Abstract
Objective
The goal of this research was to examine the extent to which 10-year breast cancer survivors integrated cancer into their self-concept (i.e., survivor centrality), identify predictors of survivor centrality, and determine the relation of survivor centrality to well-being.
Methods
Breast cancer survivors (n = 240) were interviewed 10 years following the initial diagnosis. They completed measures of survivor centrality, illness valence (i.e., positive or negative views of illness), and well-being (positive and negative affect, mental and physical functioning, psychological distress, benefit-finding).
Results
There were few predictors of the kinds of women who were more likely to integrate breast cancer into their self-concepts, but survivor centrality was related to engaging in behaviors that suggested survivorship was relevant to women’s daily lives, such as becoming involved in breast cancer activities. Survivor centrality was related to three markers of negative psychological well-being: more negative affect, poorer mental functioning, and greater psychological distress. However, in the case of negative affect and psychological distress, this relation was moderated by illness valence, such that survivor centrality was only related to negative psychological well-being when the illness was viewed in less positive terms.
Conclusions
Women vary in the extent to which they define themselves in terms of the breast cancer experience. Survivor centrality in and of itself is not always indicative of adjustment to disease. When women have a more negative view of being a breast cancer survivor, survivor centrality is more likely to signify potential problems.
Keywords: breast cancer, survivorship, self-concept, well-being
Scientists in the area of psycho-oncology have been studying women’s adjustment to the diagnosis and treatment of cancer for several decades now (1–4). With the improvement in early detection procedures and the development of more effective treatments, the prognosis for breast cancer has improved dramatically. Thus, researchers have begun to examine survivorship issues. Whereas most studies show that survivors, on average, do not show elevated levels of general distress, such as anxiety or depression, compared to healthy controls or population norms (5–7), moderate levels of cancer-specific worries persist (8). General quality of life measures do not capture the subtleties in long-term survivorship (7). With time, stress related to the diagnosis and treatment of cancer diminishes and some of the memories associated with the trauma wane, but other sources of stress persist. Survivorship issues have centered on fears of recurrence, the long-term effects of treatment, concerns about a shortened life span, impact on body image, practical issues of employability and insurance (8–12), and, finally, the consequences of having had cancer for one’s self-concept. It is the impact of cancer on the self-concept that is the subject of the present study.
The diagnosis and treatment for cancer leads to the development of a new social role—the role of cancer survivor—and that role persists for the rest of one’s life (13). That is, cancer is not an acute, discrete event with a defined ending, but a process that extends for the rest of the lifespan (14,15). Regardless of the type of cancer, the severity of disease, or the length of survival, cancer survivors need to figure out how cancer changes the self and what its implications are for daily life. According to Zebrack (13), the way that cancer survivors see themselves in relation to the world has consequences for their well-being. However, little is known about how or whether people integrate cancer into their self-concept, distinguish those who do integrate cancer into their self-concept from those who do not, and understand the implications of a cancer self-concept for quality of life (13). The purpose of the present research is to examine the extent to which breast cancer is central to 10-year survivors’ identity (i.e., survivor centrality), to examine the determinants of survivor centrality, and to determine if survivor centrality is associated with positive or negative indices of well-being.
There are many dimensions of illness that have been explored by previous research, such as the perception of an illness’s consequences, perceptions of personal control over illness, and attributions for illness (16–18). There also are aspects of illness self-concept that have been assessed, including the extent to which one feels good about the self and the stigma associated with illness (19,20). Researchers often ask one of two questions. First, how does cancer affect one’s self-concept, typically operationalized as self-esteem (21,22)? Second, how do aspects of self-concept affect adjustment to cancer (19,23)? One study examined illness self-concepts by asking women who had undergone bone marrow transplants (BMT) to rate a person who has had a bone marrow transplant on a set of items and then to rate themselves on the same set of items (24). Correspondence between the two sets of descriptors was considered to reflect BMT self-concept. BMT self-concept was related to greater distress, as indicated by more intrusive thoughts about the illness. However, none of these studies seem to address the extent to which an illness is integrated into the self, or survivor centrality.
Despite the lack of research on survivor centrality, health care professionals have learned that the integration of an illness, or surviving an illness, into one’s identity is a theme that emerges from discussions with cancer survivors. In a qualitative study of adult survivors of childhood cancer, survivors frequently discussed the extent to which the cancer came to identify themselves (25). In another interview study of adult survivors of childhood cancer, some individuals said that they defined themselves in terms of the cancer and saw the cancer as integral to their self (26). In one study, 15 men and women with a history of cancer were interviewed and issues of identity and changes in identity were explored (27). For some of the people, survivorship was a defining part of their identities (“I am a survivor”). In her research on chronic illness, Charmaz (28) discusses the extent to which people incorporate the illness into their self-concepts, distinguishing between people who define themselves in terms of the illness and people who try to separate the illness from the rest of their lives.
