Abstract
Aims
On the basis of the JUPITER trial, European health authorities recently approved the use of rosuvastatin to reduce first major cardiovascular events among ‘high' global risk primary prevention patients defined either by Framingham risk score >20% or European systematic coronary risk evaluation (SCORE) ≥5%. However, as these are post hoc analyses, data describing these subgroups have not previously been available to the clinical community.
Methods and results
We randomized 17 802 apparently healthy men aged ≥50 and women ≥60 with low-density lipoprotein cholesterol (LDL-C) <3.4 mmol/L, who were at an increased vascular risk due to elevated levels of C-reactive protein measured with a high-sensitivity (hs) assay to rosuvastatin 20 mg daily or placebo. Patients with high global cardiovascular risk at baseline were identified by 10-year Framingham risk score >20% or SCORE risk ≥5%. During 1.8-year median follow-up (maximum 5 years) of patients with Framingham risk >20%, the rate of myocardial infarction/stroke/cardiovascular death was 9.4 and 18.2 per 1000 person-years in rosuvastatin and placebo-allocated patients, respectively [hazard ratio (HR): 0.50, 95% confidence interval (CI): 0.27–0.93, P = 0.028]. Among patients with SCORE risk ≥5%, the corresponding rates were 6.9 and 12.0 using a model extrapolating risk for age ≥65 years (HR: 0.57, 95% CI: 0.43–0.78, P = 0.0003) and rates were 5.9 and 12.7 when risk for age was capped at 65 years (HR: 0.47, 95% CI: 0.32–0.68, P < 0.0001).
Conclusion
In primary prevention patients with elevated hs C-reactive protein who have high global cardiovascular risk (10-year Framingham risk score >20% or SCORE risk ≥5%), but LDL-C levels not requiring pharmacologic treatment, rosuvastatin 20 mg significantly reduced major cardiovascular events.
ClinicalTrial.gov Identifier: NCT00239681
Keywords: Rosuvastatin, Coronary heart disease, C-reactive protein, High risk
Introduction
The Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial was designed to investigate whether rosuvastatin decreased first major cardiovascular events among patients with levels of low-density lipoprotein cholesterol (LDL-C) <3.4 mmol/L (130 mg/dL), but who were at increased cardiovascular risk due to elevated levels of high-sensitivity (hs) C-reactive protein.1 As previously reported, after median follow-up of 1.9 years (maximum 5 years), rosuvastatin use was associated with a 54% reduction in myocardial infarction (MI), a 48% reduction in stroke, a 46% reduction in revascularization, a 43% reduction in venous thromboembolism, and a 20% reduction in total mortality.1,2 On this basis, the United States Food and Drug Administration approved the use of rosuvastatin for primary prevention of cardiovascular events among JUPITER eligible participants with elevated hs C-reactive protein and at least one additional risk factor.3 In contrast, the Dutch Medical Agency (the MEB) and 18 other European health authorities have approved rosuvastatin for the subgroup of trial participants who were considered to be at ‘high risk' either on the basis of an estimated 10-year Framingham risk score >20% or an estimated systematic coronary risk evaluation (SCORE) risk of ≥5%.4 As neither of these criteria were used in the design of the JUPITER trial, these post hoc data, not published previously, are likely to be of utility for European practitioners and are thus presented here.
Methods
JUPITER was a double-blind, placebo-controlled trial which randomized apparently healthy men aged ≥50 and women aged ≥60, with LDL-C <3.4 mmol/L (130 mg/dL) and C-reactive protein ≥2 mg/L in 26 countries. Participants did not qualify for statin therapy according to guidelines in effect in 2003, but were at increased cardiovascular risk due to evidence of systemic inflammation.5
Full details of the trial protocol, procedures, and methods of confirming clinical endpoints and ascertaining adverse events have been presented previously.1,6 Trial exclusion criteria included use within 6 weeks before screening of any lipid-lowering therapies, the current use of post-menopausal hormone replacement therapy, evidence of hepatic dysfunction, serum creatinine >177 μmol/L, diabetes mellitus, prior cardiovascular or cerebrovascular events, chronic inflammatory conditions such as severe arthritis, lupus, or inflammatory bowel disease, or other serious medical conditions that might compromise safety or successful completion of the study.
Potentially eligible subjects underwent a 4-week placebo run-in phase; those with compliance >80% were randomly allocated to rosuvastatin 20 mg daily or placebo and followed for occurrence of the primary endpoint, a composite of MI, stroke, arterial revascularization, unstable angina, or confirmed death from cardiovascular causes. An independent endpoint committee adjudicated primary endpoint events. Evaluation of all-cause mortality, MI/stroke/cardiovascular death, fatal/non-fatal MI, and fatal/non-fatal stroke was pre-specified.
