Abstract
It is widely accepted that non-endogenous compounds that target CB1 and/or CB2 receptors possess therapeutic potential for the clinical management of an ever growing number of disorders. Just a few of these disorders are already treated with Δ9-tetrahydrocannabinol or nabilone, both CB1/CB2 receptor agonists, and there is now considerable interest in expanding the clinical applications of such agonists and also in exploiting CB2-selective agonists, peripherally restricted CB1/CB2 receptor agonists and CB1/CB2 antagonists and inverse agonists as medicines. Already, numerous cannabinoid receptor ligands have been developed and their interactions with CB1 and CB2 receptors well characterized. This review describes what is currently known about the ability of such compounds to bind to, activate, inhibit or block non-CB1, non-CB2 G protein-coupled receptors such as GPR55, transmitter gated channels, ion channels and nuclear receptors in an orthosteric or allosteric manner. It begins with a brief description of how each of these ligands interacts with CB1 and/or CB2 receptors.
Keywords: Δ9-tetrahydrocannabinol, rimonabant, AM251, cannabinoid receptors, GPR55, G protein-coupled receptors, transmitter gated and ion channels, the nuclear receptors PPARα, PPARγ
INTRODUCTION
It now generally accepted that mammalian tissues contain an endogenous cannabinoid system that consists of at least two types of cannabinoid receptor, CB1 and CB2, of endogenous cannabinoids, also known as “endocannabinoids”, that can activate these G protein-coupled receptors and of processes responsible for endocannabinoid biosynthesis, cellular uptake and metabolism (reviewed in [1–3]). It is also generally accepted that there are certain disorders in which the density or coupling efficiency of cannabinoid CB1 and/or CB2 receptors or the release of endocannabinoids onto these receptors changes in a manner that is sometimes “autoprotective”, as for example in multiple sclerosis, and sometimes harmful, as for example in obesity and type-2 diabetes (reviewed in [4–6]). As a result, there is currently a lot of interest in the idea of developing new ligands that can be administered exogenously to mimic the protective effects of endocannabinoids by directly activating cannabinoid CB1 and/or CB2 receptors or to prevent unwanted effects of endocannabinoids by blocking these receptors.
Two mixed CB1/CB2 cannabinoid receptor agonists entered the clinic several years before the discovery of the endocannabinoid system (reviewed in [6]). These are Δ9-THC, the main psychoactive constituent of cannabis, and nabilone, a synthetic analogue of Δ9-THC. Nabilone (Cesamet®) was licensed in 1981 for the suppression of nausea and vomiting produced by chemotherapy. Δ9-THC first entered the clinic, as Marinol® (dronabinol), in 1985 for anti-emesis and in 1992 for the stimulation of appetite, for example in AIDS patients experiencing excessive loss of body weight. Δ9-THC is also a major constituent of the more recently developed medicine, Sativex®. This is prescribed for the symptomatic relief of neuropathic pain in adults with multiple sclerosis and as an adjunctive analgesic treatment for adult patients with advanced cancer (reviewed in [6]).
Continually emerging new information about the autoprotective roles of the endocannabinoid system has sparked a search for additional therapeutic uses for cannabinoid receptor agonists. It has also prompted the development of a second generation of synthetic cannabinoid receptor agonists, particularly CB1/CB2 receptor agonists that do not readily cross the blood-brain barrier and a large and ever growing population of CB2-selective ligands, including several with a proven ability to activate the CB2 receptor (reviewed in [6–8]). CB2 receptor-selective agonists and peripherally restricted CB1/CB2 receptor agonists are both thought to possess potential therapeutic applications, not least for the management of inflammatory, neuropathic and cancer pain, and are expected to have better benefit-to-risk ratios than centrally active CB1 receptor agonists (reviewed in [6]).
With regard to strategies for combating harmful effects that appear in some instances to be mediated by the endocannabinoid system, a lot of attention has been directed recently at SR141716A (rimonabant; Acomplia®). This CB1-selective antagonist entered European clinics in 2006 for the management of obesity ([9]). Unfortunately, however, safety concerns about the adverse effects observed in patients taking rimonabant, particularly an increased incidence of depression and suicidality, prompted the European Medicines Agency to recommend in 2008 that sales of this drug be halted. Consequently, the interest in developing other CB1 receptor antagonists as new medicines displayed by a number of pharmaceutical companies in the recent past seems to have waned considerably. Even so, this once intense interest has led to the development of a large number of new CB1-selective antagonists (reviewed in [10, 11]).
According to peer-reviewed literature published up to August 2009, the pharmacological characterization of most cannabinoid receptor ligands has been concerned primarily with establishing their relative abilities to bind to CB1 and CB2 receptors or to target these receptors as agonists or antagonists. Thus, relatively few such ligands have been investigated for their abilities to interact with other kinds of receptor, let alone with other kinds of pharmacological targets such as enzymes or carrier molecules that transport ions or small organic molecules across cell membranes. Yet, the extent to which a particular ligand displays such additional activity, particularly at those concentrations at which it can activate or block CB1 and/or CB2 receptor, could well of course influence its overall pharmacological profile and therapeutic potential to a significant degree.
The main aim of this review is to consider what is currently known about the extent to which established CB1 and CB2 receptor ligands target non-CB1, non-CB2 receptors. It focuses particularly on non-endogenous cannabinoid receptor ligands, since it is from this group of compounds that medicines have already been developed and new medicines are very likely to emerge. The first three sections of this review contain brief descriptions of the CB1 and CB2 receptor pharmacology of each of the rather small group of CB1/CB2 receptor ligands that has been investigated to-date for its ability to target certain non-CB1, non-CB2 receptors. Brief mention is also made in the first part of this review of the cannabinoid CB1 and CB2 receptor pharmacology both of the endocannabinoids, arachidonoylethanolamide (anandamide) and 2-arachidonoyl glycerol, and of a few particularly notable non-endogenous CB1/CB2 receptor ligands, the ability of which to target non-CB1, non CB2 receptors has yet to be explored.
AGONISTS THAT TARGET CB1 AND CB2 RECEPTORS WITH SIMILAR POTENCY
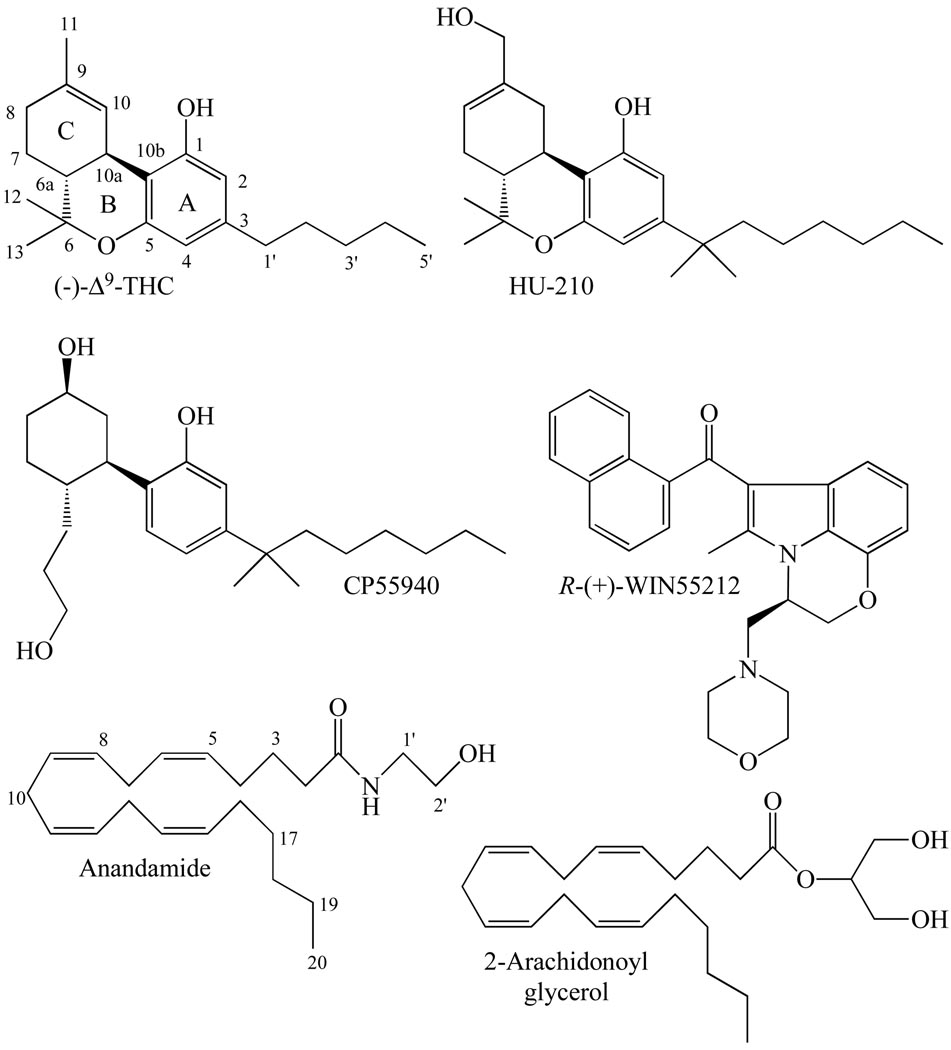
Several of the established cannabinoid receptor agonists discussed in this review that bind more or less equally well to CB1 and CB2 receptors (Table 1) are widely used as research tools. In terms of their chemical structures (Fig. 1), these compounds fall essentially into four main groups: classical, nonclassical, eicosanoid and aminoalkylindole (reviewed in [1, 3, 12, 13]).
Table 1.
A Selection of Ki Values of Cannabinoid CB1/CB2 Receptor Agonists and Antagonists for the In Vitro Displacement of [3H]CP55940 or [3H]HU-243 from CB1- and CB2-Specific Binding Sites
| Ligand | CB1 Ki value (nM) | CB2 Ki value (nM) |
|---|---|---|
| Agonists with similar CB1 and CB2 affinities | ||
| (−)-Δ9-THC | 5.05 to 80.3 | 3.13 to 75.3 |
| HU-210 | 0.06 to 0.73 | 0.17 to 0.52 |
| CP55940 | 0.5 to 5.0 | 0.69 to 2.8 |
| R-(+)-WIN55212 | 1.89 to 123 | 0.28 to 16.2 |
| Anandamide | 61 to 543 | 279 to 1940 |
| 2-Arachidonoyl glycerol | 58.3, 472 | 145, 1400 |
| Agonists with higher CB1 than CB2 affinity | ||
| ACEA | 1.4, 5.29 | 195, >2000 |
| ACPA | 2.2 | 715 |
| R-(+)-methanandamide | 17.9 to 28.3 | 815 to 868 |
| 2-Arachidonyl glyceryl ether (noladin ether) | 21.2 | >3000 |
| Agonists with higher CB2 than CB1 affinity | ||
| JWH-133 | 677 | 3.4 |
| HU-308 | >10000 | 22.7 |
| JWH-015 | 383 | 13.8 |
| AM1241 | 280 | 3.4 |
| Antagonists with higher CB1 than CB2 affinity | ||
| Rimonabant (SR141716A) | 1.8 to 12.3 | 514 to 13200 |
| AM251 | 7.49 | 2290 |
| AM281 | 12 | 4200 |
| LY320135 | 141 | 14900 |
| Taranabant | 0.13, 0.27 | 170, 310 |
| Antagonists with higher CB2 than CB1 affinity | ||
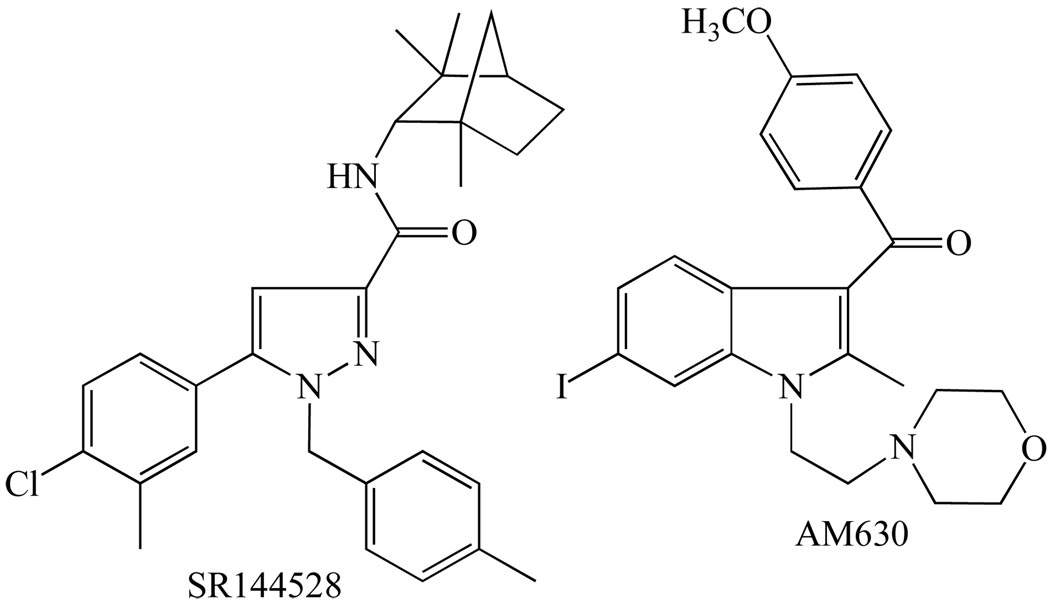
| SR144528 | 50.3 to >10000 | 0.28 to 5.6 |
| AM630 | 5152 | 31.2 |
Fig. (1).
The structures of the CB1/CB2 cannabinoid receptor agonists, (−)-Δ9-tetrahydrocannabinol [(−)-Δ9-THC], HU-210, CP55940, R-(+)-WIN55212, anandamide and 2-arachidonoyl glycerol.
The classical group of CB1/CB2 cannabinoid receptor agonists consists of dibenzopyran derivatives, two particularly notable examples being (−)-Δ9-tetrahydrocannabinol (Δ9-THC), which is the main psychoactive constituent of cannabis, and (−)-11-hydroxy-Δ8-THC-dimethylheptyl (HU-210), which is a synthetic analogue of (−)-Δ8-THC. HU-210 has relatively high affinity for both CB1 and CB2 receptors and displays particularly high efficacy and potency as a cannabinoid receptor agonist, all propertes that are due largely to its dimethylheptyl side chain. It also has an exceptionally long duration of action when administered in vivo. Δ9-THC displays much lower CB1 and CB2 affinity and efficacy than HU-210, exhibiting even less efficacy at CB2 than at CB1 receptors.
The nonclassical group of CB1/CB2 cannabinoid receptor agonists are close relatives of the classical cannabinoids, consisting as they do of bicyclic and tricyclic analogues of tetrahydrocannabinol that lack a pyran ring. One member of this group is particularly widely used as an experimental tool. This is CP55940 which possesses HU-210-like CB1 and CB2 receptor efficacy. It has been found to have slightly lower CB1 and CB2 affinities than HU-210 in some investigations but, even so, binds to these receptors at concentrations in the low nanomolar range and is therefore quite potent.
Moving on to the eicosanoid group of CB1/CB2 cannabinoid receptor agonists, these have markedly different structures from both classical and nonclassical cannabinoids. The prototypical members of this group are the endocannabinoids, anandamide and 2-arachidonoyl glycerol. Anandamide binds with slightly higher affinity to CB1 than to CB2 receptors. It resembles Δ9-THC both in its behaviour as a CB1 and CB2 receptor partial agonist and in possessing lower CB2 than CB1 efficacy. Its CB1 receptor affinity is also similar to that of this classical cannabinoid, though it does have lower affinity than Δ9-THC for the CB2 receptor. 2-Arachidonoyl glycerol binds with more or less equal affinity to CB1 and CB2 receptors. It seems to have greater CB1 receptor efficacy than anandamide or CP55940, greater CB1 and CB2 receptor potency than anandamide but less CB1 receptor potency than CP55940. Because this review is focusing on non-endocannabinoids, it is important to note that this group does also contain non-endogenous synthetic eicosanoids. Of these, the ones that have been investigated for their ability to target non-CB1, non-CB2 receptors are all CB1-selective agonists and are therefore mentioned in the next section (CB1-selective and CB2-selective cannabinoid receptor agonists).