Wiebe et al. (29) suggested that the impact of illness centrality on health outcomes would depend on the individual’s attitude toward the illness, that is, whether the individual perceives the illness in positive or negative terms. In a study of children with diabetes, they predicted and found that the centrality of diabetes was related to more depressive symptoms and poor metabolic control only when the illness was perceived in highly negative terms. In another study of adolescents with diabetes, this pattern of findings was replicated—but only for females (30).
In the case of breast cancer, will women’s attitude toward having had this disease be positive or negative? Research shows that people without cancer tend to have more negative perceptions of cancer and stigmatize the illness more than people with cancer (31). This is partly due to the negative portrayal of cancer and exaggeration of the consequences of cancer by the media (32). Studies have shown that women without breast cancer perceive cancer as less controllable and overestimate the negative consequences (physical, social, economic) compared to women with breast cancer (33,34). This is one of the reasons that cancer is stigmatized (35). Cancer also is stigmatized because it is an illness that is poorly understood, greatly feared, and evokes images of mortality (35–37). Several studies have shown that people without cancer tend to make more internal attributions and less chance attributions than people with cancer (33,34), most likely because locating the blame within another person removes the threat to the self. Thus, one author concluded that people with cancer end up being stigmatized for having cancer and for causing cancer (34).
However, the heightened survivorship from cancer has led to a decrease in the stigma attached to cancer (15). There is a body of research that suggests cancer survivors perceive the illness in positive terms, in part by experiencing positive changes from the illness (38). This literature has come to be known as benefit-finding, stress-related growth, and post-traumatic growth (39). Persons with cancer commonly report an enhanced appreciation of life, closer relationships, increased spirituality, a shift in priorities, and increased personal strength (38). In terms of identities, a study of women under active treatment for cancer showed that many women described themselves in positive terms that they would not have ascribed to themselves prior to the cancer, such as “strong” and “fighter” (40). An emerging controversy in that literature is the extent to which these reported positive experiences are actual or illusory (41,52).
What are the implications of defining oneself in terms of an illness or illness survivorship for adjustment to disease? Is it psychologically adaptive to view cancer survivorship as part of the self, or to place having had cancer in the past and separate it from the self? Are the breast cancer survivors who seem to identify themselves with surviving the illness by wearing pink ribbons, openly discussing their illness experience with newly diagnosed women, and participating in breast cancer activities better or worse off for having adopted these attitudes and behaviors? One might believe that these women are successfully adjusting to their disease because they are confronting it rather than denying it. However, one also could suggest that these women have not accepted their disease, are constantly reliving it, and are unable to put it behind them and move on with their lives. To the extent that having had breast cancer is viewed as a stigmatizing condition, the stigma literature predicts that survivor centrality will be related to more psychosocial difficulties (42). However, from the previous literature it is not clear that cancer survivors today view cancer as a stigmatizing condition.
There were three goals of the present study. First, we described the extent to which having had breast cancer is integrated into survivors’ self-concept versus segregated from the rest of their lives. The survivors are 10 years post diagnosis, making this study unique as most survivorship studies do not exceed 5 years (7). Second, we determined whether there are certain groups of women who are more or less likely to perceive themselves in terms of cancer survivorship by examining whether demographic or medical variables predict survivor centrality. One might expect that women who were diagnosed with more advanced disease or that women who have suffered a recurrence define themselves more in terms of the breast cancer. Younger women also might be more likely to identify with cancer survivorship because the experience of cancer is more stressful for younger women (8). Among cancer survivors, younger age has been related to worse mental health (7), but better physical health (7) and greater stress-related growth (43). Finally, we examined whether survivor centrality is related to well-being measures, specifically positive and negative affect, quality of life, intrusive and avoidant thoughts, and benefit-finding. We hypothesize that the extent to which women view breast cancer in positive or negative terms will moderate these associations. That is, survivor centrality will only be related to poor outcomes if the illness is perceived in negative terms.
Method
Participants
The data examined in this paper are drawn from 10-year follow-up interviews of women who were diagnosed with Stages 1, 2, or 3 breast cancer, treated with surgery followed by adjuvant chemotherapy, had no history of cancer (except skin cancer), and lived within a 1-hour radius of Pittsburgh. The majority (86%, or 312 or 364) of the women who were recruited into the study at diagnosis agreed to be randomly assigned to one of four arms of a clinical trial of support interventions (44). The remaining 14% continued to complete questionnaires over the course of the study. This study is based on our attempts to contact all of the women approximately 10 years after the initial interview.
At 10-year follow-up, we interviewed 240 of the original 364 (66%) women enrolled in the study. The other women had either died (22%), withdrawn from the study in the past or refused the 10-year interview (10%), or were unable to be located (2%). Neither participation in the randomized support intervention trial nor the specific support condition was associated with survival or participation in the 10-year follow-up interview. Of the 240 women we interviewed, 37 had sustained a recurrence and 10 had had another cancer. Demographic characteristics for these 240 women are shown in Table 1.