The trial's monitoring plan called for two interim efficacy analyses with the O'Brien–Fleming stopping boundaries determined by means of the Lan–DeMets approach. The stopping boundary was crossed at the first planned efficacy evaluation, and on 29 March 2008, the independent data and safety monitoring board recommended termination of the trial. The steering committee accepted that recommendation and only major cardiovascular events occurring prior to 30 March 2008 are included in this analysis. Reporting of adverse events and all-cause mortality continued in a blinded manner until each participant appeared for a closeout visit and discontinued study medication.
Clinic physicians reported adverse events as verbatim terms, which were coded to Medical Dictionary for Regulatory Activities (MedDRA) preferred terms7 by an automated system, over-read by trained coders. We report here treatment-emergent adverse events, i.e. events that either began or worsened after randomization.
Participants provided fasting blood samples for lipid profiles at baseline, annually thereafter, and at the final visit. Alanine aminotransferase was measured at baseline, 3 and 6 months after randomization, semi-annually thereafter, and at the final visit. Serum creatinine was measured at baseline, 1 year after randomization, and at the final visit. We estimated glomerular filtration rate by the Modification of Diet in Renal Disease study equation.8
Statistical analyses
For consistency with post hoc analyses performed for the European health authorities, all study participants were classified according to 10-year global risk estimates using the Framingham risk score9 and the European SCORE risk algorithm.10 The Framingham risk score estimates the 10-year risk of MI/coronary death on the basis of age, gender, smoking status, blood pressure, and total and high-density lipoprotein cholesterol (HDL-C), whereas the SCORE algorithm estimates the global 10-year risk of cardiovascular death on the basis of age, gender, smoking status, systolic blood pressure, total cholesterol, and geographic region. The regional risk was based on each participant's country of enrolment; those enrolled in Belgium, Canada, Chile, Israel, Mexico, the Netherlands, and Switzerland were categorized as low risk. Patients enrolled in Argentina, Brazil, Bulgaria, Colombia, Costa Rica, Denmark, El Salvador, Estonia, Germany, Norway, Panama, Poland, Romania, Russia, South Africa, UK, USA, Uruguay, and Venezuela were categorized as high risk.
The SCORE model is limited to ages 45–64 years. To account for the large proportion of subjects enrolled in JUPITER older than 65 years (n = 10 237), a modification of the SCORE model was used in which the risk conferred by age was extrapolated for patients aged 65 or older (extrapolated model). Extrapolation was based on the contribution of age to the risk of cardiovascular death as assessed in the algorithm for patients up to 65 years of age. The extrapolated model is the model cited in the European CRESTOR Summary of the Product Characteristics.4 An analysis was also performed using the SCORE algorithm capped at 65 years (capped model), in which all subjects 65 years or older were assigned the risk of a 64-year old. This more conservative approach resulted in fewer patients being classified as high risk; 52% of the JUPITER participants had SCORE risk ≥5% using the extrapolated model compared with 35% using the capped model. Analyses for patients with SCORE risk <5% are included as Supplemental data.
Analyses were performed on an intention-to-treat basis. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated from the Cox proportional hazard models. Changes in lipoproteins and hs C-reactive protein levels were compared by the Wilcoxon signed-rank test. Baseline hs C-reactive protein is the mean of each participant's values at the screening and randomization visits. Analyses were performed using SAS version 8.2 (SAS Institute, Inc., Cary, NC, USA).
Results
JUPITER enrolled 17 802 men and women (6515 in Europe), and randomized 8901 each to rosuvastatin 20 mg daily and placebo. At baseline, 9% of the cohort was considered to be at ‘high risk’ for a first cardiovascular event on the basis of having a 10-year Framingham risk of MI/coronary death above 20%; 52% were considered to be at ‘high risk’ on the basis of having a 10-year SCORE risk of cardiovascular death of 5% or higher using the extrapolated model, and 35% were considered to be at ‘high risk' using the capped SCORE model. Baseline characteristics of these high-risk patients are shown by treatment allocation (Table 1). As anticipated, when compared with the entire JUPITER cohort, the higher-risk patients were older, more often male and more likely to smoke, have hypertension and low levels of HDL-C. Differences between the high-risk Framingham and SCORE groups reflect the patient characteristics included in the risk algorithms and their weighted contribution to individuals' estimated global cardiovascular risk. In particular, metabolic syndrome was more prevalent among high-risk Framingham patients.
Table 1.