As to the aminoalkylindole group of CB1/CB2 cannabinoid receptor agonists, these have structures quite unlike those of classical, nonclassical or eicosanoid cannabinoids. The best known member of this group is R-(+)-WIN55212 which displays CP55940- and HU-210-like efficacy at both CB1 and CB2 receptors, though in contrast to CP55940, HU-210 and anandamide, it has been found in some investigations to have slightly higher CB2 than CB1 affinity. Interestingly, R-(+)-WIN55212 is thought to bind differently to the CB1 receptor than either HU-210 or CP55940 (reviewed in [1, 14]). Even so, mutual displacement between R-(+)-WIN55212 and non-aminoalkylindole cannabinoids at CB1 binding sites does still occur.
All of the classical, nonclassical and aminoalkylindole CB1/CB2 cannabinoid receptor agonists just mentioned contain chiral centres and exhibit greater pharmacological activity in cannabinoid receptor bioassays than their stereoisomers (reviewed in [1, 3]). Thus, classical and nonclassical cannabinoids with the same absolute stereochemistry as (−)-Δ9-THC at 6a and 10a, trans (6aR, 10aR), are generally more active than their cis (6aS, 10aS) enantiomers, and R-(+)-WIN55212 is more active than S-(−)-WIN55212. In contrast neither anandamide nor 2-arachidonoyl glycerol contain any chiral centres. However, some synthetic eicosanoid cannabinoids, for example the CB1-selective agonist, R-(+)-methanandamide, are chiral molecules. As is indicated later on in this review, each of the non-endogenous CB1/CB2 receptor agonists mentioned in this section has been investigated for its ability to activate or block non-CB1, non-CB2 receptors.
CB1-SELECTIVE AND CB2-SELECTIVE CANNABINOID RECEPTOR AGONISTS
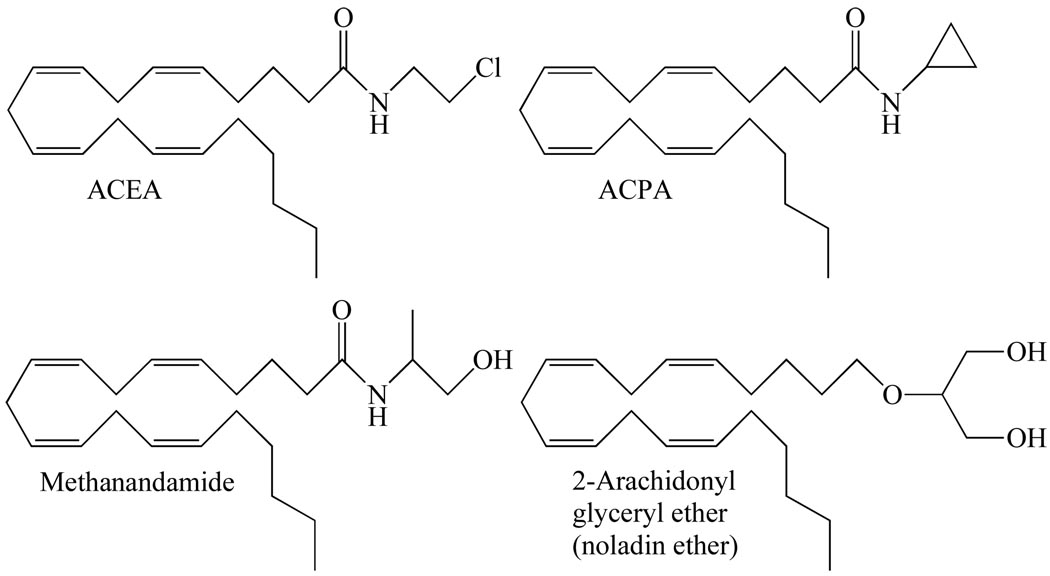
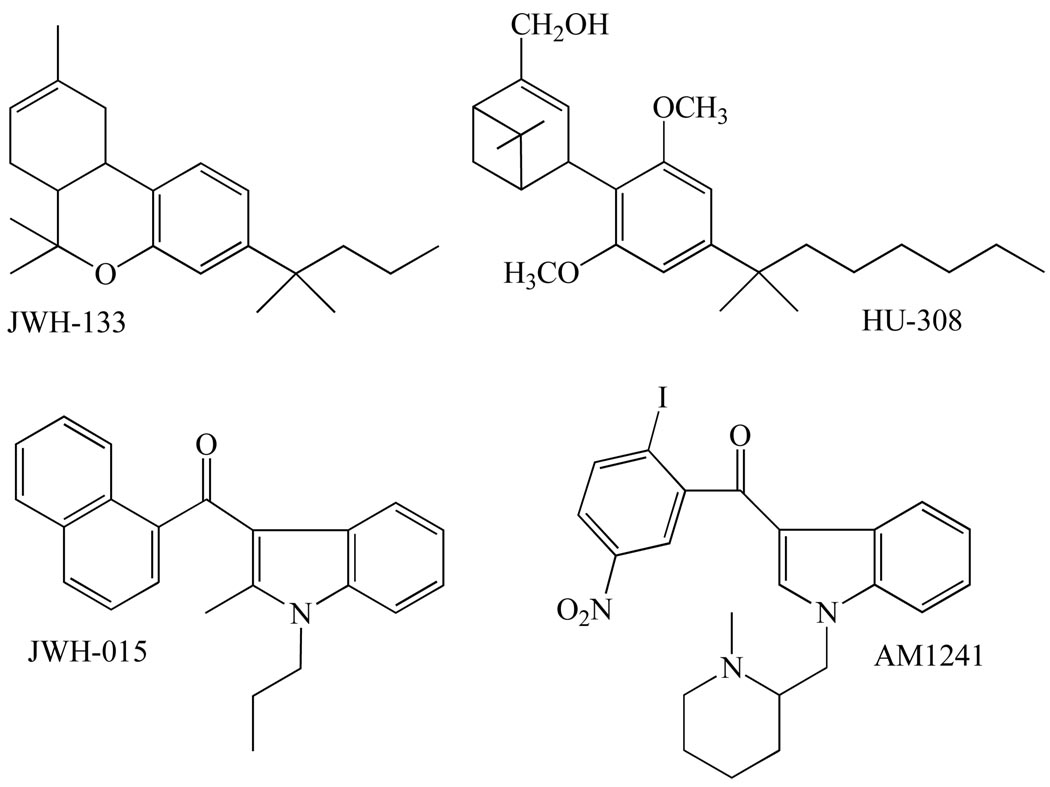
Four CB1-selective compounds, all synthetic eicosanoid cannabinoids, have most often been used in research directed at activating CB1 receptors selectively in vitro or in vivo (Table 1 and Fig. 2); reviewed in [1, 3, 12, 13]). These are the three synthetic anandamide analogues, R-(+)-methanandamide, arachidonyl-2’-chloroethylamide (ACEA) and arachidonylcyclopropylamide (ACPA) [15, 16], and a single analogue of 2-arachidonoyl glycerol, noladin ether (2-arachidonyl glyceryl ether) [17], which may or may not be an endocannabinoid (reviewed in [18]). All three anandamide analogues possess significant potency and efficacy as CB1 receptor agonists. Importantly, however, whereas R-(+)-methanandamide is not readily hydrolysed by the anandamide-metabolizing enzyme, fatty acid amide hydrolase, ACEA and ACPA do not display such resistance to this enzyme. Turning now to noladin ether, this has been reported to exhibit CP55940-like CB1 receptor efficacy but less CB1 efficacy than 2-arachidonoyl glycerol and less CB1 receptor potency than either CP55940 or 2-arachidonoyl glycerol [19–22]. As to ligands most frequently used in research directed at activating CB2 receptors selectively there are again four of these (Table 1 and Fig. 3): the classical cannabinoid, JWH-133, the nonclassical cannabinoid HU-308, and the aminoalkylindoles, JWH-015 and AM1241 (reviewed in [1, 3, 12, 13]). With the exception of ACPA, all the CB1-selective and CB2-selective agonists mentioned in this section have been investigated to at least some extent for their ability to target one or more types of established non-CB1, non-CB2 receptor and so feature again in the second part of this review.
Fig. (2).
The structures of ACEA, ACPA, methanandamide and noladin ether, each of which activates CB1 receptors more potently than CB2 receptors.
Fig. (3).
The structures of JWH-133, HU-308, JWH-015 and AM1241, each of which activates CB2 receptors more potently than CB1 receptors.
CB1-SELECTIVE AND CB2-SELECTIVE COMPETITIVE CANNABINOID RECEPTOR ANTAGONISTS
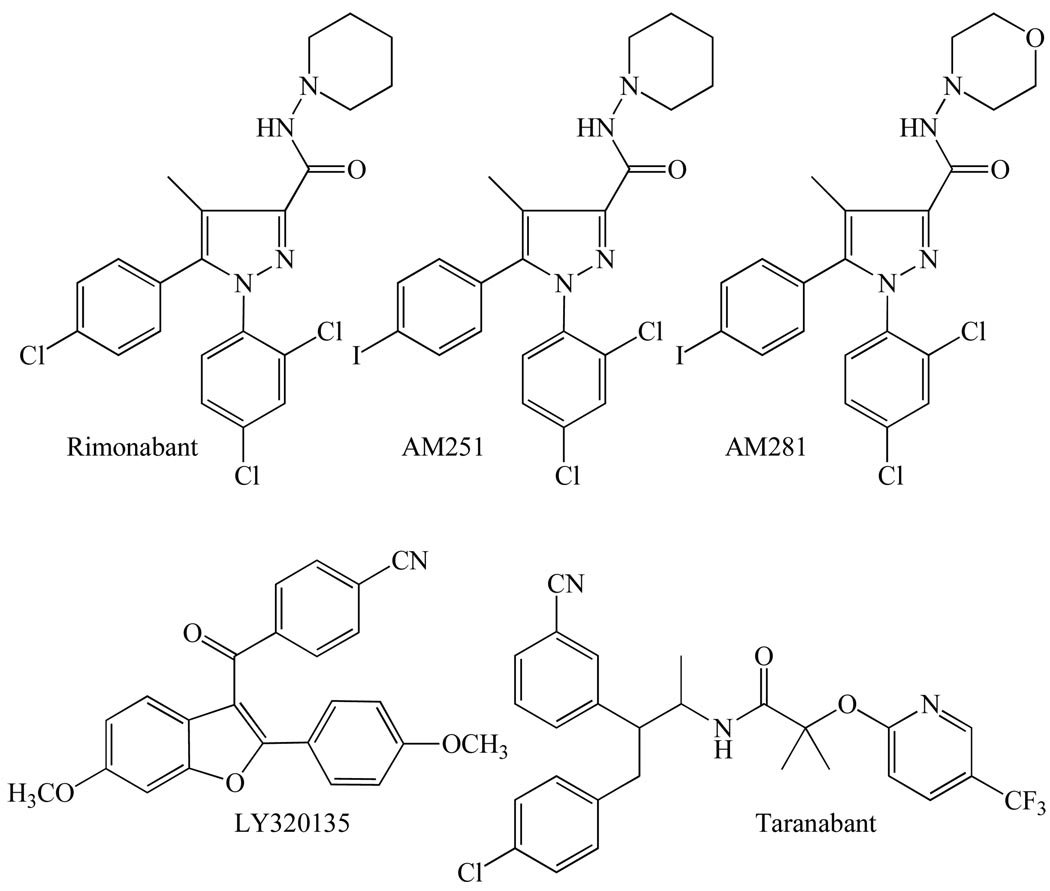
Several compounds have been developed that can be used in research to block agonist-induced activation of cannabinoid CB1 receptors in a competitive manner both in vitro and in vivo (reviewed in [1, 3, 12, 13]). The most widely used of these compounds are the diarylpyrazole, rimonabant, and its structural analogues, AM251 and AM281 (Fig. 4). Another compound with a rimonabant-like structure, LY320135 (Fig. 4), is also sometimes used for research purposes, although it has less affinity for the CB1 receptor than rimonabant, AM251 or AM281 (Table 1).
Fig. (4).
The structures of the cannabinoid CB1 receptor antagonists/inverse agonists, rimonabant, AM251, AM281, LY320135 and taranabant, each of which blocks CB1 receptors more potently than CB2 receptors.
All these compounds possess significantly greater affinity for cannabinoid CB1 than CB2 receptors (Table 1) and lack any ability to activate cannabinoid receptors. There is evidence, however, that when administered alone they can trigger responses in some CB1 receptor-containing tissues that are actually opposite in direction from those induced by CB1 receptor agonists. In some instances this may of course be the result of direct competitive antagonism of responses that are being evoked at CB1 receptors by released endocannabinoids. In other instances, however, they may well be acting as inverse agonists, producing inverse cannabimimetic effects by somehow decreasing the spontaneous coupling of CB1 receptors to their effector mechanisms that it is thought can occur in the absence of exogenously added or endogenously released CB1 agonists (reviewed in [23]). The ability to behave both as a competitive antagonist and as an inverse agonist at the CB1 receptor is shared by the Merck compound, taranabant (Fig. 4 and Table 1). This CB1-selective compound was developed as a potential medicine for the treatment of obesity and is mentioned again later in this review as its ability to bind to several non-CB1, non-CB2 receptors has been investigated [24, 25]. Interestingly, experiments performed with rimonabant have yielded data suggesting that it, and hence possibly also AM251, AM281 and taranabant, can produce inverse cannabimimetic effects not only by targeting the cannabinoid CB1 receptor but also through one or more CB1 receptor-independent mechanisms [21, 26, 27].
Turning now to the compounds that are most often used in research to block the CB2 receptor (reviewed in [1, 3, 12, 13]), these are 6-iodopravadoline (AM630) and the diarylpyrazole, SR144528 (Fig. 5), each of which displays much higher affinity for CB2 than for CB1 receptors (Table 1). As well as blocking CB2 receptors competitively, both these compounds can by themselves produce inverse cannabimimetic effects in tissues expressing these receptors. They are therefore thought to be CB2 receptor inverse agonists rather than neutral antagonists.
Fig. (5).
The structures of the cannabinoid CB2 receptor antagonists/inverse agonists, SR144528 and AM630, both of which block CB2 receptors more potently than CB1 receptors.
Other notable examples of CB2-selective cannabinoid receptor antagonists/inverse agonists include JTE-907 [28] and the triaryl bis-sulphones, Sch.225336, Sch.356036 and Sch.414319 (reviewed in [29]). The question of whether these ligands target non-CB1, non CB2 receptors as agonists or antagonists remains to be addressed. CB2 receptor inverse agonists are of interest as they may well have important therapeutic applications. Thus, there is evidence that they can inhibit inflammatory cell migration in a manner that would make these compounds effective against dermatitis, inflammation of the central nervous system and bone damage in antigen-induced mono-articular arthritis [29, 30].