Table 1.
Descriptive Statistics on Demographic Variables (n = 240)
| Age | M = 58.58 (SD = 8.95) |
| Education | 2.1% less than high school |
| 32.3% high school graduate | |
| 25.1% some college | |
| 25.1% college graduate | |
| 15.5% post graduate school | |
| Race | 96% Caucasian |
| 4% African American | |
| .4% Hispanic | |
| Marital status | 69.2% married |
| 10.4% single | |
| 10.4% divorced | |
| 3.8% separated | |
| 6.3% widowed | |
| Work status | 60% currently working |
| 40% not working | |
| Stage at diagnosis | 31.3% stage I |
| 65% stage II | |
| 3.8% stage III | |
| Number of positive lymph nodes | 1.63 (SD = 2.85) |
| Type of surgery | 1.7% bilateral mastectomy |
| 30% mastectomy | |
| 68.3% lumpectomy | |
| Health status | 80.4% no evidence of cancer |
| 15.4% recurrent breast cancer | |
| 4.2% another type of cancer |
Procedure
We contacted women by phone about 10 years following their initial interview. The initial interview took place 4 months after diagnosis (M = 119 days), and the 10-year follow-up interview took place on average 10.58 years after diagnosis. We interviewed women in their homes. All of the instruments described below were administered at the 10-year follow-up interview.
Instruments
Survivor centrality
We modified a measure of survivor centrality that has been used in the area of diabetes (29,30) by replacing diabetes with breast cancer survivor. This measure included 7 items (e.g., Being a breast cancer survivor is an important part of who I am; I think of being a breast cancer survivor when I think of who I am; Having had breast cancer is a small part of my life [reverse-scored]). The internal consistency was good (alpha = .72).
We also included several other markers of survivor centrality that we could use to validate this measure. We asked survivors: (1) whether they had been involved in any activities related to breast cancer over the past year (e.g., volunteering, Race for the Cure; 1 = not at all; 4 = a lot); (2) whether they owned anything with a pink ribbon on it (yes or no); (3) whether they liked having symbols of breast cancer, like the pink ribbon, around them (1 = dislike a lot; 5 = like a lot); (4) whether they currently do anything to mark or celebrate anniversaries of their breast cancer diagnosis (yes or no), and (5) whether having had breast cancer was the most stressful thing that had ever happened to them.
Valence
We adapted an open-ended measure of valence used in the area of diabetes to breast cancer (29,30). Survivors were asked to think about how they see themselves as breast cancer survivors. Then, they were asked to fill in the blank of the following sentence: “I am a __________ person because I had breast cancer.” They were asked to give up to 5 responses. Two persons independently rated each of these responses as having either a positive valence (e.g., stronger, more mature), a negative valence (e.g., less patient, more angry), or neutral valence (e.g., more of a risk taker; vague items that could not be clearly determined to be positive or negative). The two raters agreed in 77% of the cases (kappa = .59 which indicates moderate agreement). Discrepancies were resolved by a third independent rater. The majority of disagreements centered around one rater assigning a neutral value and the other rater assigning a positive value. If clear positive valence could not be established, we assigned the response to the neutral category.
To validate our open-ended measure of valence, we included two valence statements with which respondents disagreed (1) or agreed (5): “Being a breast cancer survivor makes me feel good about myself” and “I feel bad about being a breast cancer survivor.”
Positive and negative affect
Positive and negative affect over the past week were measured with the 20-item Positive and Negative Affect Scale (45). Each item is rated on a 5-point scale (1 = not at all; 5 = extremely). The internal consistency of the positive affect scale was high (alpha = .89), as was the negative affect scale (alpha = .86).
Quality of life
We administered the SF-36 from the Medical Outcomes Study (46). This instrument has excellent reliability and validity and has been used to evaluate functional status in depressed, chronically ill, and healthy populations (47). The SF-36 consists of 36 items that form eight multi-item scales. The internal consistencies of the scales were all good (alphas ranged from .70 to .92). These scales have been reduced to two composite scores, the mental health component score which represents mental and emotional functioning and the physical health component score which represents physical functioning (48). Higher scores indicate better functioning.
Intrusive and avoidant thoughts
We measured illness-related intrusive and avoidant thoughts over the past month with the Impact of Events Scale (49). This scale measures intrusive, undesirable, uncontrollable thoughts about a stressful experience as well as tendencies to avoid thinking about a stressful experience. The internal consistency of the intrusive thoughts measure was high (alpha = .83) as was the avoidant thoughts measure (alpha = .79). Because the two scales were highly correlated (r = .57, p < .001), we combined the two into a distress index by taking the average.