Baseline characteristics according to the estimated 10-year risk defined by the Framingham risk score or the systematic coronary risk evaluation risk algorithm
| Entire cohort | Framingham 10-year risk >20% |
SCORE 10-year risk ≥5% |
|||||
|---|---|---|---|---|---|---|---|
| Extrapolated model |
Capped model |
||||||
| Rosuvastatin | Placebo | Rosuvastatin | Placebo | Rosuvastatin | Placebo | ||
| n | 17 802 | 786 | 772 | 4619 | 4683 | 3130 | 3177 |
| Age (years) | 66 | 74 | 74 | 70 | 70 | 67 | 67 |
| Female (%) | 38 | 17 | 15 | 32 | 31 | 12 | 11 |
| Race or ethnic group (%) | |||||||
| White | 71 | 68 | 67 | 72 | 72 | 74 | 74 |
| Black | 13 | 15 | 14 | 14 | 14 | 14 | 14 |
| Hispanic | 13 | 14 | 17 | 10 | 10 | 7 | 7 |
| Other | 4 | 2 | 2 | 2 | 3 | 4 | 4 |
| Hypertension (%) | 57 | 87 | 86 | 67 | 67 | 69 | 68 |
| Current smoker (%) | 16 | 32 | 31 | 21 | 22 | 30 | 31 |
| Family history premature CHDa (%) | 12 | 8 | 11 | 10 | 10 | 10 | 10 |
| HDL-C < 1.0 mmol/L (%) | 23 | 60 | 60 | 22 | 22 | 24 | 24 |
| Body mass index (kg/m2) | 28 | 28 | 28 | 28 | 28 | 28 | 28 |
| Metabolic syndromeb (%) | 41 | 68 | 69 | 41 | 41 | 40 | 40 |
| Framingham 10-year risk score | 10 | 25 | 25 | 16 | 16 | 16 | 16 |
| SCORE 10-year risk | 5 | 14 | 14 | 9 | 9 | 10 | 10 |
Values are median or n (%). SCORE, systematic coronary risk evaluation; CHD, coronary heart disease; HDL-C, high-density lipoprotein cholesterol.
aCHD in a male first-degree relative before age 55 or in a female first-degree relative before age 65.
bMetabolic syndrome defined as three or more of the following: waist circumference >102 cm (men) and 89 cm (women); triglycerides ≥1.7 mmol/L; HDL-C < 1.0 mmol/L (men) and 1.3 mmol/L (women); blood pressure ≥85 mmHg diastolic or 130 mmHg systolic or treated hypertension; fasting glucose ≥5.6 mmol/L.
For the entire JUPITER cohort, rosuvastatin lowered LDL-C by 50%, triglycerides by 17% and hs C-reactive protein by 37%, whereas it increased HDL-C by 4% compared with placebo (P < 0.001 for all from baseline to year 1).1 In the high-risk groups, effects of rosuvastatin on lipoproteins and hs C-reactive protein were similar to those seen for the entire cohort (Table 2), with significant reductions in LDL-C, triglycerides, and hs C-reactive protein (P< 0.0001 vs. placebo for all) and a significant increase in HDL-C (P < 0.0001).
Table 2.
Lipoprotein and high-sensitivity C-reactive protein levels in high-risk subgroups
| SCORE 10-year risk ≥5% |
||||||
|---|---|---|---|---|---|---|
| Framingham 10-year risk >20% |
Extrapolated model |
Capped model |
||||
| Rosuvastatin | Placebo | Rosuvastatin | Placebo | Rosuvastatin | Placebo | |
| LDL-C (mmol/L) | ||||||
| Baseline | 2.8 (2.5–3.1) | 2.8 (2.5–3.1) | 2.8 (2.5–3.1) | 2.8 (2.5–3.1) | 2.8 (2.5–3.1) | 2.8 (2.5–3.1) |
| Year 1 | 1.3 (1.1–1.9) | 2.8 (2.4–3.2) | 1.4 (1.1–1.8) | 2.8 (2.4–3.2) | 1.4 (1.1–1.9) | 2.8 (2.4–3.2) |