NEUTRAL COMPETITIVE CANNABINOID RECEPTOR ANTAGONISTS
Neutral competitive antagonists are compounds that can displace an agonist from the orthosteric site of a receptor but lack the ability to modulate any signalling that is triggered by this receptor in the absence of any exogenously administered or endogenously released agonist. Ligands that have been reported to behave as neutral cannabinoid receptor antagonists include
two CB1-selective analogues of rimonabant, AM6527, which is orally active, and AM4113, which is not [31, 32];
two other rimonabant analogues, VCHR [33, 34] and NESS O327 [35, 36];
a set of 3-alkyl-5,5'-diphenylimidazolidinediones [37];
O-2654 [3, 38] which is a structural analogue of the plant cannabinoid, cannabidiol;
O-2050, a sulphonamide analogue of Δ8-THC [3] and
There is evidence that of these compounds, AM4113, AM6527 and NESS O327 display markedly higher affinity for CB1 than for CB2 receptors [31, 32, 35], whereas O-2654 and Δ9-tetrahydrocannabivarin do not [38, 39]. However, the extent to which any of these apparent neutral antagonists targets non-CB1, non CB2 receptors as an agonist or antagonist has yet to be investigated.
Neutral competitive cannabinoid receptor antagonists are important research tools as they are expected to produce only dextral shifts in the log concentration-response curve of a cannabinoid receptor agonist. Cannabinoid receptor antagonists/inverse agonists, on the other hand, often produce downward as well as dextral shifts in such curves, particularly in bioassay systems employing cells or cell membanes in which CB1 or CB2 receptors are significantly overexpressed. The absence of such a downward shift makes it much easier to calculate an apparent KB value of an antagonist from the dextral shift it has induced and hence to establish whether newly developed agonists are indeed acting on CB1 or CB2 receptors (e.g. see [38]). It should also be possible to use neutral antagonists to distinguish between tonic cannabimimetic activity arising from ongoing endocannabinoid release onto CB1 or CB2 receptors, which they should oppose, and tonic activity arising from spontaneous CB1 or CB2 receptor signalling, which they should not alter. Neutral CB1 receptor antagonists may also have higher benefit-to-risk ratios than CB1 receptor antagonists/inverse agonists when they are used as medicines. Thus, for example, there is evidence that the neutral CB1 receptor-selective antagonist, AM4113, shares the ability of the CB1 receptor antagonist/inverse agonist, AM251, to suppress food intake and food-reinforced behaviour in rats and that, in contrast to AM251, it produces this effect at doses that do not induce signs of nausea [32]. AM4113 has also been found to lack the ability of AM251 to potentiate morphine-6-glucuronide-induced vomiting in ferrets [42]. Whether neutral CB1 receptor-selective antagonists also differ from CB1 receptor antagonists/inverse agonists by not triggering signs of depression or anxiety when administered once or repeatedly has yet to be established.
No neutral antagonist that selectively targets the CB2 receptor has yet been developed. It is noteworthy, however, that (S)-(−)-WIN55212 which has similar, albeit rather low, affinity for human CB1 and CB2 receptors [43] has been found in one investigation to behave in vitro as a neutral human CB2 receptor antagonist [44]. In other experiments though, performed with cell membranes in which human CB2 receptors were more highly expressed, this compound was found to behave as an inverse agonist [43]. Two synthetic analogues of olivetol/resorcinol have also been reported to behave in vitro as neutral CB2 receptor antagonists [45, 46]. These compounds, each of which binds with significant potency to both CB1 and CB2 receptors, were found to antagonize CP55940-induced stimulation of [35S]GTPαS binding to human CB2-expressing Chinese hamster ovary (CHO) cell membranes at concentrations of either 100 nM or 1 µM.
NON-CB1, NON-CB2 RECEPTORS TARGETED BY NON-ENDOGENOUS ESTABLISHED CANNABINOID RECEPTOR LIGANDS
The remainder of this review focuses on data obtained from experiments with certain established non-endogenous CB1 and CB2 receptor ligands that were directed at investigating the ability of these compounds to bind to a selection of non-cannabinoid receptors or to target them as agonists or antagonists. As will become apparent, the data obtained so far suggest that one or more of these CB1/CB2 receptor ligands can target orthosteric or allosteric sites on the following G protein-coupled receptors, transmitter-gated channels, ion channels and nuclear receptors:
GPR55;
certain other G protein-coupled receptors, including β-adrenoceptors and 5-hydroxytryptamine, muscarinic acetylcholine, opioid, adenosine and imidazoline-like receptors;
nicotinic acetylcholine, ionotropic glutamate and 5-HT3 receptors;
glycine receptors;
calcium channels;
potassium channels;
sodium channels
TRPV1, TRPV2 and TRPA1 channels and/or
the nuclear receptors, peroxisome proliferator-activated receptors α and γ (PPARα and PPARγ).
GPR55
As indicated in Table 2, there have been several reports that certain CB1 and/or CB2 receptor ligands can target GPR55 as agonists or antagonists when administered in vitro at concentrations in the low nanomolar or low micromolar range, and hence at or above concentrations at which they are normally used to activate or block CB1 or CB2 receptors. These ligands fall essentially into two groups. In the first group are the cannabinoid CB1/CB2 receptor agonists, Δ9-THC and HU-210, the cannabinoid CB1 receptor agonists, noladin ether and R-(+)-methanandamide, the cannabinoid CB2 receptor agonist, JWH-015, and the cannabinoid CB1 receptor antagonists, AM251 and AM281. Each of these ligands has been found to activate GPR55 in at least one investigation, though with the exception of noladin ether which has only been investigated once, not in all investigations. There has also been a GlaxoSmithKline patent (WO01/86305) claiming that AM251 targets GPR55 in a yeast-based assay [47]. The second group contains the CB1/CB2 agonist, CP55940, and the cannabinoid CB1 receptor antagonist, rimonabant both of which have been reported to activate GPR55 in at least one bioassay but to lack such activity or to behave as GPR55 antagonists in other bioassays.
Table 2.
Evidence that Certain Established Cannabinoid Receptor Agonists and Antagonists Activate and/or Block GPR55
| Compound | Assay and reference |
Concentration/potency for agonism |
Assay and reference |
Concentrations at which no agonism detected |
Assay and reference |
Concentration for antagonism |
|---|---|---|---|---|---|---|
| Δ9-THC | 1 | EC50 = 8 nM | 4 | 1 µM | – | – |
| 2a, 2b, 2c | 5 µM | 8a | 30 µM | |||
| 6a | <1 µM (low efficacy) | |||||
| 7 | Slight agonism | |||||
| HU210 | 1 | EC50 = 26 nM | 4 | 1 µM | – | – |
| 6a | ca 1 nM to >10 µM | |||||
| 7 | 10 µM | |||||
| 8a | 30 µM | |||||
| R-(+)-MethAEA | 2a | 5 µM | 8a | 30 µM | – | – |
| Noladin ether | 1 | EC50 = 10 nM | – | – | – | – |
| JWH-015 | 2a, 2c | 3 µM | 8a | 30 µM | – | – |
| AM251 | 1 | EC50 = 39 nM | 8c | 30 µM | – | – |
| 3 | EC50 = 612 nM | |||||
| 6a | EC50 = 2700 nM | |||||
| 6b | EC50 = 3400 nM | |||||
| 8a | EC50 = 9.6 µM | |||||
| 8b | 10 µM | |||||
| 9 | No data | |||||
| AM281 | 3 | 3 to 30 µM | 1 | up to 30 µM | – | – |
| 9 | No data | 8a | 30 µM | |||
| CP55940 | 1 | EC50 = 5 nM* | 2a | 5 µM | 3 | 3 µM (vs LPI) |
| 8b | 10 µM (low efficacy) | 3 | up to 3 µM | 8a | IC50 = 678 nM (vs LPI) | |
| 4 | 1 µM | 8b | 10 µM (vs LPI) | |||
| 6a, 6b | ca 1 nM to >10 µM§ | |||||
| 7 | 10 µM | |||||
| 8a | 30 µM | |||||
| 8c | 10 µM | |||||
| Rimonabant | 3 | 100 nM to 30 µM | 2a | 2 µM | 2a | 2 µM vs methAEA |
| 6a | 9.3 µM | 4 | 1 µM | 2a, 2c | 2 µM vs Δ9-THC, JWH-015 | |
| 6b | 10.9 µM | 7 | 10 µM | |||
| 8a | EC50 = 3.9 µM | 8c | 30 µM | |||
| 8b | 10 µM | |||||
| 9 | No data | |||||
| R-(+)-WIN55212 | – | – | 2a | 5 µM | – | – |
| 1 | up to 30 µM | |||||
| 4 | 1 µM | |||||
| 5 | 1 µM | |||||
| 6a, 6b | ca 1 nM to >10 µM | |||||
| 7 | 10 µM | |||||
| 8a | 30 µM | |||||
| AM630 | – | – | 3 | up to 30 µM | – | – |
| 6a, 6b | ca 1 nM to >10 µM | |||||
| SR144528 | – | – | 2c | 2 µM† | – | – |
| 8a | 30 µM | |||||
| In vitro assay/measured response | Reference | |
|---|---|---|
| 1 | Stimulation of [35S]GTPγS binding to membranes of HEK293s cells transiently transfected with human GPR55 | [57] |
| 2 | Elevation of intracellular Ca2+
|
[48] |
| 3 | Elevation of intracellular Ca2+ in HEK293 cells stably expressing human GPR55 | [52, 53] |
| 4 | Induction of phosphorylation of ERK in HEK293 cells stably transfected with human GPR55 | [50, 51] |
| 5 | Stimulation of [35S]GTPγS binding to membranes of HEK293T cells transiently transfected with human GPR55 | [58] |
| 6 |
|
[47] |
| 7 | Elevation of intracellular Ca2+ in GPR55-expressing microglial cells (BV2) | [54] |
| 8 |
|
[55] |
| 9 | Reporter gene assays performed with HEK293 cells stably expressing GPR55 | [60] |
ERK, extracellular signal-regulated kinase; LPI, lysophosphatidylinositol; R-(+)-MethAEA, R-(+)-methanandamide.
SR144528 (2 µM) was also found not to antagonize JWH-015 in assays 1a and 1c.
CP55940 at >10 µM induced signs of inverse agonism in assay 6b.
In assay 1, CP55940 was antagonized by cannabidiol (IC50 = 445 nM) whereas in assay 8a LPI was not antagonized by cannabidiol (10–30 µM).
Some established CB1/CB2 receptor ligands have so far been found not to activate GPR55 in any GPR55 bioassay in which they have been investigated (Table 2). This additional group of compounds consists of the CB1/CB2 receptor agonist, R-(+)-WIN55212, and the cannabinoid CB2 receptor antagonists, AM630 and SR144528, the second of which has also been reported not to block ligand-induced activation of GPR55 [48]. Conversely, several non-CB1, non-CB2 receptor ligands have been found to activate GPR55 (reviewed in [49]). Among these are the endogenous phospholipid, lysophosphatidylinositol [47, 48, 50–56] and two synthetic analogues of cannabidiol, O-1602 [56–58] and abnormal cannabidiol [57, 58]. It should be noted, however, that there have also been reports that in some bioassay systems, GPR55 is not activated by O-1602 [47, 51, 55] or abnormal cannabidiol [47, 48, 50, 55]. Cannabidiol itself has been found, albeit so far only in two investigations, to behave in vitro as a reasonably potent GPR55 antagonist. In one of these [57], this plant cannabinoid was found to antagonize CP55940-induced activation of human GPR55 transiently transfected into human embryonic kidney (HEK293) cells (IC50 = 445 nM). In the other investigation, cannabidiol at 0.5 or 1 µM was found to oppose lysophosphatidylinositol and O-1602-induced activation of GPR55 naturally expressed in human or mouse cultured osteoclasts [56]. There has, however, also been a report that, at 10–30 µM, cannabidiol does not antagonize lysophosphatidylinositol in a β-arrestin assay performed with U2OS cells stably transfected with human GPR55 [55].
Importantly, in those investigations in which some CB1 and/or CB2 receptor ligands have been found not to activate or block GPR55, positive results were obtained with at least one other compound, that therefore served as an "active control". These compounds included other established cannabinoid receptor ligands that did appear to activate GPR55 in the same bioassay [47, 48, 52, 53, 57] and/or lysophosphatidylinositol, which seems to be a particularly reliable active control [47, 48, 50–55]. Consistent with the evidence that R-(+)-WIN55212 does not appear to activate GPR55, are findings, first that at a concentration of 50 nM [3H]R-(+)-WIN55212 does not share the ability of [3H]CP55940 or tritiated rimonabant to bind to human GPR55 [57]. There has also been an AstraZeneca patent (WO2004074844) claiming that GPR55 is not activated by R-(+)-WIN55212, or indeed by the CB2-selective agonist, JWH-133 (reviewed in [59]).
The mixed GPR55 agonism/non-agonism or mixed GPR55 agonism/non-agonism/antagonism displayed by some CB1/CB2 receptor ligands could be an indication that these compounds are partial agonists. Thus, the ability of a partial agonist to activate GPR55 is expected to be determined by the expression level and coupling efficiency of this receptor in a particular bioassay system. Conversely, since AM251 seems usually to produce a strong activation of GPR55 [47, 52, 55, 57], it could well be a relatively high-efficacy GPR55 agonist. The manner in which GPR55 signals may also be a significant variable as preliminary evidence has emerged suggesting that agonist-induced GPR55 signalling is ligand dependent. Thus, there has been one recent report that GPR55 signals differently when activated by AM251, AM281 or rimonabant than when activated by lysophosphatidylinositol [53] and another report that it signals differently when activated by AM281 than when activated by AM251 or lysophosphatidylinositol [60]. There has also been a report that AM251 and rimonabant share the ability of lysophosphatidylinositol to activate human GPR55 both in a β-arrestin assay and in an assay that measures G-protein-dependent activation of PKCΔII but not in an assay that depends on induced phosphorylation of extracellular signal-regulated kinase (ERK) [55]. It will be of interest to investigate whether there are any disorders in which GPR55 expression upregulates or downregulates or in which GPR55 signalling or constitutive activity alters, as such changes might well shape the pharmacology and therapeutic potential of at least some GPR55 ligands, particularly of any ligands that display mixed agonist/antagonist activity and are indeed GPR55 partial agonists. Since evidence has already emerged that GPR55 mediates inflammatory mechanical hyperalgesia, the release of inflammatory cytokines and impairment of bone function [56, 61], future pharmacological research is likely to focus particularly on establishing whether cannabidiol really is a GPR55 antagonist and on whether GPR55 antagonists do indeed have important therapeutic applications.
Finally, although it is possible that the coupling efficiency of GPR55 may not have been the same in all bioassays listed in Table 2, it is unlikely that inter-bioassay variations in its expression level accounted for the apparent ability of certain compounds to produce detectable activation of GPR55 in some bioassays but not in others. Thus, most of these bioassays were performed with transfected cells in which GPR55 was highly expressed.
OTHER G PROTEIN-COUPLED RECEPTORS
Results obtained from experiments with bovine cerebral cortical synaptic membranes showed that, at 10 µM, 11-hydroxy-Δ8-THC and 11-oxo-Δ8-THC but not Δ8-THC can reduce [3H]5-HT binding to 5-HT1A, 5-HT1B, 5-HT1D and 5-HT1E receptors but not [3H]ketanserin binding to 5-HT2A or 5-HT2B receptors [62]. In addition, there have been reports, first that at 500 nM the cannabinoid receptor agonist, HU-210, produces a slight enhancement of high-affinity 5-HT binding to 5-HT2 receptors in rat cerebral cortical membranes labelled with [3H]ketanserin [63], and second, that at 3 µM and/or 10 µM but not higher or lower concentrations, Δ9-THC and 11-hydroxy-Δ9-THC increase the affinity of [3H]dihydroalprenolol for α-adrenoceptors in mouse cerebral cortical membranes [64].