Benefit-finding
We administered the 16-item Benefit-Finding Scale (50). Five positive growth domains are represented: personal priorities (e.g., more grateful for each day), daily activities (e.g., interest in new activities), family (e.g., more sensitive to family issues), world views (e.g., more concerned for the future of humankind), and relationships (e.g., closer to people). Participants rated the extent to which each of these changes had occurred as a result of having had breast cancer on a 4-point scale, ranging from 1 (not at all) to 4 (very much). The internal consistency was very high (alpha = .92). The average score was 2.51 (SD = .76).
Results
Descriptive Statistics
The mean score for survivor centrality was 3.58 (SD = .82) on a 5-point scale ranging from 1 to 5, which indicates a moderate amount of survivor centrality and good variability in the measure. Higher survivor centrality was related to younger age, r = −.27, p < .001, and to current work status (more likely to be working), F (1, 237) = 7.88, p < .01, eta2 = .03, but was not related to any other demographic variable shown in Table 1. Because younger women are more likely to be working, we examined whether the relation to work status was independent of age. When age was statistically controlled, there was no relation of survivor centrality to work status. Survivor centrality also was not related to stage of disease, number of positive lymph nodes, or whether survivors had sustained a recurrence.
Next, we calculated a valence score. The proportion of positive responses made to the open-ended valence prompt was high (76%). The rest of the responses were negative (10%) or neutral (14%). We calculated the valence score by substracting the proportion of negative responses from the proportion of positive responses for each person. We used the proportion rather than the number of responses because women differed in the number of responses that they made. Thus, positive scores reflect a more positive than negative valence. The average valence score was .73 (SD = .39), indicating an overall positive valence. Valence was not related to any of the demographic or medical variables shown in Table 1.
Construct Validity for Survivor Centrality and Valence
To establish construct validity for our measure of survivor centrality, we measured several behaviors that we thought would be indicative of someone who viewed the illness as part of themselves. Survivor centrality was related to owning something with a pink ribbon on it, F (1, 237) = 5.67, p < .05, eta2 = .02, and observing breast cancer anniversaries, F(1, 220) = 5.13, p < .05, eta2 = .02. Survivor centrality also was related to breast cancer activism, r = .25, p < .001, and to liking to have symbols of breast cancer around, r = .28, p < .001. In addition, women who said that breast cancer was the most stressful thing that had ever happened to them (41% of sample) scored higher in survivor centrality (M = 3.77; SD = .73) than women who did not agree with this statement (M = 3.44, SD = .85), F(1, 236) = 9.41, p < .01, eta2 = .04.
To establish construct validity for our measure of valence, we examined its relation to the two self-report valence items. The valence measure was positively related to the positive valence statement (r = .24, p < .001) and negatively related to the negative valence statement (r = −.17, p < .05).
Links to Well-Being Outcomes
As shown in Table 2, survivor centrality was related to greater negative affect, but was not related to positive affect. Survivor centrality was related to poorer mental functioning, but was unrelated to physical functioning. Survivor centrality was related to greater distress, but also greater benefit-finding.
Table 2.
Relations (Correlations) of Survivor Centrality and Valence to Well-Being Indices
| Survivor Centrality | Positive Valence | |
|---|---|---|
| Negative Affect | .20** | −.05 |
| Positive Affect | −.01 | .29*** |
| Mental Functioning | −.13* | .05 |
| Physical Functioning | .01 | .30*** |
| Distress Index | .31*** | −.15* |
| Benefit-Finding | .28*** | .23*** |
Note: p < .05;
p < .01;
p < .001
Positive valence was linked to indices of favorable well-being (see Table 2). Positive valence was related to greater positive affect, but not negative affect. Positive valence was related to greater physical functioning, but not mental functioning. Positive valence was related to less distress and greater benefit-finding.
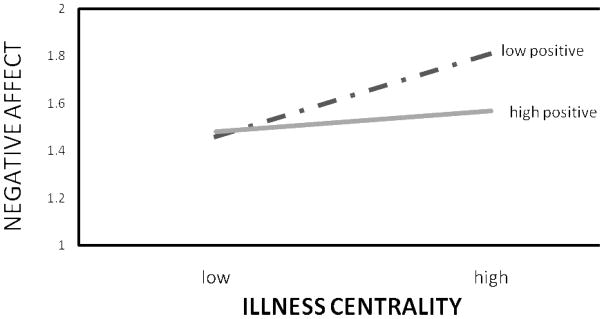
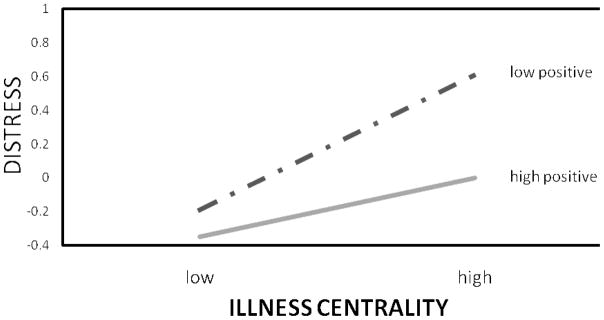
Next, we tested the interaction between centrality and valence in predicting these same outcomes with multiple regression analysis. Survivor centrality and positive illness valence were modestly positively related (r = .13, p = .05). We entered the main effects for centrality and valence on the first step (centered) and the interaction between the two on the second step. The interaction predicted negative affect and distress, as shown in Table 3. To interpret the interactions, we used Aiken and West’s (51) procedure of plotting the dependent variable scores at plus and minus one standard deviation for the two independent variables. As shown in Figure 1, survivor centrality was related to greater negative affect, especially when positive valence was low. The same pattern of findings held for distress, shown in Figure 2. Survivor centrality was related to greater distress, especially when positive valence was low. Thus, survivor centrality was only associated with negative outcomes when the illness was viewed in less positive terms. The interaction did not predict positive affect, quality of life, or benefit-finding.