| % change | −51 | 0 | −49 | +2 | −49 | +2 |
| P-value | <0.0001 | <0.0001 | <0.0001 | |||
| Non-HDL-C (mmol/L) | ||||||
| Baseline | 3.7 (3.3–4.0) | 3.7 (3.3–4.0) | 3.5 (3.1–3.87) | 3.5 (3.1–3.8) | 3.5 (3.1–3.8) | 3.5 (3.1–3.8) |
| Year 1 | 2.0 (1.7–2.6) | 3.6 (3.2–4.1) | 2.0 (1.6–2.5) | 3.5 (3.0–4.0) | 2.0 (1.7–2.5) | 3.5 (3.0–4.0) |
| % change | −45 | 0 | −43 | +2 | −42 | +2 |
| P-value | <0.0001 | <0.0001 | <0.0001 | |||
| HDL-C (mmol/L ) | ||||||
| Baseline | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 1.3 (1.1–1.6) | 1.3 (1.1–1.6) | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) |
| Year 1 | 1.1 (1.0–1.3) | 1.0 (0.9–1.2) | 1.3 (1.1–1.7) | 1.3 (1.1–1.6) | 1.3 (1.1–1.6) | 1.3 (1.0–1.6) |
| % change | +9 | +3 | +6 | 0 | +6 | 0 |
| P-value | <0.0001 | <0.0001 | <0.0001 | |||
| Triglycerides (mmol/L) | ||||||
| Baseline | 1.7 (1.3–2.5) | 1.7 (1.2–2.5) | 1.3 (0.9–1.9) | 1.3 (0.9–1.9) | 1.3 (0.9–1.9) | 1.3 (0.9–1.9) |
| Year 1 | 1.3 (1.0–1.8) | 1.6 (1.2–2.4) | 1.1 (0.8–1.5) | 1.3 (1.0–1.8) | 1.1 (0.8–1.6) | 1.3 (1.0–1.9) |
| % change | −22 | −3 | −16 | 0 | −16 | +1 |
| P-value | <0.0001 | <0.0001 | <0.0001 | |||
| hs C-reactive protein (mg/L) | ||||||
| Baseline | 4.6 (3.0–7.9) | 4.6 (3.1–7.8) | 4.2 (2.9–7.1) | 4.4 (2.9–7.3) | 4.2 (2.8–6.9) | 4.4 (2.9–7.2) |
| Year 1 | 2.5 (1.3–4.8) | 3.7 (2.3–6.7) | 2.3 (1.3–4.6) | 3.6 (2.0–6.4) | 2.2 (1.2–4.5) | 3.5 (2.0–6.4) |
| % change | −49 | −17 | −46 | −21 | −46 | −21 |
| P-value | <0.0001 | <0.0001 | <0.0001 | |||
Values are median (interquartile range) or median (%); SCORE, systematic coronary risk evaluation; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; hs, high sensitivity.
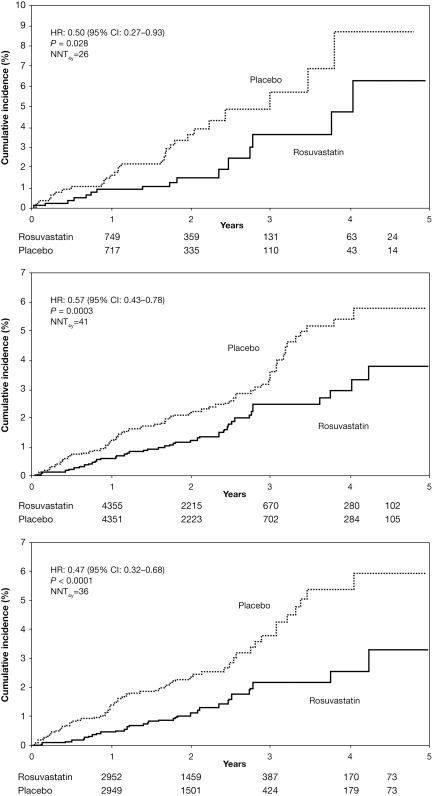
At study closure (median follow-up 1.8 years; maximal follow-up 5 years), the occurrence of MI/stroke/cardiovascular death was lower among high-risk subjects allocated to rosuvastatin compared with placebo (HR: 0.50, 95% CI: 0.27–0.93 for Framingham risk score >20%; HR: 0.57, 95% CI: 0.43–0.78 for SCORE risk ≥5% extrapolated model; HR: 0.47, 95% CI: 0.32–0.68 for SCORE risk ≥5% capped model; Figure 1, Table 3). The proportional reduction in MI/stroke/cardiovascular death with rosuvastatin was similar for patients with Framingham risk score above or below 20% (P for interaction = 0.95), or SCORE risk above or below 5% (P for interaction = 0.37 capped model, 0.25 extrapolated model).
Figure 1.
Cumulative incidence of myocardial infarction/stroke/cardiovascular death in high-risk patients. The cumulative incidence of myocardial infarction/stroke/cardiovascular death is shown by the treatment group for patients with a 10-year Framingham risk score >20% (upper panel), 10-year systematic coronary risk evaluation risk ≥5% using the extrapolated model (middle panel), and systematic coronary risk evaluation risk ≥5% using the capped model (lower panel). NNT, number needed to treat.
Table 3.