R-(+)-methanandamide has been found to reduce binding of the muscarinic acetylcholine receptor antagonists, [3H]N-methylscopolamine and [3H]quinuclidinyl benzilate, to sites on adult human frontal cerebrocortical membranes with IC50 values of 15 and 44 µM, respectively [65]. These reductions in binding were rimonabant-insensitive and were not mimicked by R-(+)-WIN55212 at concentrations of up to 5 µM. R-(+)-methanandamide also modulated the binding of [3H]oxotremorine to these membranes, its effect being inhibitory at concentrations above 100 µM but stimulatory at lower micromolar concentrations. It is possible that these binding changes were induced allosterically as all the reductions in binding induced by R-(+)-methanandamide at maximal concentrations were slightly less than 100%. Similarly, Christopoulos and Wilson [66] have found that at concentrations in the micromolar range, R-(+)-methanandamide but not R-(+)-WIN55212, can displace [3H]N-methylscopolamine or [3H]quinuclidinyl benzilate from human M1 and M4 muscarinic acetylcholine receptors expressed in CHO cell membranes. Rimonabant too was found to induce some displacement of [3H]quinuclidinyl benzilate, though with somewhat less potency. It is noteworthy that anandamide also produced such displacement, since this seemed to be noncompetitive and hence possibly allosteric in nature.
There is more conclusive evidence that Δ9-THC can bind to allosteric sites on opioid receptors. Thus, in experiments with rat brain cerebral cortical membranes, it has been found that this CB1/CB2 receptor agonist can accelerate the dissociation of [3H]DAMGO ([3H]D-Ala2, NMePhe4,Gly-ol] enkephalin) and [3H]naltrindol, presumably from μ and δ opioid receptors, respectively [67]. This acceleration was induced by Δ9-THC only at rather high concentrations (EC50 = 21.4 µM and 10 µM, respectively) and was no more than two-fold. Interestingly, the non-psychoactive plant cannabinoid, cannabidiol, was more effective though not more potent than Δ9-THC at accelerating [3H]DAMGO and [3H]naltrindol dissociation in this bioassay. Rimonabant at 10 µM did not affect the dissociation rates of these tritiated ligands but did appear to displace [3H]DAMGO in a competitive manner (IC50 = 4.1 µM). It displayed less potency as an inhibitor of [3H]naltrindol binding, suggesting that it possesses some degree of selectivity for the μ opioid receptor. These findings are in line with previous results obtained in equilibrium binding experiments with rat whole brain membranes which also suggest that, at concentrations in the low micromolar range, Δ9-THC and cannabidiol can undergo noncompetitive/allosteric interactions with μ and δ̣ opioid receptors, though not with κ opioid receptors or σ/phencyclidine receptors [68]. Binding to μ opioid receptors was also found to be inhibited in this much earlier investigation by (+)-Δ9-THC, equatorial hexahydrocannabinol, cannabinol, D- and L-nantradol and 11-hydroxy-Δ9-THC, the relative inhibitory potencies displayed these compounds and by (−)-Δ9-THC (IC50 = 7 µM) and cannabidiol (IC50 = 7 µM) suggesting a lack of correlation between their potencies as inhibitors of μ opioid receptor binding and their CB1 receptor affinities.
There have been two recent papers confirming that rimonabant can induce radioligand displacement from μ opioid receptors, its reported IC50 values for this displacement being 3 μM [25] and 5.7 µM [27]. One of these papers also describes evidence that, at concentrations in the low micromolar range, rimonabant can bind to several other non-CB1, non-CB2 G protein-coupled receptors [25]. Thus, rimonabant was found to induce radiolabelled ligand displacement from κ opioid receptors, α2A- and α2C-adrenoceptors, prostanoid EP4, FP and IP receptors and serotonergic 5-HT6, angiotensin AT1, adenosine A3 and tachykinin NK2 receptors with IC50 values ranging from 1.5 to 7.2 µM. The corresponding IC50 values of taranabant were found to exceed 10 µM at all of these receptors apart from the tachykinin NK2 and adenosine A3 receptor (IC50 = 0.5 and 3.4 µM, respectively) [25]. At concentrations in the low µM range, taranabant was also found to induce radiolabelled ligand displacement from dopamine D1 and D3 receptors (Ki = 3.4 and 1.9 µM, respectively) and, in contrast to rimonabant (IC50 = >10 µM), from melatonin MT1 receptors (IC50 = 7.5 µM) [24, 25]. There is evidence too that ligand-induced activation of human melatonin MT1 and human muscarinic acetylcholine M4 receptors can be opposed by (S)-(−)-WIN55212 at the rather high concentration of 100 µM [44]. No such antagonism was detected at human P2Y12 or endogenous lysophosphatidic acid receptors or at rat brain μ opioid, sphingosine-1-phosphate, GABAB or adenosine A1 receptors.
There is evidence that rimonabant and its structural analogue, AM251, can also each target the adenosine A1 receptor. Thus, at 10 µM, both compounds have been reported to oppose the activation of A1 receptors in rat cerebellar membranes and also to inhibit basal [35S]GTPγS binding to these membranes in a manner that can be prevented by the selective adenosine A1 receptor antagonist, DPCPX [21]. These findings prompted the hypothesis that rimonabant and AM251 can inhibit [35S]GTPγS binding by blocking the activation of A1 receptors by endogenously released adenosine [21]. That these two CB1 receptor ligands can induce an inverse effect independently of CB1 receptors is supported by reports first, that rimonabant can inhibit basal [35S]GTPγS binding to brain membranes obtained from CB1 −/− mice, and second, that this inhibitory effect is not antagonized by the CB1 receptor neutral antagonist, O-2050, in brain membranes obtained from either CB1+/+ or CB1−/− mice [26, 27].
Finally, as discussed in greater detail elsewhere [69–71], there is evidence that, at concentrations in the nanomolar or low micromolar range, CP55940 and rimonabant can interact with a non-I1, non-I2 subtype of the putative imidazoline receptor that may belong to a family of G protein-coupled sphingosine-1-phosphate/lysophosphatidic acid receptors originally known as endothelial differentiation gene (EDG) receptors. This is a putative receptor subtype that appears to be both CB1 receptor-like and α2-adrenoceptor-like and to mediate inhibition of evoked noradrenaline release, for example from the terminals of sympathetic neurons supplying cardiovascular tissue [69, 70, 72]. There is also evidence first, that this putative receptor subtype can be activated by CP55940 at 300 nM and by R-(+)-WIN55212 at 10 and 100 µM although not by R-(+)-WIN55212 at 1 µM or by S-(−)-WIN55212 at 100 µM [71], and second, that its activation by CP55940, by the α2-adrenoceptor agonist, clonidine, and by two agonists of the putative non-I1, non-I2 imidazoline receptor can be antagonized by rimonabant at 1 or 10 µM and/or by LY320135 at 0.1, 1 or 10 µM [71, 73]. Rauwolscine, which behaves as an antagonist of the putative non-I1, non-I2 imidazoline receptor subtype at 30 µM, has at this concentration also been found to oppose the activation of this putative receptor subtype by CP55940 [73].
NICOTINIC ACETYLCHOLINE, IONOTROPIC GLUTAMATE AND 5-HT3 RECEPTORS
There is evidence that R-(+)-methanandamide and CP55940 (IC50 = 183 nM and 3.4 µM, respectively) but not Δ9-THC or R-(+)-WIN55212 can each produce complete inhibition of currents induced by acetylcholine in α7-nicotinic acetylcholine receptors expressed in Xenopus oocytes [74]. Evidence has also been obtained that R-(+)-methanandamide (1 µM) but not Δ9-THC can enhance activation of ionotropic glutamate receptors by N-methyl-D-aspartate [75].
As to the 5-HT3 receptor, evidence has been obtained that some synthetic cannabinoid receptor ligands can antagonize 5-HT-induced activation of this receptor and that the rank order of the potency displayed by these ligands as 5-HT3 antagonists does not correlate with the rank order of their potency for binding to cannabinoid CB1 or CB2 receptors. This evidence, which is discussed in greater detail elsewhere [69, 76], has come in part from experiments with rat nodose ganglion neurons [77] in which CP55940 and R-(+)-WIN55212 were found to behave as 5-HT3 receptor antagonists (IC50 = 94 and 310 nM, respectively). It has also come from experiments with HEK293 cells stably transfected with the functional 3A subunit of the human 5-HT3 receptor [78]. These experiments showed that 5-HT3A-mediated currents induced by 5-HT could be inhibited by the CB1/CB2 receptor agonists, Δ9-THC, R-(+)-WIN55212, anandamide and CP55940 (IC50 = 38, 104, 130 and 648 nM, respectively), by the CB2-selective agonist, JWH-015 (IC50 = 147 nM) and by the CB1-selective antagonist, LY320135 (IC50 = 523 nM), although not by rimonabant or S-(−)-WIN55212 at 1 µM. Two additional findings, first, that R-(+)-WIN55212 antagonized 5-HT in an insurmountable manner, and second, that R-(+)-WIN55212, CP55940 and anandamide did not displace [3H]GR65630 from specific binding sites on membranes obtained from the 5-HT3A-transfected HEK293 cells [78], raise the possibility that cannabinoids inhibit currents mediated by the 5-HT3A receptor by targeting an allosteric site on this receptor. It is noteworthy, however, that CP55940 at 10 µM was found to alter neither the rate of dissociation of [3H]GR65630 from the 5-HT3A receptor nor its rate of association [78].
GLYCINE RECEPTOR
There is evidence that at concentrations in the nanomolar range, Δ9-THC can potentiate glycine receptor activation, possibly in an allosteric manner [79]. Thus, it has been found that this CB1/CB2 receptor agonist can potentiate glycine-activated currents in both homomeric α1 and heteromeric α1β1 subunits of human glycine receptors that had been transfected into Xenopus laevis oocytes (EC50 = 86 nM and 73 nM, respectively) and in native glycine receptors expressed by neurons obtained from the ventral tegmental area of rat brain (EC50 = 115 nM) [79]. More recently, the ability of three other non-endocannabinoids to modulate glycineinduced in vitro activation of human α1, α1β, α2 and α3 glycine receptor subunits recombinantly expressed in HEK293 cells was investigated [80]. These cannabinoids were the CB1/CB2 receptor agonists, HU-210 and R-(+)-WIN55212, and the CB2-selective agonist, HU-308. It was found first, that α1 activation was potentiated by HU-210 (EC50 = 270 nM), unaffected by R-(+)-WIN55212 (30 µM) and weakly inhibited by HU-308 (30 µM), second, that α2 activation was inhibited with similar potency by HU-210 and R-(+)-WIN55212 (IC50 = 90 nM and 220 nM, respectively) and less potently by HU-308 (IC50 = 1.13 µM), and third, that α3 activation was inhibited with similar potency by HU-210, R-(+)-WIN55212 and HU-308 (IC50 = 50 nM, 86 nM and 97 nM, respectively). As to α1β glycine receptor subunit currents, these were enhanced by HU-210 at 30 µM, unaffected by R-(+)-WIN55212 at 30 µM and inhibited by HU-308 at 30 µM. Results obtained in experiments with rat isolated hippocampal pyramidal neurons also suggest that R-(+)-WIN55212 (5 µM) can inhibit glycine activated currents [81]. That HU-210 can enhance the activation of α1 glycine receptor subunits has been confirmed by the demonstration that this compound potentiates glycine-induced activation of strychnine-sensitive α1 subunits transiently transfected into HEK293 cells (EC50 = 5.1 µM) [82]. It was also found in this investigation that at concentrations above 10 µM, HU-210 can activate these glycine receptor subunits in the absence of added glycine (EC50 = 189 µM).
CALCIUM CHANNELS
Shen and Thayer [83] have found that R-(+)-WIN55212 can inhibit N- and P/Q-type voltage gated calcium channels in rat cultured hippocampal neurons. It seemed to produce this effect at nanomolar concentrations only via cannabinoid CB1 receptors (IC50 = 14 nM), though at higher concentrations both R-(+)- and S-(−)-WIN55212 appeared to target these channels directly. Currents through these channels were not affected by rimonabant at 300 nM, a concentration at which it does block the CB1 receptor.
Evidence has also been obtained that R-(+)-methanandamide at 1 µM, HU-210 at 10 but not 1 µM and rimonabant at 100 nM and 1 µM can inhibit currents through cloned α1H subunits of low-voltage-activated (T-type) calcium channels in cultured cells [84]. No such inhibition was induced by R-(+)-WIN55212, CP55940 or Δ9-THC at 10 µM. R-(+)-methanandamide (1 µM) but not R-(+)-WIN55212 (1 µM) also inhibited native T-channel currents in neuroblastoma NG108-15 cells. More recently, it was demonstrated that, at 10 µM, both R-(+)-methanandamide and ACEA inhibit currents through the cloned T-channels, human CaV3.1, CaV3.2 and CaV3.3, expressed in tsA-201 cells [85]. There has also been a report that at 10 µM, R-(+)-methanandamide can inhibit specific binding of a calcium L-type channel ligand of the dihydropyridine class (IC50 = 7.1 µM) and depolarization-induced Ca2+ efflux through voltage-gated calcium channels in transverse tubule membrane vesicles obtained from rabbit skeletal muscle [86]. No effects of this kind were produced by R-(+)-WIN55212, CP55940 or Δ9-THC at concentrations of up to 10 or 100 µM [86]. Nor indeed have they been detected in response to rimonabant at 1 µM [87], though this concentration of rimonabant has been found to inhibit potassium-evoked Ca2+ influx into neonatal rat cultured dorsal root ganglion sensory neurons [88]. Rimonabant has also been found to displace cis-(+)-[n-methyl-3H]diltiazem from L-type calcium channels, as indeed has taranabant (IC50 = 6.1 µM, and 300 nM respectively) [24, 25].
Contrasting with negative results obtained previously with Δ9-THC in experiments with cultured cells expressing T-type calcium channels encoded by the CaV3 gene family [84], is a recent report that this cannabinoid can inhibit CaV3 channels [89]. An inhibitory effect of Δ9-THC (1 µM) was observed both on channels that had been stably transfected into HEK293 cells and on channels expressed naturally in mouse trigeminal ganglion sensory neurons. Interestingly, such inhibition was also produced at 1 µM by the non-psychoactive plant cannabinoid, cannabidiol, and both these cannabinoids were found to inhibit CaV3.1 and CaV3.2 currents more potently than CaV3.3 currents. At 3 µM, the CB1-selective antagonist, AM251, inhibited the naturally-expressed channels too, but did not diminish the inhibitory effects of subsequently administered Δ9-THC or cannabidiol [89]. The same investigation also yielded data suggesting that Δ9-THC but not cannabidiol may increase the amount of calcium entry following T-type channel activation by stabilizing open states of the channel and that cannabidiol but not Δ9-THC interacts significantly with closed states of the CaV3.1 channel.
POTASSIUM CHANNELS
Evidence has been obtained from experiments with murine fibroblasts stably transfected with KV1.2 cDNA that Δ9-THC (IC50 = 2.4 µM) can inhibit Shaker-related voltage gated KV1.2 potassium channels in a CB1/CB2 receptor independent manner [90]. It also appears that potassium channels can be targeted by certain other synthetic cannabinoid CB1/CB2 receptor agonists. Thus, Van den Bossche and Vanheel [91] have obtained evidence from experiments with rat aortic smooth muscle cells that R-(+)-methanandamide (10 µM) can block delayed rectifier potassium (KV) channels in a rimonabant-insensitive manner. This it seems to do by binding to an external site on or near this channel. They also found that this channel could be blocked by both R-(+)-WIN55212 (20 µM) and rimonabant (10 µM). More recently, it has been found that rimonabant and taranabant can induce radiolabelled ligand displacement from rapid delayed rectifier potassium channels (IC50 = 2.5 µM and 2.3 µM, respectively) [24, 25], and that R-(+)-methanandamide (10 µM) can inhibit cromakalim-induced activation of ATP-sensitive inward-rectifier (KATP) channels in Xenopus oocytes [92].