Table 3.
Regression: Testing Interactions Between Survivor Centrality and Valence
| Beta | Change in R2 | Total R2 | |
|---|---|---|---|
| Negative Affect | |||
| Step 1: | |||
| survivor centrality | .22*** | ||
| positive valence | −.07 | .05 | .05 |
| Step 2: | |||
| survivor centrality | .21** | ||
| positive valence | −.10 | ||
| centrality X valence | −.13* | .02 | .06 |
| Distress Index | |||
| Step 1: | |||
| survivor centrality | .33*** | ||
| positive valence | −.19** | .12 | .12 |
| Step 2: | |||
| survivor centrality | .32*** | ||
| positive valence | −.22*** | ||
| centrality X valence | −.13* | .02 | .14 |
Note: p < .05;
p < .01;
p < .001
Figure 1.
Figure 2.
Discussion
First, we examined the extent to which 10-year breast cancer survivors viewed having had breast cancer as central to their self-concepts. Average scores on this measure indicated a moderate amount of survivor centrality. This is consistent with the perspective that cancer is not an acute event but a process that extends throughout the lifespan (14,15). Although previous researchers have discussed the idea that some women are more likely than others to define themselves in terms of having had breast cancer, this is the first study that has tried to quantify this effect and attempted to identify determinants. In terms of who was more or less likely to identify themselves in terms of having had breast cancer, with the exception of age, no demographic variable was predictive. Younger women were more likely to define themselves in terms of breast cancer survivorship, perhaps because there is a greater incongruence between the experience of a life-threatening illness and a young age. Illness-related variables associated with treatment or prognosis were not predictive. Even the experience of a recurrence was not diagnostic of survivor centrality. Thus, we did not learn a lot about who is more likely to define themselves in terms of cancer survivorship—but, it is certainly not women who had a worse prognosis or women who are experiencing more illness-related problems.
However, there were characteristic behaviors associated with survivor centrality. Women who defined themselves in terms of their illness were more likely to engage in behaviors that indicated that breast cancer was self-relevant, such as owning something with a pink ribbon on it and involving themselves in breast cancer activities. In addition, women who said that breast cancer was the most stressful thing that had ever happened to them (slightly less than half of the sample) were more likely to define themselves in terms of their illness. Thus, we established some construct validity for the concept of survivor centrality by showing that it was linked to the self-report of behaviors that one would expect to reflect identifying with having had breast cancer. Future research should continue to examine the concept of survivor centrality to see how it changes over time. Is this a stable construct that is set in motion after diagnosis, does it emerge with time, or does it fluctuate with other events in one’s life?
Next, we examined whether survivor centrality had implications for well-being. Overall, there was some evidence that greater survivor centrality was associated with poorer indices of well-being. The one exception was benefit-finding. Women who scored higher in survivor centrality were more likely to perceive that they had grown in positive ways from the illness. The experience of personal growth from traumatic events does not necessarily occur without the experience of psychological distress. Again, we remind the reader that there is controversy in the literature as to whether reports of personal growth are actual or illusory (41,52).
We also examined the valence that women attached to being a breast cancer survivor. The majority of women viewed survivorship in positive terms, which is consistent with the literature that shows women hold more positive views of cancer than laypersons (31). However, there may have been some demand characteristics to reporting one’s views of survivorship aloud to an interviewer. Positive valence was related to positive indices of well-being. Future research should examine the source of illness valence. Interestingly, valence was not related to specific medical variables—stage at diagnosis, type of surgery, or even recurrence status. The valence one attaches to survivorship could be related to interactions with social network members or health care professionals, or even be a product of exposure to the media as suggested by some researchers (32).
