 1988 Sep 25;263(27):13655–13662. [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kayano T., Burant C. F., Fukumoto H., Gould G. W., Fan Y. S., Eddy R. L., Byers M. G., Shows T. B., Seino S., Bell G. I. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 1990 Aug 5;265(22):13276–13282. [PubMed] [Google Scholar]
- Kayano T., Fukumoto H., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988 Oct 25;263(30):15245–15248. [PubMed] [Google Scholar]
- Klip A., Ramlal T., Bilan P. J., Marette A., Liu Z., Mitsumoto Y. What signals are involved in the stimulation of glucose transport by insulin in muscle cells? Cell Signal. 1993 Sep;5(5):519–529. doi: 10.1016/0898-6568(93)90047-p. [DOI] [PubMed] [Google Scholar]
- Klip A., Tsakiridis T., Marette A., Ortiz P. A. Regulation of expression of glucose transporters by glucose: a review of studies in vivo and in cell cultures. FASEB J. 1994 Jan;8(1):43–53. doi: 10.1096/fasebj.8.1.8299889. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Lagnado C. A., Brown C. Y., Goodall G. J. AUUUA is not sufficient to promote poly(A) shortening and degradation of an mRNA: the functional sequence within AU-rich elements may be UUAUUUA(U/A)(U/A). Mol Cell Biol. 1994 Dec;14(12):7984–7995. doi: 10.1128/mcb.14.12.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucine-zipper motif update. Nature. 1989 Jul 13;340(6229):103–104. doi: 10.1038/340103a0. [DOI] [PubMed] [Google Scholar]
- Maher F., Simpson I. A. The GLUT3 glucose transporter is the predominant isoform in primary cultured neurons: assessment by biosynthetic and photoaffinity labelling. Biochem J. 1994 Jul 15;301(Pt 2):379–384. doi: 10.1042/bj3010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher F., Vannucci S. J., Simpson I. A. Glucose transporter proteins in brain. FASEB J. 1994 Oct;8(13):1003–1011. doi: 10.1096/fasebj.8.13.7926364. [DOI] [PubMed] [Google Scholar]
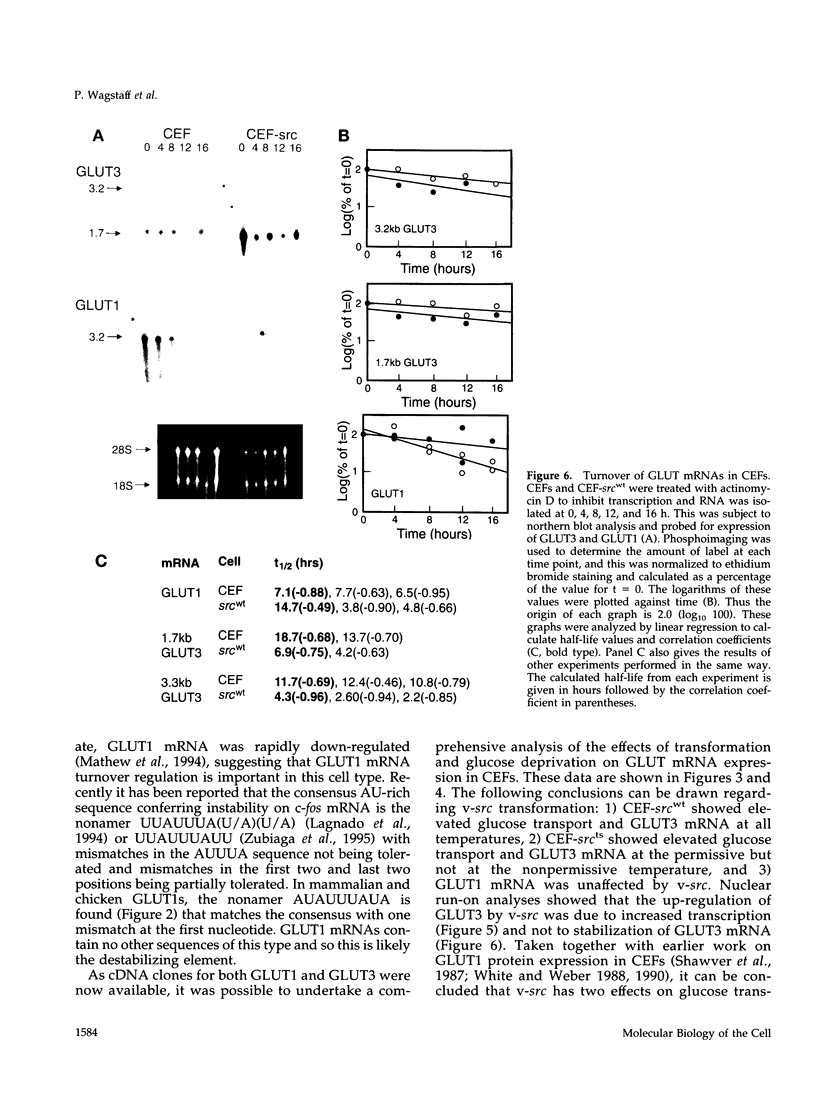
- Mathew A., Grdisa M., Robbins P. J., White M. K., Johnstone R. M. Loss of glucose transporters is an early event in differentiation of HD3 cells. Am J Physiol. 1994 May;266(5 Pt 1):C1222–C1230. doi: 10.1152/ajpcell.1994.266.5.C1222. [DOI] [PubMed] [Google Scholar]
- McCubrey J. A., Smith S. R., Algate P. A., DeVente J. E., White M. K., Steelman L. S. Retroviral infection can abrogate the factor-dependency of hematopoietic cells by autocrine and non-autocrine mechanisms depending on the presence of a functional viral oncogene. Oncogene. 1993 Nov;8(11):2905–2915. [PubMed] [Google Scholar]
- Mellanen P., Minn H., Grénman R., Härkönen P. Expression of glucose transporters in head-and-neck tumors. Int J Cancer. 1994 Mar 1;56(5):622–629. doi: 10.1002/ijc.2910560503. [DOI] [PubMed] [Google Scholar]
- Mountjoy K. G., Housey G. M., Flier J. S. Overproduction of the beta 1 form of protein kinase C enhances phorbol ester induction of glucose transporter mRNA. Mol Endocrinol. 1989 Dec;3(12):2018–2027. doi: 10.1210/mend-3-12-2018. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994 Feb 1;219(3):713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- Murakami T., Nishiyama T., Shirotani T., Shinohara Y., Kan M., Ishii K., Kanai F., Nakazuru S., Ebina Y. Identification of two enhancer elements in the gene encoding the type 1 glucose transporter from the mouse which are responsive to serum, growth factor, and oncogenes. J Biol Chem. 1992 May 5;267(13):9300–9306. [PubMed] [Google Scholar]
- Nagamatsu S., Kornhauser J. M., Burant C. F., Seino S., Mayo K. E., Bell G. I. Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem. 1992 Jan 5;267(1):467–472. [PubMed] [Google Scholar]