Major cardiovascular events and all-cause mortality in high-risk subgroups
| Rosuvastatin |
Placebo |
ARR | HR (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|
| No. events | Event ratea | No. events | Event rate | ||||
| Entire JUPITER cohort (n = 8901 rosuvastatin, 8901 placebo) | |||||||
| Primary endpoint | 142 | 7.7 | 251 | 13.6 | 5.9 | 0.56 (0.46–0.69) | <0.0001 |
| MI/stroke/CV death | 83 | 4.5 | 157 | 8.5 | 4.0 | 0.53 (0.40–0.69) | <0.0001 |
| Total mortality | 198 | 10.0 | 247 | 12.5 | 2.5 | 0.80 (0.67–0.97) | 0.02 |
| Baseline Framingham >20% (n = 786 rosuvastatin, 772 placebo) | |||||||
| Primary endpoint | 29 | 17.2 | 38 | 24.1 | 6.9 | 0.70 (0.43–1.14) | 0.155 |
| MI/stroke/CV death | 16 | 9.4 | 29 | 18.2 | 8.8 | 0.50 (0.27–0.93) | 0.028 |
| Total mortality | 31 | 17.2 | 40 | 23.6 | 6.3 | 0.73 (0.46–1.17) | 0.193 |
| Baseline SCORE ≥5% (extrapolated model; n= 4619 rosuvastatin, 4683 placebo) | |||||||
| Primary endpoint | 111 | 11.5 | 183 | 18.8 | 7.3 | 0.61 (0.48–0.78) | <0.0001 |
| MI/stroke/CV death | 67 | 6.9 | 118 | 12.0 | 5.1 | 0.57 (0.43–0.78) | 0.0003 |
| Total mortality | 149 | 14.4 | 185 | 17.5 | 3.2 | 0.82 (0.66–1.02) | 0.076 |
| Baseline SCORE ≥5% (age capped at 65 years; n = 3130 rosuvastatin, 3177 placebo) | |||||||
| Primary endpoint | 71 | 11.1 | 130 | 20.1 | 9.0 | 0.56 (0.42–0.74) | <0.0001 |
| MI/stroke/CV death | 38 | 5.9 | 83 | 12.7 | 6.9 | 0.47 (0.32–0.68) | <0.0001 |
| Total mortality | 97 | 15 | 135 | 20.6 | 5.6 | 0.74 (0.57–0.96) | 0.022 |
MI, myocardial infarction; CV, cardiovascular; HR, hazard ratio; CI, confidence interval; SCORE, systematic coronary risk evaluation; ARR, absolute rate reduction. Primary endpoint, time to occurrence of first MI/stroke/cardiovascular death/arterial revascularization/unstable angina.
Data for the entire JUPITER cohort are from reference 1. Extrapolated and capped SCORE models are described in the ‘Methods’ section.
aRates are per 1000 person-years.
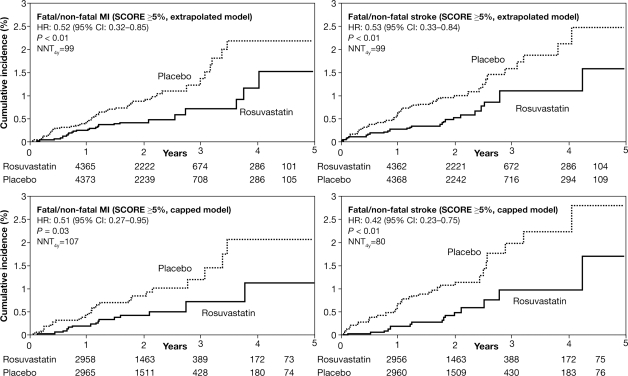
Rosuvastatin significantly reduced the occurrence of the primary composite endpoint of MI/stroke/arterial revascularization/unstable angina/cardiovascular death as well as fatal/non-fatal MI and fatal/non-fatal stroke among patients with SCORE ≥5% (in both the extrapolated and capped models; Table 3, Figure 2) and reduced all-cause mortality in the capped SCORE model (Table 3).
Figure 2.
Cumulative incidence of fatal/non-fatal myocardial infarction and stroke in high-risk patients. The cumulative incidence of fatal/non-fatal myocardial infarction and fatal/non-fatal stroke are shown by the treatment group among patients with a 10-year systematic coronary risk evaluation risk ≥5% using the extrapolated (upper panel) and capped models (lower panel).
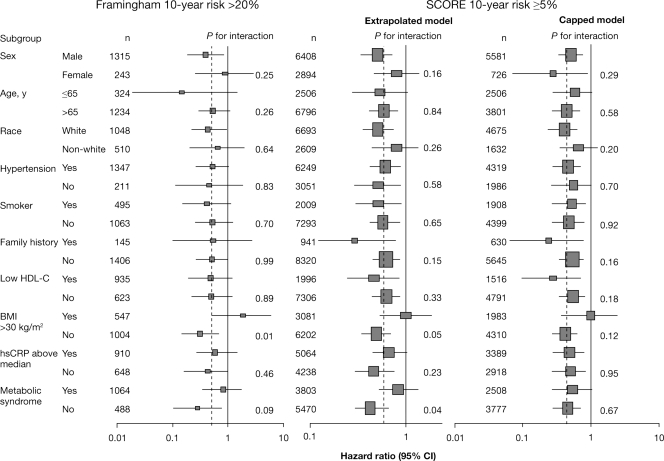
In the higher-risk patients, there was no evidence of heterogeneity for the endpoint of MI/stroke/cardiovascular death in subgroups by gender, age, race/ethnicity, hypertension, smoking, family history of premature coronary heart disease, baseline HDL-C, or C-reactive protein (Figure 3). Patients with body mass index >30 kg/m2 at baseline appeared to benefit less from rosuvastatin treatment compared with non-obese patients, but this interaction between treatment assignment and body mass index was not observed for the JUPITER cohort as a whole and thus is likely to be more apparent than real.