Importantly, and as predicted, for some outcomes the relation of survivor centrality to well-being depended on illness valence. For negative affect and psychological distress, survivor centrality was only associated with poor outcomes if the woman viewed the illness in less positive (or more negative) terms. It is unclear why this interactive effect did not appear for the other outcomes. The centrality by valence interaction is consistent with previous research on stigma in general (42) as well as the specific work in the area of diabetes that demonstrated this same finding (30). Identifying with a domain is only problematic if that domain is associated with a stigma. Thus, knowing whether a women defines herself in terms of survivorship—wears emblems related to breast cancer survivorship, commemorates survivorship markers, and participates in survivorship activities—is not necessarily indicative of problems coping with having had breast cancer or difficulties moving on with life after the diagnosis and treatment of breast cancer. It is moreso that when these same women view survivorship in negative terms that there may be psychological difficulties.
The research on cancer survivorship needs to continue to examine the centrality of having had breast cancer to survivors’ self-concepts as well as whether survivorship is viewed in positive or negative terms. This research will help us to understand the implications of having had breast cancer for one’s self views and the implications of self-concept for quality of life. Rather than focus on whether survivorship is associated with stigma at a societal level, here we focused on whether the women themselves associate survivorship with stigma. We note that viewing survivorship in more negative terms is not a proxy for illness severity, as it was not the case that women who had more severe disease or who had experienced a recurrence viewed the illness in more negative terms.
The question arises as to whether survivor centrality or the valence associated with survivorship reflect stable personality traits, such as neuroticism, rather than responses to illness. Although we did not measure neuroticism in this study, we did measure negative affect at both study start and 10-year follow-up. Perhaps, somewhat surprisingly, illness valence was unrelated to negative affect at either time of assessment. That is, those who viewed cancer survivorship in more negative terms 10 years later were not the same people who experienced more negative emotions. However, as noted in the results section, survivor centrality was related to negative affect at 10-year follow-up. We also found that survivor centrality was related to negative affect at baseline (r = .27, p < .001)--although it was not related to changes in negative affect over time. Thus, the possibility exists that people who experience more negative emotions are more likely to define themselves in terms of their illness. Because the data are cross-sectional, we do not know if survivor centrality leads to negative affect or a more stable personality trait related to negative affect drives survivor centrality.
The findings from this research have implications for health care professionals who see women over the course of diagnosis, treatment, and survivorship. Whether women seem to be integrating cancer into their identities or separating it from the rest of their lives is only partially diagnostic of adjustment difficulties. The women who may be at greatest risk for psychological distress are those who have integrated breast cancer into their identities and perceive the illness in negative terms. Therefore, when treatment is terminated and women move on to the survivorship phase of illness, health care professionals should examine women’s views of their situations whether they perceive survivorship in positive or negative terms. Those who attach a negative label to survivorship may be at risk for psychological distress. The question remains as to whether educational groups, peer support groups, cognitive behavioral therapy, or other types of counseling can affect illness valence. Before such recommendations can be made, further research needs to be conducted on the sources and malleability of survivor centrality and survivorship valence.
Acknowledgments
This research was supported by NIH R21 CA104078. The author would like to thank Abigail Kunz-Vaughn, Elizabeth Muia, and Dara Stern for conducting the 10-year follow-up interviews with the patients for this study. The author also is grateful for the dedication that the patients in this study showed over the 10 years.
References
- 1.Brennan J. Adjustment to cancer: Coping or personal transition? Psycho-Oncology. 2001;10:1–18. doi: 10.1002/1099-1611(200101/02)10:1<1::aid-pon484>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 2.Glanz K, Lerman C. Psychosocial impact of breast cancer: A critical review. Annals of Behavioral Medicine. 1992;14:204–212. [Google Scholar]
- 3.Meyerowitz BE. Psychosocial correlates of breast cancer and its treatments. Psychological Bulletin. 1980;87:108–131. [PubMed] [Google Scholar]