There is also evidence that R-(+)-methanandamide can inhibit the leak or background potassium channel, TASK-1 (IC50 = 700 nM) as indicated by results obtained from experiments with COS-7 cells transfected with cDNA encoding this channel [93]. At 10 µM, R-(+)-WIN55212 and CP55940, but not Δ9-THC or HU-210, were found to share the ability of R-(+)-methanandamide to inhibit the TASK-1 channel. Rimonabant (10 µM) also produced a slight inhibition of TASK-1 but did not antagonize R-(+)-WIN55212. More recently, it has been found in experiments with transfected HEK293 cells that R-(+)-methanandamide (1, 3 and/or 10 µM) can block not only TASK-1 channels, but also another member of this subfamily of two pore domain potassium channels, TASK-3 [94, 95]. R-(+)-methanandamide inhibited rat TASK-1 slightly more than rat TASK-3 [95] whereas human TASK-3 was inhibited significantly more by this cannabinoid than either human TASK-1 or mouse TASK-3 [94]. In addition, R-(+)-methanandamide (1 and 10 µM) has been found to inhibit heteromeric TASK-1/TASK-3 channels [95]. Given these findings, it is also noteworthy that genetic deletion of TASK-1 or TASK-3 has been shown to reduce the ability of R-(+)-WIN55212 to produce antinociception in mice in the hot plate test though not in the tail flick test [96, 97]. Deletion of TASK-1 but not of TASK-3 also decreased R-(+)-WIN55212-induced hypomotility and hypothermia [96, 97].
Finally, it has been reported that R-(+)-methanandamide (0.3 to 3 µM) but not JWH-133 (1 µM) can increase the activity of Ca2+-activated potassium (BK) channels in a CB1/CB2 receptor independent manner. These findings came mainly from experiments with HEK293 cells transfected with one or two subunits of the BK channel [98].
SODIUM CHANNELS
Evidence has been obtained that R-(+)-WIN55212 can inhibit voltage-gated sodium channels [99]. This was indicated by its ability to inhibit depolarization of mouse brain synaptoneurosomes induced by the sodium channel site 2-selective neurotoxin, veratridine, (IC50 = 21.1 µM) as well as by its ability to inhibit veratridine-dependent release of L-glutamic acid (IC50 = 12.2 µM) and γ-aminobutyric acid (IC50 = 14.4 µM) from mouse purified brain synaptosomes and the binding of [3H]batrachotoxinin A 20-α-benzoate ([3H]BTX-B) to mouse brain synaptoneurosomal voltage-gated sodium channels (IC50 = 19.5 µM). Additionally, R-(+)-WIN55212 (1 µM) but not Δ9-THC (10 µM) was found to block sustained repetitive firing in rat cultured cortical neurons that could also be blocked by the sodium channel-selective blocker, tetrodotoxin. None of these effects of R-(+)-WIN55212 were affected by AM251 at 1 or 2 µM. Indeed, subsequent research by the same group showed that this cannabinoid CB1 receptor antagonist shares the ability of R-(+)-WIN55212 both to inhibit veratridine-dependent depolarization of mouse brain synaptoneurosomes (IC50 = 8.9 µM) and release of L-glutamic acid (IC50 = 8.5 µM) and γ-aminobutyric acid (IC50 = 9.2 µM) from mouse purified brain synaptosomes and to displace [3H]BTX-B from mouse brain synaptoneurosomal membranes (IC50 = 11.2 µM) [100]. AM251 may interact allosterically with sodium channels as it was also found to accelerate the dissociation of [3H]BTX-B from its binding sites. This research group has also discovered that the ability of R-(+)-WIN55212 to displace [3H]BTX-B from mouse brain synaptoneurosomal voltage-gated sodium channels in a manner not opposed by AM251 at 2 µM is shared by another potent cannabinoid CB1/CB2 receptor agonist, CP55940 (IC50 = 22.3 µM) [101]. Like AM251 but not R-(+)-WIN55212, this nonclassical cannabinoid seems to inhibit [3H]BTX-B binding through an allosteric mechanism. There have been reports too that R-(+)-WIN55212 (10 µM) and noladin ether (50 µM) inhibit voltage-gated sodium currents in frog parathyroid cells in a cannabinoid receptor-independent manner [102] and that R-(+)-WIN55212 (IC50 = 17.8 µM) inhibits such currents slightly in rat cultured trigeminal ganglion neurons, albeit in a manner that could be partly blocked by AM251 [103]. Interestingly, in the second of these investigations it was also found that at 10 nM, though not at higher concentrations, R-(+)-WIN55212 induced a slight enhancement of voltage-gated sodium currents that was not blocked by AM251. There is also evidence that rimonabant and taranabant can target the human sodium channel site 2 as both these compounds have been found to induce radiolabelled ligand displacement from such channels (IC50 = 5.1 µM and 1.9 µM, respectively) [24, 25].
TRPV1, TRPV2 AND TRPA1 CHANNELS
The chemical similarity of anandamide to capsaicin and olvanil, first noted by Di Marzo, Bisogno, Melck, Ross, Brockie, Stevenson, Pertwee and De Petrocellis [104], prompted research that led to the discovery that anandamide can activate TRPV1 channels, albeit with significantly lower efficacy than capsaicin (reviewed in [69]). R-(+)-methanandamide can activate these channels too, though its potency and efficacy are even less than those of anandamide [105–107]. It has also been found to displace [3H]resiniferatoxin from rat TRPV1 receptors, again with a potency less than that of anandamide (Ki = 21.4 and 1.7 µM, respectively) ([105]. Another synthetic analogue of anandamide that behaves as a TRPV1 partial agonist is ACEA. This has been shown to mimic the ability of capsaicin to stimulate calcitonin gene-related peptide (CGRP) release from rat cultured trigeminal ganglion (TG) sensory neurons (EC50 = 14 µM) and to do this in a manner that can be antagonized by the TRPV1 antagonists, iodo-resiniferatoxin and capsazepine [108]. Interestingly, evidence was also obtained in this investigation that rimonabant may be a low-potency TRPV1 mixed agonist/antagonist. Thus, it stimulated CGRP release when administered by itself at concentrations above 50 µM but inhibited capsaicin-evoked CGRP release at concentrations of 10 and 30 µM. Evidence that rimonabant can block TRPV1 channels at concentrations in the low micromolar range has also been obtained from experiments with TRPV1-transfected HEK cells [109], isolated blood vessels [106] and hippocampal neurons [110].
Although ACEA appears to activate TRPV1 channels it has been found at 25 µM not to activate TRPA1 channels [111]. However, there is evidence that TRPA1 channels can be activated by Δ9-THC and by another CB1/CB2 partial agonist and plant cannabinoid, cannabinol. Thus, for example, it has been found that both Δ9-THC (20 µM) and cannabinol (20 µM) but not capsaicin (1 µM) increase intracellular free calcium in HEK293 cells transfected with human or rat TRPA1 (ANKTM1) channels and that both these compounds are antagonized by the non-selective cation channel blocker, ruthenium red [112]. There have also been reports first, that Δ9-THC (400 µM) elicits robust inward currents in human TRPA1-expressing oocytes [113], and second, that Δ9-THC (20 µM) activates both mouse TRPA1 channels transiently transfected into HeLa cells and rat TRPA1 channels naturally expressed in cultured TG sensory neurons in a manner that can be antagonized by ruthenium red [114]. Importantly, Δ9-THC appeared to induce much faster and more efficaceous activation of these receptors when applied directly to the cytoplasmic side than to the outside of HeLa cells or TG neuronal membranes [114]. Evidence has also emerged that Δ9-THC can activate rat and human TRPV2 channels. More specifically, there has been a report that Δ9-THC induces calcium mobilization in rat and human TRPV2-transfected HEK293 cells with EC50 values of 16 µM and 43 µM, respectively [115]. Its effect in both cell lines was antagonized by ruthenium red. Further evidence that Δ9-THC can target non-TRPV1 TRP ion channels comes from the finding that it inhibits electrically-evoked CGRP release and subsequent vasorelaxation (IC50 = 1.55 µM) in the rat mesenteric arterial bed in a manner that can be blocked by ruthenium red but not by AM251, AM630 or capsazepine [116]. Ruthenium red has also been found to oppose the ability of both Δ9-THC and cannabinol at high nanomolar or low micromolar concentrations to reverse phenylephrine-induced contractions of rat arterial segments [117]. Δ9-THC-induced relaxation of these segments was not blocked by rimonabant or AM251, or mimicked by HU-210 or CP55940. It was also not blocked by capsazepine and was detectable in arterial segments obtained from TRPV1−/− mice.
There is strong evidence that the ability of Δ9-THC to activate TRPA1 channels is shared by the aminoalkylindoles, R-(+)- and S-(−)-WIN55212, though not by two other CB1/CB2 receptor agonists, the classical cannabinoid, HU-210 and the nonclassical cannabinoid, CP55940 [112]. Thus, it has been found that CGRP release from rat cultured TG sensory neurons can be evoked by both R-(+)-WIN55212 (EC50 = 26 µM) and S-(−)-WIN55212 (at 25 µM), and that the effect of R-(+)-WIN55212 can be blocked by ruthenium red but not by TRPV1- or CB1-selective antagonists [118]. Results obtained in this investigation also showed that R-(+)- and S-(−)-WIN55212 are more potent at inhibiting potassium- evoked CGRP release in these cells (IC50 = 1.7 µM and 2.7 µM, respectively). The inhibitory effect of R-(+)-WIN55212 was not blocked by CB1-selective antagonists. However, it was blocked by the CB2-selective antagonist, AM630, albeit at 10 µM but not 1 µM.
That the non-TRPV1 TRP channel targeted by R-(+)-WIN55212 in rat TG sensory neurons is the TRPA1 channel is suggested in particular by the finding that R-(+)-WIN55212 (25 µM) generates inward currents in mouse TRPA1-transfected CHO cells but, in contrast to capsaicin, not in CHO cells transfected with rat TRPV1 or indeed with rat TRPV2 or TRPV4 or mouse TRPV3 or TRPM8 [111]. Similar results have been obtained with another aminoalkylindole, AM1241 (30 µM). Interestingly, unlike R-(+)-WIN55212, AM1241 generated larger currents in CHO cells co-transfected with TRPA1 and TRPV1 receptors than in CHO cells expressing only TRPA1 receptors. It has also been found that inhibition of capsaicin-induced inward currents can be induced by R-(+)-WIN55212 and AM1241 in CHO cells cotransfected with TRPA1 and TRPV1 receptors [111]. Currents generated by R-(+)-WIN55212 exhibit faster activation kinetics in TRPA1-expressing CHO cells than in TRPA1/TRPV1 coexpressing cells [119]. This could possibly be because such coexpression opposes the onset of desensitization.
PEROXISOME PROLIFERATOR-ACTIVATED RECEPTORS
O'Sullivan, Tarling, Bennett, Kendall and Randall [120] have discovered that Δ9-THC is a PPARγ agonist. More specifically they found that, like the established PPARγ-selective agonist, rosiglitazone, Δ9-THC (10 µM) can cause slowly developing vasorelaxation in rat isolated aortae in a manner that can be antagonized by the selective PPARγ antagonist, GW9662, but not by the cannabinoid CB1 receptor antagonist, AM251. They also found that GW9662 antagonizes Δ9-THC-induced relaxation in rat superior mesenteric arteries (G0) and that at concentrations of 100 nM and above, Δ9-THC activates PPARγ in HEK293 cells transiently expressing this receptor. There has been a preliminary report too that in HEK293 cells also expressing another nuclear receptor, retinoid X receptor α, PPARγ can be activated at 10 µM not only by Δ9-THC but also by the CB1/CB2 receptor synthetic agonist, CP55940, by the CB1 receptor antagonists, rimonabant and AM251, and by anandamide and cannabidiol, though not by Δ9-tetrahydrocannabivarin or R-(+)-WIN55212 [113].
More recently, however, evidence has emerged suggesting that PPARγ can be activated by R-(+)-WIN55212 [122]. Thus, it has been found that at 100 nM, R-(+)-WIN55212 decreases vascular cell adhesion molecule-1 expression in mouse brain endothelial cell cultures, either infected with Theiler’s murine encephalomyelitis virus or uninfected, and that this effect can be opposed by the PPARγ antagonist, GW9662 but not by rimonabant or SR144528. There is evidence too that apoptosis and downregulation of survivin and two other survival factors induced in hepatoma HepG2 cells by R-(+)-WIN55212 at 10 µM is PPARγ-mediated. More specifically, it has been shown that the first of these effects can be antagonized by the PPARγ antagonists, GW9662 and T0070907, and that GW9662 also antagonizes the second effect [123]. Other cannabinoid receptor ligands that appear to activate PPARγ include two cannabinoid CB1 receptor agonists, noladin ether and R-(+)-methanandamide. With regard to noladin ether, it has been found that the inhibition of interleukin-2 secretion by human Jurkat T cells that can be induced by this cannabinoid at 20 µM is antagonized by the PPARγ antagonist, T0070907 [124]. As to R-(+)-methanandamide, its ability to induce apoptosis in human cervical carcinoma cells at 10 µM has been found to be reduced by both GW9662 and knockdown of PPARγ in response to siRNA transfection [125].
Finally, evidence has emerged that certain cannabinoid CB1/CB2 receptor agonists can activate PPARα [126]. More particularly, it has been found first, that both R-(+)-WIN55212 (1 µM) and noladin ether (10 µM) appear to stimulate PPARα-mediated gene transcription in HeLa cells expressing these receptors, and second, that at concentrations in the low micromolar range both these cannabinoids can displace cis-parinaric acid from the PPARα receptor. Similar results were obtained with anandamide. In contrast, Δ9-THC was found to alter neither the transcriptional activity of PPARα at 10 µM nor cis-parinaric acid binding to this receptor at 1 to 32 µM.
CONCLUSIONS AND FUTURE DIRECTIONS
In conclusion, there is now good evidence that a number of non-endogenous CB1/CB2 receptor ligands, including several that are widely used as research tools, can interact with certain established non-CB1, non-CB2 G protein-coupled receptors, transmitter gated channels, ion channels and/or nuclear receptors (Tables 2 to 5). Importantly, it has been found that
many of these receptors and channels are affected by CB1/CB2 receptor ligands only when these are applied at concentrations well above those at which they can activate or block CB1 and/or CB2 receptors;
certain of these receptors and channels seem to be targeted by some CB1/CB2 receptor agonists but not by others and
certain CB1/CB2 receptor agonists seem to target allosteric rather than orthosteric sites on at least some of the following receptors or channels: muscarinic acetylcholine receptors, μ and δ opioid receptors, 5-HT3 receptors, glycine receptors and sodium channels.
Table 5.