- Nagamatsu S., Sawa H., Wakizaka A., Hoshino T. Expression of facilitative glucose transporter isoforms in human brain tumors. J Neurochem. 1993 Dec;61(6):2048–2053. doi: 10.1111/j.1471-4159.1993.tb07441.x. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessino A., Hebert D. N., Woon C. W., Harrison S. A., Clancy B. M., Buxton J. M., Carruthers A., Czech M. P. Evidence that functional erythrocyte-type glucose transporters are oligomers. J Biol Chem. 1991 Oct 25;266(30):20213–20217. [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Usher P., Flier J. S. Platelet-derived growth factor regulates glucose transporter expression. J Biol Chem. 1988 Nov 15;263(32):16523–16526. [PubMed] [Google Scholar]
- Salter D. W., Baldwin S. A., Lienhard G. E., Weber M. J. Proteins antigenically related to the human erythrocyte glucose transporter in normal and Rous sarcoma virus-transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1540–1544. doi: 10.1073/pnas.79.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. W., Weber M. J. Glucose-specific cytochalasin B binding is increased in chicken embryo fibroblasts transformed by Rous sarcoma virus. J Biol Chem. 1979 May 10;254(9):3554–3561. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shawver L. K., Olson S. A., White M. K., Weber M. J. Degradation and biosynthesis of the glucose transporter protein in chicken embryo fibroblasts transformed by the src oncogene. Mol Cell Biol. 1987 Jun;7(6):2112–2118. doi: 10.1128/mcb.7.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Waddell I. D., Zomerschoe A. G., Voice M. W., Burchell A. Cloning and expression of a hepatic microsomal glucose transport protein. Comparison with liver plasma-membrane glucose-transport protein GLUT 2. Biochem J. 1992 Aug 15;286(Pt 1):173–177. doi: 10.1042/bj2860173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- White M. K., Bramwell M. E., Harris H. Hexose transport in hybrids between malignant and normal cells. Nature. 1981 Nov 19;294(5838):232–235. doi: 10.1038/294232a0. [DOI] [PubMed] [Google Scholar]
- White M. K., Rall T. B., Weber M. J. Differential regulation of glucose transporter isoforms by the src oncogene in chicken embryo fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4448–4454. doi: 10.1128/mcb.11.9.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. K., Weber M. J. The src oncogene can regulate a human glucose transporter expressed in chicken embryo fibroblasts. Mol Cell Biol. 1990 Apr;10(4):1301–1306. doi: 10.1128/mcb.10.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. K., Weber M. J. Transformation by the src oncogene alters glucose transport into rat and chicken cells by different mechanisms. Mol Cell Biol. 1988 Jan;8(1):138–144. doi: 10.1128/mcb.8.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. A., Birnbaum M. J. The rat facilitated glucose transporter gene. Transformation and serum-stimulated transcription initiate from identical sites. J Biol Chem. 1988 Dec 25;263(36):19513–19518. [PubMed] [Google Scholar]
- Wilson T., Treisman R. Removal of poly(A) and consequent degradation of c-fos mRNA facilitated by 3' AU-rich sequences. Nature. 1988 Nov 24;336(6197):396–399. doi: 10.1038/336396a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Seino Y., Fukumoto H., Koh G., Yano H., Inagaki N., Yamada Y., Inoue K., Manabe T., Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990 Jul 16;170(1):223–230. doi: 10.1016/0006-291x(90)91263-r. [DOI] [PubMed] [Google Scholar]
- Zubiaga A. M., Belasco J. G., Greenberg M. E. The nonamer UUAUUUAUU is the key AU-rich sequence motif that mediates mRNA degradation. Mol Cell Biol. 1995 Apr;15(4):2219–2230. doi: 10.1128/mcb.15.4.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]