- 4.Ranchor AV, Sanderman R, Steptoe A, Wardle J, Miedema I, Ormel J. Pre-morbid predictors of psychological adjustment to cancer. Quality of Life Research. 2002;11:101–113. doi: 10.1023/a:1015053623843. [DOI] [PubMed] [Google Scholar]
- 5.Ganz PA, Demond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. Journal of the National Cancer Institute. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 6.Helgeson VS, Tomich PL. Surviving cancer: A comparison of 5-year disease free breast cancer survivors with healthy women. Psycho-Oncology. 2005;14:307–317. doi: 10.1002/pon.848. [DOI] [PubMed] [Google Scholar]
- 7.Zebrack BJ, Yi J, Petersen L, Ganz PA. The impact of cancer and quality of life for long-term survivors. Psycho-oncology. 2008;17:891–900. doi: 10.1002/pon.1300. [DOI] [PubMed] [Google Scholar]
- 8.Bowman KF, Smerglia VL, Deimling GT. A stress model of cancer survivorship in older long-term survivors. Journal of Mental Health and Aging. 2004;10(3):163–182. [Google Scholar]
- 9.Alfano CM, Rowland JH. The experience of survival for patients: Psychosocial adjustment. In: Miller SM, Bowen DJ, Croyle RT, Rowland JH, editors. Handbook of Cancer Control and Behavioral Science: A Resource for Researchers, Practitioners, and Policymakers. Vol. 1. Washington, D.C: American Psychological Association; 2009. [Google Scholar]
- 10.Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psycho-oncology. 2006;15:306–320. doi: 10.1002/pon.955. [DOI] [PubMed] [Google Scholar]
- 11.Deimling GT, Kahana B, Bowman KF, Schaefer ML. Cancer survivorship and psychological distress in later life. Psycho-oncology. 2002;11:479–494. doi: 10.1002/pon.614. [DOI] [PubMed] [Google Scholar]
- 12.Henderson PA. Psychosocial adjustment of adult cancer survivors: Their needs and counselor interventions. Journal of Counseling and Development. 1997;75(3):188–194. [Google Scholar]
- 13.Zebrack BJ. Cancer survivor identity and quality of life. Cancer Practice. 2000;8(5):238–242. doi: 10.1046/j.1523-5394.2000.85004.x. [DOI] [PubMed] [Google Scholar]
- 14.Bowman KF, Deimling GT, Smerglia V, Sage P, Kahana B. Appraisal of the cancer experience by older long-term survivors. Psycho-oncology. 2003;12:226–238. doi: 10.1002/pon.630. [DOI] [PubMed] [Google Scholar]
- 15.Clark EJ, Stovall EL. Advocacy: The cornerstone of cancer survivorship. Cancer Practice. 1996;4:239–244. [PubMed] [Google Scholar]
- 16.Henselmans I, Sanderman R, Helgeson VS, de Vries J, Smink A, Ranchor AV. Personal control over the cure breast cancer: Adaptiveness, underlying beliefs, and correlates. In: Henselmans I, editor. Psychological well-being and perceived control after a breast cancer diagnosis. Groningen, The Netherlands: University of Groningen; 2009. [Google Scholar]
- 17.Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ-R) Psychology and Health. 2002;17:1–16. [Google Scholar]
- 18.Weinman J, Petrie KJ, Moss-Morris R, Horne R. The illness perception questionnaire: A new method for assessing the cognitive representation of illness. Psychology and Health. 1996;11:431–440. [Google Scholar]
- 19.Curbow B, Somerfield M, Legro M, Sonnega J. Self-concept and cancer in adults: Theoretical and methodological issues. Social Science and Medicine. 1990;31:115–128. doi: 10.1016/0277-9536(90)90053-u. [DOI] [PubMed] [Google Scholar]
- 20.Esplen MJ, Stuckless N, Berk T, Butler K, Gallinger S. The FAP self-concept scale (adult form) Familial Cancer. 2009;8:39–50. doi: 10.1007/s10689-008-9204-x. [DOI] [PubMed] [Google Scholar]
- 21.Kullmer U, Stenger K, Milch W, Zygmunt M, Sachsse S, Munstedt K. Self-concept, body image, and use of unconventional therapies in patients with gynecological malignancies in the state of complete remission and recurrence. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1999;82:101–106. doi: 10.1016/s0301-2115(98)00226-7. [DOI] [PubMed] [Google Scholar]
- 22.Seitzman RL, Glover DA, Meadows AT, Mills JL, Nicholson HS, Robison LL, et al. Self-concept in adult survivors of childhood acute lymphoblastic leukemia: A cooperative children’s cancer group and national institutes of health study. Pediatric Blood and Cancer. 2004;42:230–240. doi: 10.1002/pbc.10434. [DOI] [PubMed] [Google Scholar]
- 23.Millar K, Purushotham AD, McLatchie E, George WD, Murray GD. A 1-year prospective study of individual variation in distress, and illness perceptions, after treatment for breast cancer. Journal of Psychosomatic Research. 2005;58:335–342. doi: 10.1016/j.jpsychores.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Beanlands HJ, Lipton JH, McCay EA, Schimmer AD, Elliott ME, Messner HA, et al. Self-concept as a “BMT patient”, illness intrusiveness, and engulfment in allogenic bone marrow transplant recipients. Journal of Psychosomatic Research. 2003;55:419–425. doi: 10.1016/s0022-3999(03)00509-9. [DOI] [PubMed] [Google Scholar]
- 25.Drew S. ‘Having cancer changed my life, and changed my life forever’: Survival, illness legacy, and service provision following cancer in childhood. Chronic Illness. 2007;3:278–295. doi: 10.1177/1742395307085236. [DOI] [PubMed] [Google Scholar]