Evidence that Rimonabant, Taranabant, AM251 and LY320135 can Target Certain Established Receptors and/or Channels other than Cannabinoid CB1 or CB2 Receptors or GPR55
| Ligand | Receptor or channel | Effect§ | Concentration | Reference |
|---|---|---|---|---|
| Rimonabant | acetylcholine (muscarinic M1 & M4) | Displacement | >1 µM or >10 µM | [66] |
| adenosine A1 | Antagonism | 10 µM | [21] | |
| adenosine A3 | Displacement | IC50 = 1.5 µM | [25] | |
| α2A-adrenoceptors | Displacement | IC50 = 7.2 µM | [25] | |
| a2C-adrenoceptors | Displacement | IC50 = 3.6 µM | [25] | |
| angiotensin AT1 | Displacement | IC50 = 7.2 µM | [25] | |
| 5-HT6 | Displacement | IC50 =2.8 µM | [25] | |
| imidazoline | Antagonism | 1 µM | [73] | |
| μ opioid | Displacement | IC50 = 3.0 µM | [25] | |
| μ opioid | Displacement | IC50 = 4.1 µM | [67] | |
| μ opioid | Displacement | IC50 = 5.7 µM | [27] | |
| κ opioid | Displacement | IC50 = 3.9 µM | [25] | |
| prostanoid EP4 | Displacement | IC50 = 3.9 µM | [25] | |
| prostanoid FP | Displacement | IC50 = 2 µM | [25] | |
| prostanoid IP | Displacement | IC50 = 4.9 µM | [25] | |
| tachykinin NK2 | Displacement | IC50 = 2 µM | [25] | |
| TRPV1 | Antagonism | 2.5 & 5 µM | [109] | |
| TRPV1 | Antagonism | 10 & 30 µM | [108] | |
| TRPV1 | Activation | >50 µM | [108] | |
| L-type calcium (CaV1) channels | Displacement | IC50 = 6.1 µM | [25] | |
| T-type calcium (CaV3) channels | Inhibition | 100 nM, 1 µM | [84] | |
| potassium TASK-1 channels | Inhibition | 10 µM | [93] | |
| potassium KV channels | Inhibition | 10 µM | [91] | |
| potassium KV channels | Displacement | IC50 = 2.5 µM | [25] | |
| type-2 sodium channels | Displacement | IC50 = 5.1 µM | [25] | |
| PPARγ | Activation | 10 µM | [121] | |
| Taranabant | adenosine A3 | Displacement | IC50 = 3.4 µM | [25] |
| dopamine D1 | Displacement | Ki= 3.4 µM | [24] | |
| dopamine D3 | Displacement | Ki= 1.9 µM | [24] | |
| melatonin MT1 | Displacement | IC50 = 7.5 µM | [25] | |
| tachykinin NK2 | Displacement | IC50 = 500 nM | [25] | |
| L-type calcium (CaV1) channels | Displacement | IC50 = 300 nM | [25] | |
| potassium KV channels | Displacement | IC50 = 2.3 µM | [25] | |
| type-2 sodium channels | Displacement | IC50 = 1.9 µM | [25] | |
| AM251 | adenosine A1 | Antagonism | 10 µM | [21] |
| T-type calcium (CaV3) channels | Inhibition | 3 µM | [89] | |
| type-2 sodium channels | Inhibition | IC50 = 8.5, 8.9, 9.2 µM | [100] | |
| type-2 sodium channels | Displacement | IC50 = 11.2 µM | [100] | |
| type-2 sodium channels | Dissociation | 25 & 50 µM | [100] | |
| PPARγ | Activation | 10 µM | [121] | |
| LY320135 | imidazoline | Antagonism | 0.1 & 1 µM | [73] |
| 5-HT3A | Antagonism | IC50 = 523 nM | [78] |
Activation, activation of a receptor; Antagonism, antagonism of agonist-induced activation of a receptor; Displacement, displacement of a radioligand from a specific binding site; Dissociation, acceleration of dissociation of a radioligand from a specific binding site; Inhibition, signs of inhibition of channel currents.
Since the CB1 receptor can signal through certain ion channels (reviewed in [1]), it should be noted as well that nearly all of the ligand-ion channel interactions described in this review seem not to have been mediated by this receptor.
It is also noteworthy that some channels and non-CB1, non-CB2 receptors appear to be activated by CB1/CB2 receptor antagonists or blocked by CB1/CB2 receptor agonists. This is exemplified by the antagonism of GPR55 and 5-HT3 receptors and the inhibition of certain ion channels that has been reported to be induced by some CB1/CB2 receptor agonists (Tables 2 to 4), and by the activation of GPR55 (Table 2) and PPARγ (Table 5) apparently induced by some CB1 receptor antagonists, at least in some investigations. It has also been found that not all CB1 agonists appear to affect particular non-CB1/CB2 targets in the same way. Thus, for example, glycine receptor subunit α1 activation seems to be potentiated by HU-210, inhibited by HU-308 and unaffected by R-(+)-WIN55212 (Tables 3 and 4) and there is also some evidence that R-(+)-WIN55212 does not share the ability of certain other CB1/CB2 receptor agonists to activate GPR55 (Table 2). It is noteworthy too that the rank order of the potencies displayed by CB1/CB2 receptor agonists, at least at some channels and non-CB1, non-CB2 receptors, differs from the rank order of the potencies they display as CB1 and/or CB2 receptor agonists. Thus, for example, there is a marked difference between the rank order of the potencies with which Δ9-THC, R-(+)-WIN55212, CP55940 and JWH-015 bind to CB1 or CB2 receptors (Table 1) and the rank order of the potencies with which these ligands block the 5-HT3 receptor (Tables 3 and 4).
Table 4.
Evidence that R-(+)-Methanandamide, ACEA, Noladin Ether, HU-210, JWH-015, HU-308 and AM1241 can Target Certain Established Receptors and/or Channels other than Cannabinoid CB1 or CB2 Receptors or GPR55
| Ligand | Receptor or channel | Effect§ | Concentration | Reference |
|---|---|---|---|---|
| R-(+)-MethAEA | acetylcholine (muscarinic) | Displacement | IC50 = 15 or 44 µM | [65] |
| acetylcholine (muscarinic M1 & M4) | Displacement | 3 & 10 µM | [66] | |
| acetylcholine (α7-nicotinic) | Inhibition | IC50 = 183 nM | [74] | |
| ionotropic glutamate | Potentiation | 1 µM | [75] | |
| L-type calcium (CaV1) channels | Displacement | IC50 = 7.1 µM | [86] | |
| T-type calcium (CaV3) channels | Inhibition | 1 & 10 µM | [84] [85] | |
| potassium (KATP) channels | Inhibition | 10 µM | [92] | |
| potassium TASK-1 channels | Inhibition | IC50 = 700 nM | [93] | |
| potassium TASK-3 channels | Inhibition | 1 & 10 µM | [95] | |
| potassium TASK-3 channels | Inhibition | 3 µM | [94] | |
| calcium-activated potassium (BK) channels | Potentiation | 300 nM to 3 µM | [98] | |
| potassium KV channels | Inhibition | 10 µM | [91] | |
| TRPV1 | Displacement | Ki = 21.4 µM | [105] | |
| PPARγ | Activation | 10 µM | [125] | |
| ACEA | T-type calcium (CaV3) channels | Inhibition | 10 µM | [85] |
| TRPV1 | Activation | EC50 = 14 µM | [108] | |
| Noladin ether | sodium channels | Inhibition | 50 µM | [102] |
| PPARα | Activation | 10 µM | [126] | |
| PPARα | Displacement | 10 & 32 µM | [126] | |
| PPARγ | Activation | 20 µM | [124] | |
| HU-210 | 5-HT2 | Potentiation | 500 nM | [63] |
| glycine (α1) | Potentiation | EC50 = 270 nM | [80] | |
| glycine (α1) | Potentiation | EC50 = 5.1 µM | [82] | |
| glycine (α1) | Activation | EC50 = 189 µM | [82] | |
| glycine (α2) | Inhibition | IC50 = 90 nM | [80] | |
| glycine (α3) | Inhibition | IC50 = 50 nM | [80] | |
| glycine (α1β) | Potentiation | 30 µM | [80] | |
| T-type calcium channels | Inhibition | 10 µM | [84] | |
| JWH-015 | 5-HT3A | Antagonism | IC50 = 147 nM | [78] |
| HU-308 | glycine (α1) | Inhibition | 30 µM | [80] |
| glycine (α2) | Inhibition | IC50 = 1.13 µM | [80] | |
| glycine (α3) | Inhibition | IC50 = 97 nM | [80] | |
| glycine (α1β) | Inhibition | 30 µM | [80] | |
| AM1241 | TRPA1 | Activation | 30 µM | [111] |
R-(+)-MethAEA, R-(+)-methanandamide.
Activation, activation of a receptor; Antagonism, antagonism of agonist-induced activation of a receptor; Displacement, displacement of a radioligand from a specific binding site; Inhibition, signs of inhibition of channel currents; Potentiation, potentiation of the effect of an agonist or enhancement of ion channel currents or ligand binding. The structures of the compounds listed in this Table are shown in Figs. (1, 2 or 3). The CB1 and CB2 Ki values of these compounds can be found in Table 1 and any reported ability they have to target GPR55 is indicated in Table 2.
Table 3.
Evidence that Δ 9-THC, CP55940 and R-(+)-WIN55212 can Target Certain Established Receptors and/or Channels other than Cannabinoid CB1 or CB2 Receptors or GPR55
| Ligand | Receptor or channel | Effect§ | Concentration | Reference |
|---|---|---|---|---|
| Δ9-THC | μ opioid† | Displacement | IC50 = 7 µM | [68] |
| μ opioid | Dissociation | EC50 = 21.4 µM | [67] | |
| δ opioid | Dissociation | EC50 = 10 µM | [67] | |
| β-adrenoceptor | Potentiation | 3 & 10 µM | [64] | |
| 5-HT3A | Antagonism | IC50 = 38 nM | [78] | |
| glycine (α1) | Potentiation | EC50 = 86 nM | [79] | |
| glycine (α1 β1) | Potentiation | EC50 = 73 nM | [79] | |
| glycine | Potentiation | EC50 = 115 nM | [79] | |
| T-type calcium (CaV3) channels | Inhibition | 1 µM | [89] | |
| potassium KV1.2 channels | Inhibition | IC50 = 2.4 µM | [90] | |
| TRPA1 | Activation | 20 µM | [112, 114] | |
| TRPA1 | Activation | 400 µM | [113] | |
| TRPV2 | Activation | EC50 = 16 & 43 µM | [115] | |
| PPARγ | Activation | 100 nM, 10 µM | [120] | |
| CP55940 | imidazoline | Activation | 300 nM | [71] |
| imidazoline | Activation | 1 & 10 µM | [73] | |
| acetylcholine (α7-nicotinic) | Inhibition | IC50 = 3.4 µM | [74] | |
| 5-HT3 | Antagonism | IC50 = 94 nM | [77] | |
| 5-HT3A | Antagonism | IC50 = 648 nM | [78] | |
| potassium TASK-1 channels | Inhibition | 10 µM | [93] | |
| type-2 sodium channels | Displacement | IC50 = 22.3 µM | [101] | |
| PPARγ | Activation | 10 µM | [121] | |
| R-(+)-WIN55212 | imidazoline | Activation | 10 & 100 µM | [71] |
| 5-HT3 | Antagonism | IC50 = 310 nM | [77] | |
| 5-HT3A | Antagonism | IC50 = 104 nM | [78] | |
| glycine (α2) | Inhibition | IC50 = 220 nM | [80] | |
| glycine (α3) | Inhibition | IC50 = 86 nM | [80] | |
| potassium TASK-1 channels | Inhibition | 10 µM | [93] | |
| potassium KV channels | Inhibition | 20 µM | [91] | |
| sodium channels | Potentiation | 10 nM | [103] | |
| sodium channels | Inhibition | 10 µM | [102] | |
| type-2 sodium channels | Inhibition | IC50 = 12.2, 14.4, 21.1 µM | [99] | |
| type-2 sodium channels | Displacement | IC50 = 19.5 µM | [99] | |
| TRPA1 | Activation | 25 µM | [111] | |
| PPARα | Activation | 1 µM | [126] | |
| PPARα | Displacement | 10 & 32 µM | [126] | |
| PPARγ | Activation | 100 nM | [122] | |
| PPARγ | Activation | 10 µM | [123] |
Activation, activation of a receptor; Antagonism, antagonism of agonist-induced activation of a receptor; Displacement, displacement of a radioligand from a specific binding site; Dissociation, acceleration of dissociation of a radioligand from a specific binding site; Inhibition, signs of inhibition of channel currents; Potentiation, potentiation of the effect of an agonist or enhancement of ion channel currents or ligand binding. The structures of the compounds listed in this Table are shown in Fig. (1). The CB1 and CB2 Ki values of these compounds can be found in Table 1 and any reported ability they have to target GPR55 is indicated in Table 2.
Binding to μ opioid receptors was also inhibited by the cannabinoids, cannabidiol (IC50 = 7 µM), hexahydrocannabinol (IC50 = 10 µM), cannabinol (IC50 = 35 µM), D- and Lnantradol (IC50 = 70 µM), 11-hydroxy-Δ9-THC (IC50 > 50 µM) and (+)-Δ9-THC (IC50 >50 µM) [68].
A few channels and non-CB1, non-CB2 receptors seem to be targeted by some non-endogenous CB1/CB2 receptor ligands with a potency little different from that with which these same ligands target CB1/CB2 receptors. These ligands include Δ9-THC for its inhibition of the 5-HT3 receptor and activation of PPARγ (Table 3), and R-(+)-methanandamide for its inhibition of the α7-nicotinic acetylcholine receptor (Table 4). Additionally, in one investigation [57], Δ9-THC, CP55940 and noladin ether were found to be no less potent as apparent GPR55 agonists than as CB1/CB2 receptor ligands (Tables 1 and 2).
The question of whether the G protein-coupled receptor, GPR55, really is targeted by CB1/CB2 receptor agonists and antagonists is currently a subject of heated debate (reviewed in [49, 127]). This is because several of these ligands have been found in some investigations to display activity as agonists but in other investigations to lack such activity or even to behave as GPR55 antagonists (Table 2). The potencies displayed by some of these ligands as apparent GPR55 agonists also vary widely from bioassay to bioassay. Clearly further research is required both to establish why such discrepant and variable data have been obtained and to identify a facile bioassay that will provide a reliable indication of how any particular ligand interacts with naturally expressed GPR55. It will also be important to discover a selective and potent GPR55 competitive antagonist as the availability of such a compound would make it possible to establish much more conclusively whether some CB1/CB2 receptor agonists and antagonists are or are not GPR55 agonists. One possible lead compound for such an antagonist may be cannabidiol, since as already mentioned this has been found in two investigations [56, 57], though not in a third [55], to behave as a moderately potent GPR55 antagonist.
Further research is also needed
to establish whether non-endogenous CB1/CB2 receptor agonists or antagonists activate or block any of the channels or non-CB1, non-CB2 receptors to which they have so far only been shown to bind (Tables 3 to 5);
to seek out any additional channels and non-CB1, non-CB2 receptors that are targeted by non-endogenous CB1/CB2 receptor ligands and to establish whether or not any such targeting is orthosteric or allosteric in nature and
to identify non-CB1, non-CB2 targets for any non-endogenous CB1/CB2 receptor ligands not so far investigated for their abilities to interact with such targets, focusing particularly on ligands with therapeutic potential such as certain CB1 receptor neutral antagonists, peripherally-restricted CB1/CB2 receptor agonists and antagonists, CB2-selective agonists and CB2-selective antagonists/inverse agonists.
If a non-endogenous CB1/CB2 receptor ligand interacts as potently with a channel or non-CB1, non-CB2 receptor as with the CB1 or CB2 receptor this will of course limit its value as a pharmacological tool. It may also either weaken or strengthen any therapeutic potential that it may have. Thus, such simultaneous targeting might increase the incidence or intensity either of adverse effects or of beneficial effects, there being evidence, for example, that pain relief is increased in an additive or superadditive manner by the combined activation of opioid receptors, nicotinic acetylcholine receptors or α2-adrenoceptors and cannabinoid receptors (reviewed in [6]).
