Abstract
Studies on physiological modulation of intercellular communication mediated by protein kinases are often complicated by the fact that cells express multiple gap junction proteins (connexins; Cx). Changes in cell coupling can be masked by simultaneous opposite regulation of the gap junction channel types expressed. We have examined the effects of activators and inhibitors of protein kinase A (PKA), PKC, and PKG on permeability and single channel conductance of gap junction channels composed of Cx45, Cx43, or Cx26 subunits. To allow direct comparison between these Cx, SKHep1 cells, which endogenously express Cx45, were stably transfected with cDNAs coding for Cx43 or Cx26. Under control conditions, the distinct types of gap junction channels could be distinguished on the basis of their permeability and single channel properties. Under various phosphorylating conditions, these channels behaved differently. Whereas agonists/antagonist of PKA did not affect permeability and conductance of all gap junction channels, variable changes were observed under PKC stimulation. Cx45 channels exhibited an additional conductance state, the detection of the smaller conductance states of Cx43 channels was favored, and Cx26 channels were less often observed. In contrast to the other kinases, agonists/antagonist of PKG affected permeability and conductance of Cx43 gap junction channels only. Taken together, these results show that distinct types of gap junction channels are differentially regulated by similar phosphorylating conditions. This differential regulation may be of physiological importance during modulation of cell-to-cell communication of more complex cell systems.
Full text
PDF



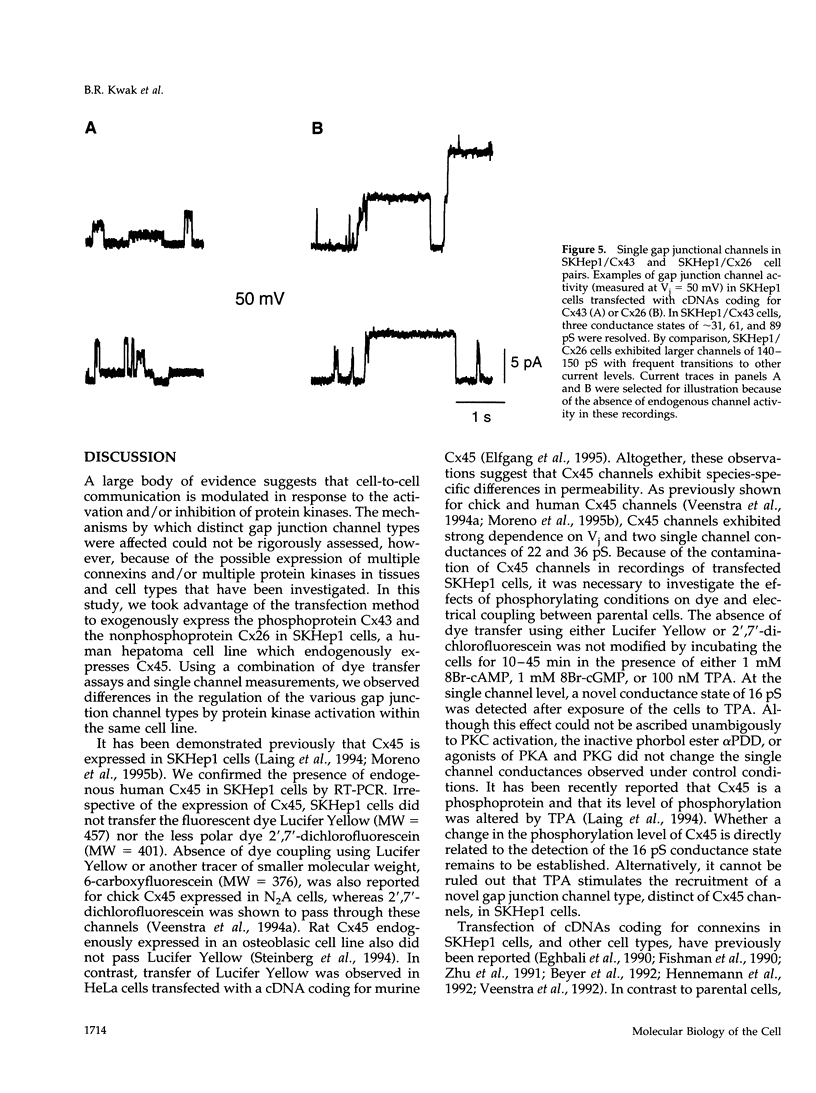
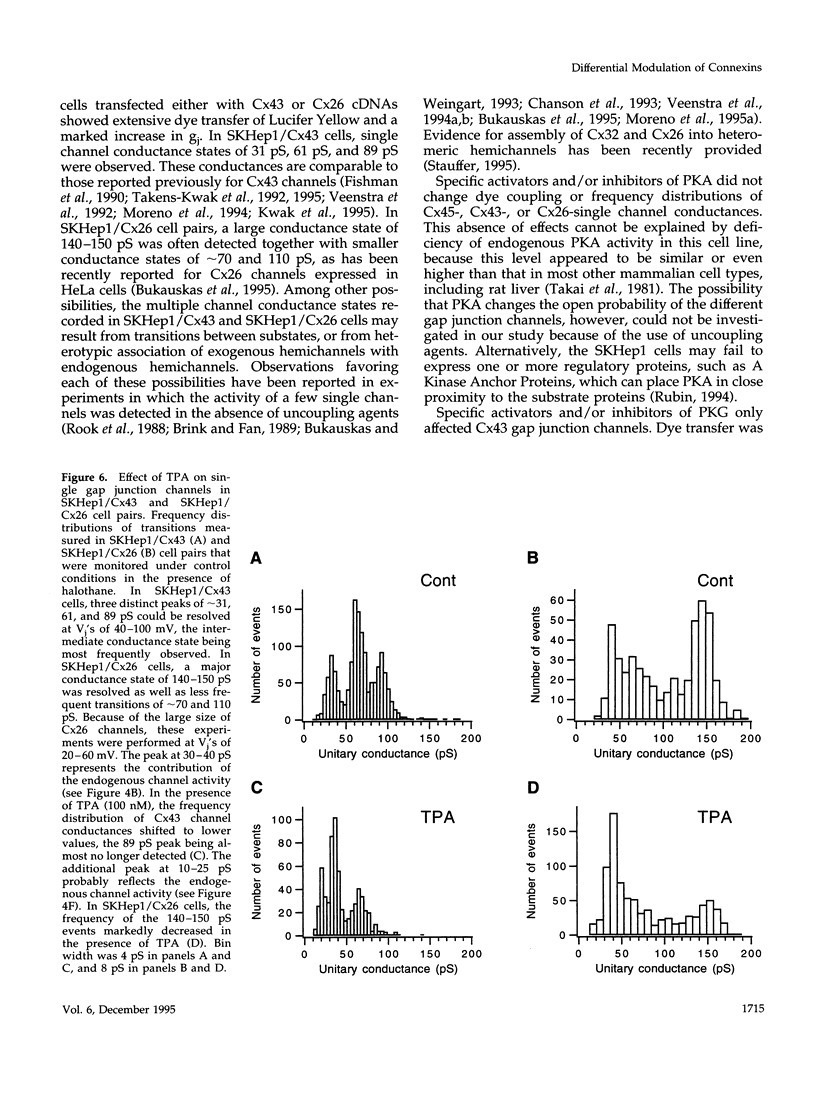
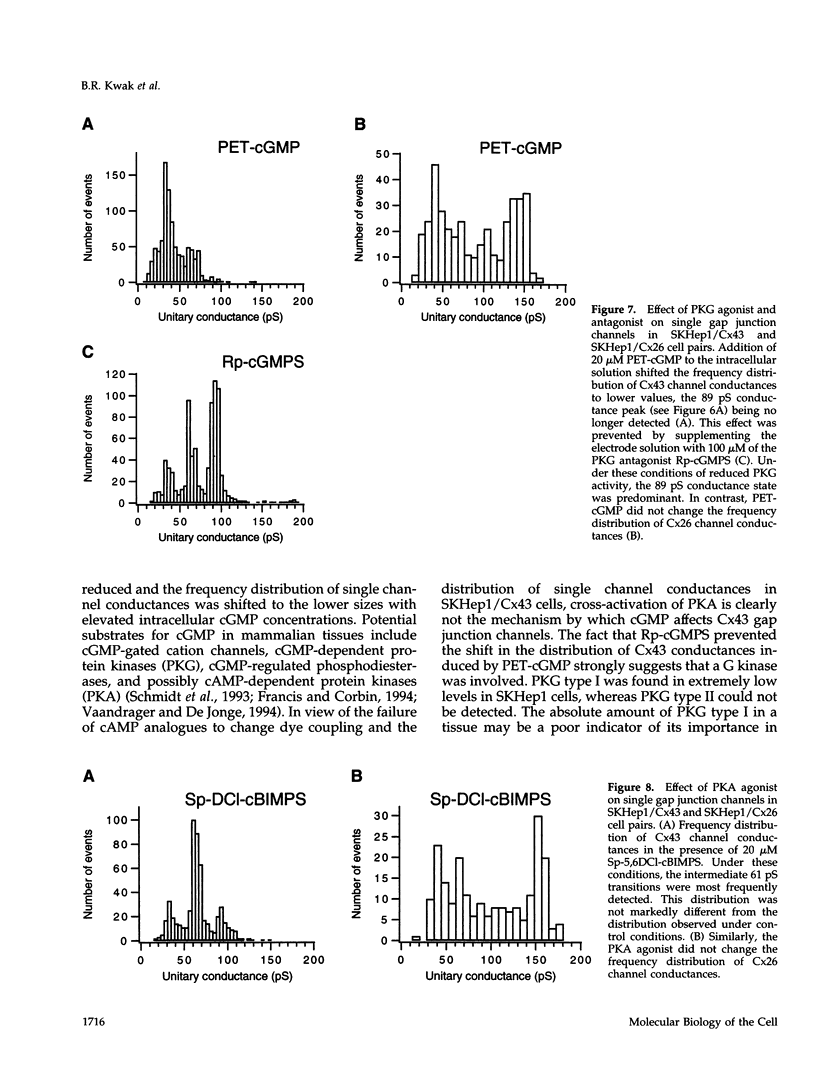



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Verselis V. K. Biophysics of gap junctions. Semin Cell Biol. 1992 Feb;3(1):29–47. doi: 10.1016/s1043-4682(10)80006-6. [DOI] [PubMed] [Google Scholar]
- Berthoud V. M., Ledbetter M. L., Hertzberg E. L., Sáez J. C. Connexin43 in MDCK cells: regulation by a tumor-promoting phorbol ester and Ca2+. Eur J Cell Biol. 1992 Feb;57(1):40–50. [PubMed] [Google Scholar]