Figure 3.
Effects of rosuvastatin on myocardial infarction/stroke/cardiovascular death in high-risk patients, according to baseline characteristics. The hazard ratios and 95% confidence intervals for rosuvastatin when compared with placebo are shown for patients with Framingham risk score >20% and systematic coronary risk evaluation risk ≥5% (extrapolated and capped models). Size of the point estimate rectangle is proportional to the number of clinical events. The dashed vertical line indicates the relative risk reduction for the entire trial cohort. Also shown are P-values for the test of an interaction between the composite endpoint and categories within each subgroup.
In the high-risk patients, serious adverse events were reported with similar frequency in rosuvastatin and placebo-allocated patients (Table 4). A small excess of myalgia was reported with rosuvastatin in patients with Framingham risk score >20% (rosuvastatin 5.9% and placebo 5.3%) or SCORE risk ≥5% (rosuvastatin 7.9%, placebo 6.5% for the extrapolated model; 7.4 vs. 5.8% for the capped model). Myopathy, myositis, and rhabdomyolysis were reported with similar frequency in the two treatment groups. In the entire JUPITER cohort, investigator-reported diabetes, a non-adjudicated outcome, was more frequent with rosuvastatin (rosuvastatin 3.0% and placebo 2.4%; P = 0.01).1 Investigator-reported diabetes was not consistently more frequent with rosuvastatin in the higher-risk patients (HR: 0.70, 95% CI: 0.41–1.19, P = 0.19 for Framingham risk score >20%; HR: 1.11, 95% CI: 0.86–1.43, P = 0.43 for extrapolated SCORE risk ≥5%; HR: 0.99, 95% CI: 0.72–1.36, P = 0.95 for capped SCORE risk). At 2 years following randomization, a 0.1% greater increase in glycosylated haemoglobin was observed with rosuvastatin compared with placebo (P < 0.001 vs. placebo for the high-risk groups). However, on-treatment fasting glucose levels were similar in the two treatment groups (P = 0.95 vs. placebo for Framingham risk score >20%; P = 0.19 for extrapolated SCORE risk ≥5%; P = 0.52 for the capped SCORE model).
Table 4.
Adverse events and laboratory abnormalities in high-risk subgroups
| Framingham risk >20% |
SCORE risk ≥5% |
|||||
|---|---|---|---|---|---|---|
| RSV | Placebo | Extrapolated model |
Capped model |
|||
| RSV | Placebo | RSV | Placebo | |||
| n | 786 | 772 | 4619 | 4683 | 3130 | 3177 |
| Any adverse event | 626 (79.6) | 617 (79.9) | 3681 (79.7) | 3704 (79.1) | 2490 (79.6) | 2510 (79.0) |
| Any serious adverse event | 154 (19.6) | 153 (19.8) | 855 (18.5) | 878 (18.7) | 544 (17.4) | 587 (18.5) |
| Muscle symptoms | ||||||
| Myalgia | 46 (5.9) | 41 (5.3) | 363 (7.9) | 303 (6.5) | 233 (7.4) | 183 (5.8) |
| Myositis | 0 | 1 (0.1) | 3 (0.1) | 3 (0.1) | 3 (0.1) | 2 (0.1) |
| Myopathy | 0 | 0 | 0 | 1 (0) | 0 | 1 (0) |
| Rhabdomyolysis | 0 | 0 | 1 (0) | 0 | 1 (0) | 0 |
| Newly diagnosed cancer | 35 (4.5) | 39 (5.1) | 195 (4.2) | 212 (4.5) | 116 (3.7) | 145 (4.6) |
| Death from cancer | 9 (1.1) | 11 (1.4) | 29 (0.6) | 48 (1.0) | 19 (0.6) | 40 (1.3) |
| Gastrointestinal disorder | 206 (26.2) | 214 (27.7) | 1184 (25.6) | 1175 (25.1) | 763 (24.4) | 737 (23.2) |
| Renal disorder | 100 (12.7) | 87 (11.3) | 487 (10.5) | 523 (11.2) | 355 (11.3) | 354 (11.1) |
| Hepatic disorder | 19 (2.4) | 14 (1.8) | 103 (2.2) | 101 (2.2) | 65 (2.1) | 57 (1.8) |
| Investigator-reported diabetes | 24 (3.1) | 34 (4.4) | 131 (2.8) | 116 (2.5) | 84 (2.7) | 83 (2.6) |
| Laboratory values | ||||||
| Creatinine >100% increase from baseline [n (%)] | 1 (0.1) | 0 | 7 (0.2) | 3 (0.1) | 6 (0.2) | 2 (0.1) |
| eGFR at 12 months (mL/min/1.73 m2 ) | 65.0 (14.2) | 64.4 (13.9) | 66.9 (14.2) | 66.4 (13.6) | 69.2 (14.3) | 68.7 (13.3) |
| ALT>3x ULN on consecutive visits [n (%)] | 3 (0.4) | 2 (0.3) | 14 (0.3) | 6 (0.1) | 12 (0.4) | 5 (0.2) |