Finally, it will be important to explore the ability of CB1/CB2 receptor ligands to interact with targets that are neither receptors nor channels, not least because there is already evidence that nanomolar concentrations of Δ9-THC can modulate the neuronal uptake of noradrenaline and serotonin and the microglial uptake of adenosine (reviewed in [41]), that rimonabant and taranabant can each interact with the noradrenaline transporter and that rimonabant can also interact both with dopamine and vesicular monoamine transporters and with 11β-hydroxysteroid dehydrogenase type 1 which catalyses the conversion of cortisone to cortisol [25].
ACKNOWLEDGEMENTS
The writing of this review was supported by grants from the National Institute on Drug Abuse (DA-03672) and from GW Pharmaceuticals.
ABBREVIATIONS
- 5-HT
5-hydroxytryptamine
- abnormal-cannabidiol
trans-4-[3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol
- ACEA
arachidonyl-2’-chloroethylamide
- ACPA
arachidonylcyclopropylamide
- anandamide
arachidonoylethanolamide
- BTX-B
batrachotoxinin A 20-α-benzoate
- CaV
voltage-gated calcium channel
- CGRP
calcitonin gene-related peptide
- CHO
Chinese hamster ovary
- DAMGO
D-Ala2, NMePhe4, Gly-ol]enkephalin
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- EDG
endothelial differentiation gene
- ERK
extracellular signal-regulated kinase
- GR65630
(3-5-methyl-1H-imidazol-4-yl)-1-(1-methyl-1H-indol-3-yl)-1-propanone)
- GTPγS
guanosine-5’-O-(3-thiotriphosphate)
- GW9662
2-chloro-5-nitro-N-phenylbenzamide
- HEK
human embryonic kidney
- KV
voltage-gated potassium channel
- LPI
lysophosphatidylinositol
- O-1602
trans-4-[3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-methyl-1,3-benzenediol
- O-2050
(6aR,10aR)-3-(1-methanesulphonylamino-4-hexyn-6-yl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran
- PKCβII
protein kinase CβII
- PPAR
peroxisome proliferator-activated receptor
- T0070907
2-chloro-5-nitro-N-4-pyridinylbenzamide
- TASK
two-pore domain acid-sensitive background potassium channel
- TG
trigeminal ganglion
- TRP
transient receptor potential cation channel
REFERENCES
- 1.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 2.Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br. J. Pharmacol. 2006;147:S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pertwee RG. Pharmacological actions of cannabinoids. In: Pertwee RG, editor. Cannabinoids. Handbook of Experimental Pharmacology. Vol. 168. Heidelberg: Springer-Verlag; 2005. pp. 1–51. [DOI] [PubMed] [Google Scholar]
- 4.Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005;7:E625–E654. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pertwee RG. Cannabinoids and multiple sclerosis. Mol. Neurobiol. 2007;36:45–59. doi: 10.1007/s12035-007-0005-2. [DOI] [PubMed] [Google Scholar]
- 6.Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poso A, Huffman JW. Targeting the cannabinoid CB2 receptor: modelling and structural determinants of CB2 selective ligands. Br. J. Pharmacol. 2008;153:335–346. doi: 10.1038/sj.bjp.0707567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marriott K-SC, Huffman JW. Recent advances in the development of selective ligands for the cannabinoid CB2 receptor. Curr. Top. Med. Chem. 2008;8:187–204. doi: 10.2174/156802608783498014. [DOI] [PubMed] [Google Scholar]
- 9.Després J-P, Lemieux I, Alméras N. Contribution of CB1 blockade to the management of high-risk abdominal obesity. Int. J. Obes. 2006;30:S44–S52. doi: 10.1038/sj.ijo.0803278. [DOI] [PubMed] [Google Scholar]
- 10.Lange JHM, Kruse CG. Cannabinoid CB1 receptor antagonists in therapeutic and structural perspectives. Chemical Record. 2008;8:156–168. doi: 10.1002/tcr.20147. [DOI] [PubMed] [Google Scholar]
- 11.Lee H-K, Choi EB, Pak CS. The current status and future perspectives of studies of cannabinoid receptor 1 antagonists as anti-obesity agents. Curr. Top. Med. Chem. 2009;9:482–503. doi: 10.2174/156802609788897844. [DOI] [PubMed] [Google Scholar]
- 12.Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- 13.Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict. Biol. 2008;13:147–159. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- 14.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 15.Hillard CJ, Manna S, Greenberg MJ, Dicamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J. Pharmacol. Exp. Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- 16.Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J. Med. Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- 17.Hanuš L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradshaw HB, Walker JM. The expanding field of cannabimimetic and related lipid mediators. Br. J. Pharmacol. 2005;144:459–465. doi: 10.1038/sj.bjp.0706093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suhara Y, Nakane S, Arai S, Takayama H, Waku K, Ishima Y, Sugiura T. Synthesis and biological activities of novel structural analogues of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Bioorg. Med. Chem. Lett. 2001;11:1985–1988. doi: 10.1016/s0960-894x(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 20.Suhara Y, Takayama H, Nakane S, Miyashita T, Waku K, Sugiura T. Synthesis and biological activities of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, and its metabolically stable ether-linked analogues. Chem. Pharm. Bull. 2000;48:903–907. doi: 10.1248/cpb.48.903. [DOI] [PubMed] [Google Scholar]
- 21.Savinainen JR, Saario SM, Niemi R, Järvinen T, Laitinen JT. An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors. Br. J. Pharmacol. 2003;140:1451–1459. doi: 10.1038/sj.bjp.0705577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savinainen JR, Järvinen T, Laine K, Laitinen JT. Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB1 receptor-dependent G-protein activation in rat cerebellar membranes. Br. J. Pharmacol. 2001;134:664–672. doi: 10.1038/sj.bjp.0704297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Fong TM, Guan X-M, Marsh DJ, Shen C-P, Stribling DS, Rosko KM, Lao J, Yu H, Feng Y, Xiao JC, Van der Ploeg LHT, Goulet MT, Hagmann WK, Lin LS, Lanza TJ, Jewell JP, Liu P, Shah SK, Qi H, Tong X, Wang J, Xu SS, Francis B, Strack AM, MacIntyre DE, Shearman LP. Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1S,2S)-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-{[5-(trifluoromethyl)pyridin-2-yl]oxy}propanamide (MK-0364), in rodents. J. Pharmacol. Exp. Ther. 2007;321:1013–1022. doi: 10.1124/jpet.106.118737. [DOI] [PubMed] [Google Scholar]
- 25.Fong TM, Shearman LP, Stribling DS, Shu J, Lao J, Huang CR-R, Xiao JC, Shen C-P, Tyszkiewicz J, Strack AM, DeMaula C, Hubert M-F, Galijatovic-Idrizbegovic A, Owen R, Huber AC, Lanning CL. Pharmacological efficacy and safety profile of taranabant in preclinical species. Drug Dev. Res. 2009;70:349–362. [Google Scholar]
- 26.Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- 27.Cinar R, Szücs M. CB1 receptor-independent actions of SR141716 on G-protein signaling: coapplication with the µ-opioid agonist Tyr-D-AlaGly-(NMe)Phe-Gly-ol unmasks novel, pertussis toxin-insensitive opioid signaling in µ-opioid receptor-Chinese hamster ovary cells. J. Pharmacol. Exp. Ther. 2009;330:567–574. doi: 10.1124/jpet.109.152710. [DOI] [PubMed] [Google Scholar]
- 28.Iwamura H, Suzuki H, Ueda Y, Kaya T, Inaba T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J. Pharmacol. Exp. Ther. 2001;296:420–425. [PubMed] [Google Scholar]
- 29.Lunn CA, Reich E-P, Fine JS, Lavey B, Kozlowski JA, Hipkin RW, Lundell DJ, Bober L. Biology and therapeutic potential of cannabinoid CB2 receptor inverse agonists. Br. J. Pharmacol. 2008;153:226–239. doi: 10.1038/sj.bjp.0707480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maekawa T, Nojima H, Kuraishi Y, Aisaka K. The cannabinoid CB2 receptor inverse agonist JTE-907 suppresses spontaneous itch-associated responses of NC mice, a model of atopic dermatitis. Eur. J. Pharmacol. 2006;542:179–183. doi: 10.1016/j.ejphar.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 31.Sink KS, Vemuri VK, Wood J, Makriyannis A, Salamone JD. Oral bioavailability of the novel cannabinoid CB1 antagonist AM6527: effects on food-reinforced behavior and comparisons with AM4113. Pharmacol. Biochem. Behav. 2009;91:303–306. doi: 10.1016/j.pbb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sink KS, McLaughlin PJ, Wood JAT, Brown C, Fan P, Vemuri VK, Pang Y, Olzewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:946–955. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurst DP, Lynch DL, Barnett-Norris J, Hyatt SM, Seltzman HH, Zhong M, Song Z-H, Nie J, Lewis D, Reggio PH. N-(Piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Mol. Pharmacol. 2002;62:1274–1287. doi: 10.1124/mol.62.6.1274. [DOI] [PubMed] [Google Scholar]
- 34.Hurst D, Umejiego U, Lynch D, Seltzman H, Hyatt S, Roche M, McAllister S, Fleischer D, Kapur A, Abood M, Shi S, Jones J, Lewis D, Reggio P. Biarylpyrazole inverse agonists at the cannabinoid CB1 receptor: importance of the C-3 carboxamide oxygen/lysine3.28(192) interaction. J. Med. Chem. 2006;49:5969–5987. doi: 10.1021/jm060446b. [DOI] [PubMed] [Google Scholar]
- 35.Ruiu S, Pinna GA, Marchese G, Mussinu J-M, Saba P, Tambaro S, Casti P, Vargiu R, Pani L. Synthesis and characterization of NESS 0327: a novel putative antagonist of the CB1 cannabinoid receptor. J. Pharmacol. Exp. Ther. 2003;306:363–370. doi: 10.1124/jpet.103.049924. [DOI] [PubMed] [Google Scholar]
- 36.Tambaro S, Mongeau R, Dessi C, Pani L, Ruiu S. Modulation of ATP-mediated contractions of the rat vas deferens through presynaptic cannabinoid receptors. Eur. J. Pharmacol. 2005;525:150–153. doi: 10.1016/j.ejphar.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 37.Ooms F, Wouters J, Oscari O, Happaerts T, Bouchard G, Carrupt P-A, Testa B, Lambert DM. Exploration of the pharmacophore of 3-alkyl-5-arylimidazolidinediones as new CB1 cannabinoid receptor ligands and potential antagonists: synthesis, lipophilicity, affinity, and molecular modeling. J. Med. Chem. 2002;45:1748–1756. doi: 10.1021/jm010896y. [DOI] [PubMed] [Google Scholar]
- 38.Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas A, Stevenson LA, Wease KN, Price MR, Baillie G, Ross RA, Pertwee RG. Evidence that the plant cannabinoid Δ9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br. J. Pharmacol. 2005;146:917–926. doi: 10.1038/sj.bjp.0706414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pertwee RG, Thomas A, Stevenson LA, Ross RA, Varvel SA, Lichtman AH, Martin BR, Razdan RK. The psychoactive plant cannabinoid, Δ9-tetrahydrocannabinol, is antagonized by Δ8- and Δ9-tetrahydrocannabivarin in mice in vivo. Br. J. Pharmacol. 2007;150:586–594. doi: 10.1038/sj.bjp.0707124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, Makriyannis A, Sharkey KA. A neutral CB1 receptor antagonist reduces weight gain in rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R2185–R2193. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- 43.Govaerts SJ, Hermans E, Lambert DM. Comparison of cannabinoid ligands affinities and efficacies in murine tissues and in transfected cells expressing human recombinant cannabinoid receptors. Eur. J. Pharm. Sci. 2004;23:233–243. doi: 10.1016/j.ejps.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Savinainen JR, Kokkola T, Salo OMH, Poso A, Järvinen T, Laitinen JT. Identification of WIN55212-3 as a competitive neutral antagonist of the human cannabinoid CB2 receptor. Br. J. Pharmacol. 2005;145:636–645. doi: 10.1038/sj.bjp.0706230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brizzi A, Brizzi V, Cascio MG, Bisogno T, Sirianni R, Di Marzo V. Design, synthesis, and binding studies of new potent ligands of cannabinoid receptors. J. Med. Chem. 2005;48:7343–7350. doi: 10.1021/jm0501533. [DOI] [PubMed] [Google Scholar]
- 46.Cascio MG, Bisogno T, Palazzo E, Thomas A, van der Stelt M, Brizzi A, de Novellis V, Marabese I, Ross R, van de Doelen T, Brizzi V, Pertwee R, Maione S, Di Marzo V. In vitro and in vivo pharmacology of synthetic olivetol- or resorcinol-derived cannabinoid receptor ligands. Br. J. Pharmacol. 2006;149:431–440. doi: 10.1038/sj.bjp.0706888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, Nguyen DG, Caldwell JS, Chen YA. Lipid G protein-coupled receptor ligand identification using β-arrestin PathHunter™ assay. J. Biol. Chem. 2009;284:12328–12338. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauckner JE, Jensen JB, Chen H-Y, Lu H-C, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross RA. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009;30:156–163. doi: 10.1016/j.tips.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 51.Oka S, Toshida T, Maruyama K, Nakajima K, Yamashita A, Sugiura T. 2-Arachidonoyl-sn-glycero-3-phosphoinositol: a possible natural ligand for GPR55. J. Biochem. 2009;145:13–20. doi: 10.1093/jb/mvn136. [DOI] [PubMed] [Google Scholar]
- 52.Henstridge CM, Balenga NAB, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand L-α-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009;23:183–193. doi: 10.1096/fj.08-108670. [DOI] [PubMed] [Google Scholar]
- 53.Henstridge C, Arthur S, Irving A. Lack of specificity for cannabinoid CB1 receptor antagonists: interactions with GPR55. 19th Annual Symposium on the Cannabinoids, Burlington, Vermont, International Cannabinoid Research Society; 2009. p. 20. [Google Scholar]
- 54.Eldeeb K, Alexander S, Pritchard D, Kendall D. LPI-evoked increases in intracellular calcium increases in microglial cells in culture. 19th Annual Symposium on the Cannabinoids, Burlington, Vermont, International Cannabinoid Research Society; 2009. p. 21. [Google Scholar]
- 55.Kapur A, Zhao PW, Sharir H, Bai YS, Caron MG, Barak LS, Abood ME. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J. Biol. Chem. 2009;284:29817–29827. doi: 10.1074/jbc.M109.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whyte LS, Ryberg E, Sims NA, Ridge SA, Mackie K, Greasley PJ, Ross RA, Rogers MJ. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. USA. 2009;106:16511–16516. doi: 10.1073/pnas.0902743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue T-L, Brown AJ, Morrison AD, Douglas SA. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br. J. Pharmacol. 2007;152:825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol. Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Balenga NS, chröder R, Platzer W, Kargl J, Henstridge CM, Irving AJ, Kostenis E, Waldhoer M. Functional selectivity of cannabinoids on the novel cannabinoid receptor GPR55. 19th Annual Symposium on the Cannabinoids, Burlington, Vermont, International Cannabinoid Research Society; 2009. p. 23. [Google Scholar]