- 26.Prouty D, Ward-Smith P, Hutto CJ. The lived experience of adult survivors of childhood cancer. Journal of Pediatric Oncology Nursing. 2006;23:143–151. doi: 10.1177/1043454206287295. [DOI] [PubMed] [Google Scholar]
- 27.Little M, Paul K, Jordens CFC, Sayers E-J. Survivorship and discourses of identity. Psycho-Oncology. 2002;11:170–178. doi: 10.1002/pon.549. [DOI] [PubMed] [Google Scholar]
- 28.Charmaz K. Near-death utopias: Now or later? Journal of Near-Death Studies. 1991;10(2):131–134. [Google Scholar]
- 29.Wiebe DJ, Berg CA, Palmer DL, Korbel C, Beveridge RM. Illness and the self: Examining adjustment among adolescents with diabetes. Paper presented at the annual Meeting of the Society of Behavioral Medicine; Washington, D.C. 2002. Apr, [Google Scholar]
- 30.Helgeson VS, Novak SA. Illness centrality and well-being among male and female early adolescents with diabetes. Journal of Pediatric Psychology. 2007;32(3):260–272. doi: 10.1093/jpepsy/jsl018. [DOI] [PubMed] [Google Scholar]
- 31.Katz I, Hass RG, Parisi N, Astone J. Lay people’s and health care personnel’s perceptions of cancer, AIDS, cardiac, and diabetic patients. Psychological Reports. 1987;60(2):615–629. doi: 10.2466/pr0.1987.60.2.615. [DOI] [PubMed] [Google Scholar]
- 32.Burke W, Olsen AH, Pinsky SE, Reynolds SE, Press NA. Misleading presentation of breast cancer in popular magazines. Effective Clinical Practice. 2001;4:58–64. [PubMed] [Google Scholar]
- 33.Anagnostopoulos F, Spanea E. Assessing illness representations of breast cancer: A comparison of patients with healthy and benign controls. Journal of Psychosomatic Research. 2005;58:327–334. doi: 10.1016/j.jpsychores.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Buick D. Illness representations and breast cancer: Coping with radiation and chemotherapy. In: Petrie KJ, Weinman JA, editors. Perceptions of Health and Illness. Amsterdam, Netherlands: Harwood Academic Publishers; 1997. pp. 379–409. [Google Scholar]
- 35.Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: Qualitative study. British Medical Journal. 2004;328:1–5. doi: 10.1136/bmj.38111.639734.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fife BL, Wright ER. The dimensionality of stigma: A comparison of its impact on the self of persons with HIV/AIDS and cancer. Journal of Health and Social Behavior. 2000;41:50–67. [PubMed] [Google Scholar]
- 37.Wilson K, Luker KA. At home in hospital? Interaction and stigma in people affected by cancer. Social Science and Medicine. 2006;62:1616–1627. doi: 10.1016/j.socscimed.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 38.Stanton AL. Psychosocial concerns and interventions for cancer survivors. Journal of Clinical Oncology. 2006;24:5132–5137. doi: 10.1200/JCO.2006.06.8775. [DOI] [PubMed] [Google Scholar]
- 39.Park C, Helgeson VS. Growth following highly stressful life events: Current status and future directions. Journal of Consulting and Clinical Psychology. 2006;74:791–796. doi: 10.1037/0022-006X.74.5.791. [DOI] [PubMed] [Google Scholar]
- 40.Kayser K, Sormanti M. Identity and the illness experience: Issues faced by mothers with cancer. Illness, Crisis, and Loss. 2002;10:10–26. [Google Scholar]
- 41.Frazier P, Tennen H, Gavian M, Park C, Tomich PL, Tashiro T. Does self-reported posttraumatic growth reflect genuine positive change? Psychological Science. 2009;20:912–919. doi: 10.1111/j.1467-9280.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- 42.Jones EE, Scott RA, Markus H. Social stigma : the psychology of marked relationships. New York: W.H. Freeman & Company; 1984. [Google Scholar]
- 43.Helgeson VS, Reynolds KA, Tomich PL. A meta-analytic review of benefit finding and growth. Journal of Consulting and Clinical Psychology. 2006;74:797–816. doi: 10.1037/0022-006X.74.5.797. [DOI] [PubMed] [Google Scholar]
- 44.Helgeson VS, Cohen S, Schulz R, Yasko J. Education and peer discussion group interventions and adjustment to breast cancer. Archives of General Psychiatry. 1999;56:340–347. doi: 10.1001/archpsyc.56.4.340. [DOI] [PubMed] [Google Scholar]
- 45.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 46.Ware JE, Snow KK, Kosinsky M, Gandek B. SF-36 Health Survey: Manual and interpretation guide. Boston: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 47.Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. Journal of the American Medical Association. 1989;262:914–919. [PubMed] [Google Scholar]
- 48.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic Medicine. 1979;41(3):209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Tomich PL, Helgeson VS. Five years later: A cross-sectional comparison of breast cancer survivors with healthy women. Psycho-Oncology. 2002;11:154–169. doi: 10.1002/pon.570. [DOI] [PubMed] [Google Scholar]
- 51.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. New York: Sage Publishing; 1991. [Google Scholar]
- 52.Helgeson VS. Corroboration of growth following breast cancer: 10 years later. Journal of Social and Clinical Psychology. doi: 10.1521/jscp.2010.29.5.546. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]