| HbA1c at 24 months (%) | 6.02 (0.53) | 5.92 (0.53) | 5.96 (0.49) | 5.86 (0.46) | 5.97 (0.48) | 5.87 (0.46) |
| Fasting glucose at 24 months (mmol/L) | 5.7 (0.9) | 5.7 (1.3) | 5.6 (1.1) | 5.6 (0.9) | 5.6 (1.0) | 5.6 (0.9) |
Values are n (%) or mean (standard deviation). SCORE, systematic coronary risk evaluation; RSV, Rosuvastatin; eGFR, estimated glomerular filtration rate; ALT, alanine aminotransferase; HbA1c, glycosylated haemoglobin; ULN, upper limit of normal.
Discussion
JUPITER investigated the effect of rosuvastatin 20 mg daily compared with placebo on major cardiovascular events in a population not requiring treatment under guidelines in effect in 2003,9,11 but at an increased cardiovascular risk on the basis of age and elevated hs C-reactive protein. To provide European practitioners access to post hoc subgroup data that were influential to the European health authorities, in this analysis, we limited the target population to select an even higher risk group using two global risk assessment algorithms, Framingham and SCORE. In these higher risk subgroups, rosuvastatin lowered LDL-C, triglycerides, and hs C-reactive protein and raised HDL-C, consistent with effects observed for the entire cohort. Rosuvastatin reduced the risk of the composite endpoint of MI/stroke/cardiovascular death by 50% in the high-risk Framingham group (P = 0.028 vs. placebo), 43% in the high-risk SCORE group using the extrapolated model (P = 0.0003) and 53% (P < 0.0001) using the capped model, consistent with the 47% reduction observed for the entire cohort.1 Adverse events and laboratory abnormalities were consistent with the known safety profile of rosuvastatin.12
Strengths of this analysis include the randomized, placebo-controlled design, broad geographic representation including a substantial number of Europeans, and inclusion of large numbers of women and ethnic minority participants. A limitation is the post hoc selection of the higher risk subgroups, which was undertaken in response to health authority requests.4 For example, analysis of JUPITER subgroups by SCORE strata was not pre-specified and since SCORE by design is limited to those under age 65, we needed to extrapolate findings for the many JUPITER participants over this age cut-off that clearly benefited from rosuvastatin.13 The subgroups by baseline characteristics in Figure 3 are drawn from within the subgroups of high-risk patients; as such, limited conclusions can be drawn from these data. Neither the Framingham nor the SCORE models include hs C-reactive protein evaluation; as all participants in JUPITER had hs C-reactive protein levels >2 mg/L and as elevated hs C-reactive protein has been shown to have a magnitude of risk prediction at least as large as that of elevated cholesterol,14 it is likely that both the Framingham and the SCORE systematically underestimate the true risk of JUPITER participants.
Also in keeping with health authority requests, the analyses here used a composite endpoint of MI/stroke/cardiovascular death rather than the pre-specified JUPITER primary endpoint. Nonetheless, the relative reduction in MI/stroke/cardiovascular death with rosuvastatin was remarkably consistent across a range of baseline participant characteristics and consistent with primary trial analyses of the full study population as pre-specified in the JUPITER protocol. Global risk prediction scores can be used to direct use of preventive therapies such as statins towards patients most likely to benefit.15,16 As expected, clinical event rates in the JUPITER placebo group were higher in the high-risk Framingham or SCORE groups compared with the entire cohort for the primary study endpoint as well as for the composite of MI/stroke/cardiovascular death and all-cause mortality. The magnitude of the absolute rate reduction for clinical events was correspondingly greater in the high-risk groups (Table 3).