- 61.Staton PC, Hatcher JP, Walker DJ, Morrison AD, Shapland EM, Hughes JP, Chong E, Mander PK, Green PJ, Billinton A, Fulleylove M, Lancaster HC, Smith JC, Bailey LT, Wise A, Brown AJ, Richardson JC, Chessell IP. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain. 2008;139:225–236. doi: 10.1016/j.pain.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Kimura T, Yamamoto I, Ohta T, Yoshida H, Watanabe K, Ho IK, Yoshimura H. Changes in 5-HT receptor binding induced by tetrahydrocannabinol metabolites in bovine cerebral cortex. Res. Commun. Alcohol Subst. Abuse. 1996;17:57–69. [Google Scholar]
- 63.Cheer JF, Cadogan A-K, Marsden CA, Fone KCF, Kendall DA. Modification of 5-HT2 receptor mediated behaviour in the rat by oleamide and the role of cannabinoid receptors. Neuropharmacology. 1999;38:533–541. doi: 10.1016/s0028-3908(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 64.Hillard CJ, Bloom AS. Δ9-tetrahydrocannabinol-induced changes in β-adrenergic receptor binding in mouse cerebral cortex. Brain Res. 1982;235:370–377. doi: 10.1016/0006-8993(82)91016-2. [DOI] [PubMed] [Google Scholar]
- 65.Lagalwar S, Bordayo EZ, Hoffmann KL, Fawcett JR, Frey WH. Anandamides inhibit binding to the muscarinic acetylcholine receptor. J. Mol. Neurosci. 1999;13:55–61. doi: 10.1385/JMN:13:1-2:55. [DOI] [PubMed] [Google Scholar]
- 66.Christopoulos A, Wilson K. Interaction of anandamide with the M1 and M4 muscarinic acetylcholine receptors. Brain Res. 2001;915:70–78. doi: 10.1016/s0006-8993(01)02825-6. [DOI] [PubMed] [Google Scholar]
- 67.Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2006;372:354–361. doi: 10.1007/s00210-006-0033-x. [DOI] [PubMed] [Google Scholar]
- 68.Vaysse PJ-J, Gardner EL, Zukin RS. Modulation of rat brain opioid receptors by cannabinoids. J. Pharmac. Exp. Ther. 1987;241:534–539. [PubMed] [Google Scholar]
- 69.Pertwee RG. Novel pharmacological targets for cannabinoids. Curr. Neuropharmacol. 2004;2:9–29. [Google Scholar]
- 70.Göthert M, Brüss M, Bönisch H, Molderings GJ. Presynaptic imidazoline receptors. New developments in characterization and classification. Ann. N. Y. Acad. Sci. 1999;881:171–184. doi: 10.1111/j.1749-6632.1999.tb09356.x. [DOI] [PubMed] [Google Scholar]
- 71.Molderings GJ, Bönisch H, Hammermann R, Göthert M, Brüss M. Noradrenaline release-inhibiting receptors on PC12 cells devoid of α2- and CB1 receptors: similarities to presynaptic imidazoline and edg receptors. Neurochem. Int. 2002;40:157–167. doi: 10.1016/s0197-0186(01)00076-6. [DOI] [PubMed] [Google Scholar]
- 72.Molderings GJ, Göthert M. Imidazoline binding sites and receptors in cardiovascular tissue. Gen. Pharmacol. 1999;32:17–22. doi: 10.1016/s0306-3623(98)00070-6. [DOI] [PubMed] [Google Scholar]
- 73.Molderings GJ, Likungu J, Göthert M. Presynaptic cannabinoid and imidazoline receptors in the human heart and their potential relationship. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:157–164. doi: 10.1007/s002109900043. [DOI] [PubMed] [Google Scholar]
- 74.Oz M, Zhang L, Ravindran A, Morales M, Lupica CR. Differential effects of endogenous and synthetic cannabinoids on α7-nicotinic acetylcholine receptor-mediated responses in Xenopus oocytes. J. Pharmacol. Exp. Ther. 2004;310:1152–1160. doi: 10.1124/jpet.104.067751. [DOI] [PubMed] [Google Scholar]
- 75.Hampson AJ, Bornheim LM, Scanziani M, Yost CS, Gray AT, Hansen BM, Leonoudakis DJ, Bickler PE. Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J. Neurochem. 1998;70:671–676. doi: 10.1046/j.1471-4159.1998.70020671.x. [DOI] [PubMed] [Google Scholar]
- 76.Oz M. Receptor-independent actions of cannabinoids on cell membranes: focus on endocannabinoids. Pharmacol. Ther. 2006;111:114–144. doi: 10.1016/j.pharmthera.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Fan P. Cannabinoid agonists inhibit the activation of 5-HT3 receptors in rat nodose ganglion neurons. J. Neurophysiol. 1995;73:907–910. doi: 10.1152/jn.1995.73.2.907. [DOI] [PubMed] [Google Scholar]
- 78.Barann M, Molderings G, Brüss M, Bönisch H, Urban BW, Göthert M. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br. J. Pharmacol. 2002;137:589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hejazi N, Zhou C, Oz M, Sun H, Ye JH, Zhang L. Δ9-Tetrahydrocannabinol and endogenous cannabinoid anandamide directly potentiate the function of glycine receptors. Mol. Pharmacol. 2006;69:991–997. doi: 10.1124/mol.105.019174. [DOI] [PubMed] [Google Scholar]
- 80.Yang Z, Aubrey KR, Alroy I, Harvey RJ, Vandenberg RJ, Lynch JW. Subunit-specific modulation of glycine receptors by cannabinoids and N-arachidonyl-glycine. Biochem. Pharmacol. 2008;76:1014–1023. doi: 10.1016/j.bcp.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 81.Lozovaya N, Yatsenko N, Beketov A, Tsintsadze T, Burnashev N. Glycine receptors in CNS neurons as a target for nonretrograde action of cannabinoids. J. Neurosci. 2005;25:7499–7506. doi: 10.1523/JNEUROSCI.0977-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Demir R, Leuwer M, de la Roche J, Krampfl K, Foadi N, Karst M, Dengler R, Haeseler G, Ahrens J. Modulation of glycine receptor function by the synthetic cannabinoid HU210. Pharmacology. 2009;83:270–274. doi: 10.1159/000209291. [DOI] [PubMed] [Google Scholar]
- 83.Shen M, Thayer SA. The cannabinoid agonist Win55,212-2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Res. 1998;783:77–84. doi: 10.1016/s0006-8993(97)01195-5. [DOI] [PubMed] [Google Scholar]
- 84.Chemin J, Monteil A, Perez-Reyes E, Nargeot J, Lory P. Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J. 2001;20:7033–7040. doi: 10.1093/emboj/20.24.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chemin J, Nargeot J, Lory P. Chemical determinants involved in anandamide-induced inhibition of T-type calcium channels. J. Biol. Chem. 2007;282:2314–2323. doi: 10.1074/jbc.M610033200. [DOI] [PubMed] [Google Scholar]
- 86.Oz M, Tchugunova Y, Dinc M. Differential effects of endogenous and synthetic cannabinoids on voltage-dependent calcium fluxes in rabbit T-tubule membranes: comparison with fatty acids. Eur. J. Pharmacol. 2004;502:47–58. doi: 10.1016/j.ejphar.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 87.Oz M, Tchugunova YB, Dunn SMJ. Endogenous cannabinoid anandamide directly inhibits voltage-dependent Ca2+ fluxes in rabbit T-tubule membranes. Eur. J. Pharmacol. 2000;404:13–20. doi: 10.1016/s0014-2999(00)00396-4. [DOI] [PubMed] [Google Scholar]
- 88.Evans RM, Scott RH, Ross RA. Multiple actions of anandamide on neonatal rat cultured sensory neurones. Br. J. Pharmacol. 2004;141:1223–1233. doi: 10.1038/sj.bjp.0705723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ross HR, Napier I, Connor M. Inhibition of recombinant human T-type calcium channels by Δ9-tetrahydrocannabinol and cannabidiol. J. Biol. Chem. 2008;283:16124–16134. doi: 10.1074/jbc.M707104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poling JS, Rogawski MA, Salem N, Vicini S. Anandamide, an endogenous cannabinoid, inhibits Shaker-related voltage-gated K+ channels. Neuropharmacology. 1996;35:983–991. doi: 10.1016/0028-3908(96)00130-x. [DOI] [PubMed] [Google Scholar]
- 91.Van den Bossche I, Vanheel B. Influence of cannabinoids on the delayed rectifier in freshly dissociated smooth muscle cells of the rat aorta. Br. J. Pharmacol. 2000;131:85–93. doi: 10.1038/sj.bjp.0703521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oz M, Yang K-H, Dinc M, Shippenberg TS. The endogenous cannabinoid anandamide inhibits cromakalim-activated K+ currents in follicle-enclosed Xenopus oocytes. J. Pharmacol. Exp. Ther. 2007;323:547–554. doi: 10.1124/jpet.107.125336. [DOI] [PubMed] [Google Scholar]
- 93.Maingret F, Patel AJ, Lazdunski M, Honoré E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Veale EL, Buswell R, Clarke CE, Mathie A. Identification of a region in the TASK3 two pore domain potassium channel that is critical for its blockade by methanandamide. Br. J. Pharmacol. 2007;152:778–786. doi: 10.1038/sj.bjp.0707436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J. Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Linden A-M, Sandu C, Aller MI, Vekovischeva OY, Rosenberg PH, Wisden W, Korpi ER. TASK-3 knockout mice exhibit exaggerated nocturnal activity, impairments in cognitive functions, and reduced sensitivity to inhalation anesthetics. J. Pharmacol. Exp. Ther. 2007;323:924–934. doi: 10.1124/jpet.107.129544. [DOI] [PubMed] [Google Scholar]
- 97.Linden A-M, Aller MI, Leppä E, Vekovischeva O, Aitta-Aho T, Veale EL, Mathie A, Rosenberg P, Wisden W, Korpi ER. The in vivo contributions of TASK-1-containing channels to the actions of inhalation anesthetics, the α2 adrenergic sedative dexmedetomidine, and cannabinoid agonists. J. Pharmacol. Exp. Ther. 2006;317:615–626. doi: 10.1124/jpet.105.098525. [DOI] [PubMed] [Google Scholar]
- 98.Sade H, Muraki K, Ohya S, Hatano N, Imaizumi Y. Activation of large-conductance, Ca2+-activated K+ channels by cannabinoids. Am. J. Physiol. Cell Physiol. 2006;290:C77–C86. doi: 10.1152/ajpcell.00482.2004. [DOI] [PubMed] [Google Scholar]
- 99.Nicholson RA, Liao C, Zheng J, David LS, Coyne L, Errington AC, Singh G, Lees G. Sodium channel inhibition by anandamide and synthetic cannabimimetics in brain. Brain Res. 2003;978:194–204. doi: 10.1016/s0006-8993(03)02808-7. [DOI] [PubMed] [Google Scholar]
- 100.Liao C, Zheng J, David LS, Nicholson RA. Inhibition of voltage-sensitive sodium channels by the cannabinoid 1 receptor antagonist AM 251 in mammalian brain. Pharmacol. Toxicol. 2004;94:73–78. doi: 10.1111/j.1742-7843.2004.pto940204.x. [DOI] [PubMed] [Google Scholar]
- 101.Duan Y, Liao C, Jain S, Nicholson RA. The cannabinoid receptor agonist CP-55,940 and ethyl arachidonate interfere with [3H]batrachotoxinin A 20 α-benzoate binding to sodium channels and inhibit sodium channel function. Comp. Biochem. Physiol., Part C. 2008;148:244–249. doi: 10.1016/j.cbpc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 102.Okada Y, Imendra KG, Miyazaki T, Hotokezaka H, Fujiyama R, Zeredo JL, Miyamoto T, Toda K. Biophysical properties of voltage-gated Na+ channels in frog parathyroid cells and their modulation by cannabinoids. J. Exp. Biol. 2005;208:4747–4756. doi: 10.1242/jeb.01967. [DOI] [PubMed] [Google Scholar]
- 103.Fu H, Xiao JM, Cao XH, Ming ZY, Liu LJ. Effects of WIN55,212-2 on voltage-gated sodium channels in trigeminal ganglion neurons of rats. Neurol. Res. 2008;30:85–91. doi: 10.1179/016164107X228642. [DOI] [PubMed] [Google Scholar]
- 104.Di Marzo V, Bisogno T, Melck D, Ross R, Brockie H, Stevenson L, Pertwee R, De Petrocellis L. Interactions between synthetic vanilloids and the endogenous cannabinoid system. FEBS Letts. 1998;436:449–454. doi: 10.1016/s0014-5793(98)01175-2. [DOI] [PubMed] [Google Scholar]
- 105.Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, Di Marzo V, Pertwee RG. Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br. J. Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 107.Ralevic V, Kendall DA, Randall MD, Zygmunt PM, Movahed P, Högestätt ED. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br. J. Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl- dopamine and arachidonyl-2-chloroethylamide. Br. J. Pharmacol. 2004;141:1118–1130. doi: 10.1038/sj.bjp.0705711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agrò A, Di Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- 110.Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–759. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J. Neurosci. 2008;28:1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jordt S-E, Bautista DM, Chuang H, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 113.Hinman A, Chuang H, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience. 2008;154:1467–1476. doi: 10.1016/j.neuroscience.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 115.Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N. Activation properties of heterologously expressed mammalian TRPV2. Evidence for species dependence. J. Biol. Chem. 2007;282:15894–15902. doi: 10.1074/jbc.M608287200. [DOI] [PubMed] [Google Scholar]
- 116.Wilkinson JD, Kendall DA, Ralevic V. Δ9-Tetrahydrocannabinol inhibits electrically-evoked CGRP release and capsaicin-sensitive sensory neurogenic vasodilatation in the rat mesenteric arterial bed. Br. J. Pharmacol. 2007;152:709–716. doi: 10.1038/sj.bjp.0707448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zygmunt PM, Andersson DA, Högestätt ED. Δ9-tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J. Neurosci. 2002;22:4720–4727. doi: 10.1523/JNEUROSCI.22-11-04720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Cannabinoid receptor-independent actions of the aminoalkylindole WIN 55,212-2 on trigeminal sensory neurons. Br. J. Pharmacol. 2004;142:257–266. doi: 10.1038/sj.bjp.0705778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur. J. Neurosci. 2009;29:1568–1578. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O'Sullivan SE, Tarling EJ, Bennett AJ, Kendall DA, Randall MD. Novel time-dependent vascular actions of Δ9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochem. Biophys. Res. Commun. 2005;337:824–831. doi: 10.1016/j.bbrc.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 121.O'Sullivan SE, Bennett AJ, Kendall DA, Randall MD. Cannabinoid ligands as activators of peroxisome proliferator-activated receptor gamma (PPARγ). 16th Annual Symposium on the Cannabinoids; Burlington, Vermont. International Cannabinoid Research Society; 2006. p. 59. [Google Scholar]
- 122.Mestre L, Docagne F, Correa F, Loría F, Hernangómez M, Borrell J, Guaza C. A cannabinoid agonist interferes with the progression of a chronic model of multiple sclerosis by downregulating adhesion molecules. Mol. Cell. Neurosci. 2009;40:258–266. doi: 10.1016/j.mcn.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 123.Giuliano M, Pellerito O, Portanova P, Calvaruso G, Santulli A, De Blasio A, Vento R, Tesoriere G. Apoptosis induced in HepG2 cells by the synthetic cannabinoid WIN: involvement of the transcription factor PPARγ. Biochimie. 2009;91:457–465. doi: 10.1016/j.biochi.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 124.Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor γ independently of cannabinoid receptors 1 and 2. Mol. Pharmacol. 2006;70:101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- 125.Eichele K, Ramer R, Hinz B. R(+)-Methanandamide-induced apoptosis of human cervical carcinoma cells involves a cyclooxygenase-2-dependent pathway. Pharm. Res. 2009;26:346–355. doi: 10.1007/s11095-008-9748-3. [DOI] [PubMed] [Google Scholar]
- 126.Sun Y, Alexander SPH, Garle MJ, Gibson CL, Hewitt K, Murphy SP, Kendall DA, Bennett AJ. Cannabinoid activation of PPARα a novel neuroprotective mechanism. Br. J. Pharmacol. 2007;152:734–743. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pertwee RG. GPR55: a new member of the cannabinoid receptor clan? Br. J. Pharmacol. 2007;152:984–986. doi: 10.1038/sj.bjp.0707464. [DOI] [PMC free article] [PubMed] [Google Scholar]