Two factors contributing to the observed absolute reduction in clinical events in JUPITER were the underlying event rate in the placebo group, enhanced in this case by selecting patients with high global cardiovascular risk and elevated hs C-reactive protein, and the relative risk reduction due to treatment, enhanced by use of a high-efficacy statin. The reductions in LDL-C (49%) and clinical events (43–53% for MI/stroke/cardiovascular death) with rosuvastatin in the JUPITER patients with SCORE risk ≥5% are greater than reported for other statins.17,18 Although these data support the use of high-efficacy statin therapy, they do not minimize the roles of diet, exercise, and smoking cessation as the most important interventions for primary prevention. Despite the benefit derived from treatment with rosuvastatin in these high-risk patients, caution should be exercised when considering treatment of patients lacking any cardiovascular risk factors. Further, long-term compliance with statin therapy is critical for efficacy among those patients where pharmacologic therapy is indicated in addition to lifestyle interventions.
Although the analyses presented here parallel those requested by European Health Authorities, they do not address most patients actually studied in the JUPITER trial. For example, among the 7340 men and women with elevated hs C-reactive protein and the Framingham risk scores of 11–20%, where the 4.5-year absolute risk of a primary endpoint was 10.6% in the placebo group, rosuvastatin was associated with a 49% reduction in risk (HR: 0.51, 95% CI: 0.39–0.68, P < 0.0001). Similarly, among the 6091 participants with entry Framingham scores of 5–10%, where the 4.5-year absolute risk was 5.3%, a 45% reduction was observed with rosuvastatin (HR: 0.55, 95% CI: 0.36–0.84, P = 0.005), and among trial participants with elevated hs C-reactive protein with SCORE risk <5%, rosuvastatin was associated with a 56% reduction in vascular risk (HR: 0.44, 95% CI: 0.29–0.68). Thus, the JUPITER trial data also indicate that many individuals with elevated hs C-reactive protein who fall outside ‘high-risk’ subgroups defined by either Framingham or SCORE have both substantive absolute risk and large relative risk reductions when treated with rosuvastatin.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The JUPITER trial was investigator-initiated and supported by AstraZeneca. The sponsor of the study collected the trial data and monitored the study sites, but had no access to unblinded data until after drafting of the trial primary report. The sponsor supported preparation of this manuscript. Trial Principal Investigator (P.M.R.) had full access to all study data; W.K. had final responsibility for the decision to submit these data for publication. Funding to pay the Open Access publication charges for this article was provided by AstraZeneca.
Conflict of interest: During the period of this project, W.K. reports receiving research support grants from Dade–Behring and Glaxo SmithKline; lecture fees from AstraZeneca, Pfizer, Novartis, and Boehringer-Ingelheim; and consulting fees from GlaxoSmithKline and Roche. P.M.R. reports having received investigator-initiated research grant support from the National Heart Lung and Blood Institute, the National Cancer Institute, the Donald W Reynolds Foundation, the Leducq Foundation, AstraZeneca, Novartis, Merck, Abbott, Roche, and sanofi-aventis; consulting fees from AstraZeneca, Novartis, Merck, Merck-Schering Plough, sanofi-aventis, ISIS, Seimens, and Vascular Biogenics; and is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease that have been licensed to Seimens and AstraZeneca.
References
- 1.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 2.Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Ridker PM. A randomized trial for rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crestor prescribing information in the United States. http://www1.astrazeneca-us.com/pi/crestor.pdf. (5 May 2010)
- 4.Crestor prescribing information in the UK. http://www.medicines.org.uk/EMC/medicine/11976/SPC/Crestor+5mg%2c+10mg%2c+20mg+and+40mg+film-coated+tablets/ (24 May 2010)
- 5.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice. A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003;108:2292–2297. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- 7.Medical Dictionary for Regulatory Activities Maintenance and Support Services Organization. http://www.meddramsso.com/index.asp. (14 April 2010)
- 8.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 9.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, Njolstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM SCORE Project Group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 11.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Manger Cats V, Orth-Gomér K, Perk J, Pyörälä K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood D. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2003;24:1601–1610. doi: 10.1016/s0195-668x(03)00347-6. [DOI] [PubMed] [Google Scholar]
- 12.Shepherd J, Vidt DG, Miller E, Harris S, Blasetto J. Safety of rosuvastatin: update on 16,876 rosuvastatin-treated patients in a multinational clinical trial program. Cardiology. 2007;107:433–443. doi: 10.1159/000100908. [DOI] [PubMed] [Google Scholar]
- 13.Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med. 2010;152:488–496. doi: 10.1059/0003-4819-152-8-201004200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Committee for Medicinal Products for Human Use. Guideline on the Evaluation of Medicinal Products for Cardiovascular Disease Prevention. Doc ref. EMEA/CHMP/EWP/311890/2007, published London 25 September 2008. [Google Scholar]
- 16.ESC Clinical Practice Guidelines. http://www.escardio.org/guidelines-surveys/esc-guidelines/Pages/cvd-prevention.aspx. (14 April 2010)
- 17.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Östergren J ASCOT Investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 18.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.