- Berthoud V. M., Rook M. B., Traub O., Hertzberg E. L., Sáez J. C. On the mechanisms of cell uncoupling induced by a tumor promoter phorbol ester in clone 9 cells, a rat liver epithelial cell line. Eur J Cell Biol. 1993 Dec;62(2):384–396. [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin family of gap junction proteins. J Membr Biol. 1990 Jul;116(3):187–194. doi: 10.1007/BF01868459. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Reed K. E., Westphale E. M., Kanter H. L., Larson D. M. Molecular cloning and expression of rat connexin40, a gap junction protein expressed in vascular smooth muscle. J Membr Biol. 1992 Apr;127(1):69–76. doi: 10.1007/BF00232759. [DOI] [PubMed] [Google Scholar]
- Brink P. R., Fan S. F. Patch clamp recordings from membranes which contain gap junction channels. Biophys J. 1989 Sep;56(3):579–593. doi: 10.1016/S0006-3495(89)82705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas F. F., Elfgang C., Willecke K., Weingart R. Heterotypic gap junction channels (connexin26-connexin32) violate the paradigm of unitary conductance. Pflugers Arch. 1995 Apr;429(6):870–872. doi: 10.1007/BF00374812. [DOI] [PubMed] [Google Scholar]
- Bukauskas F. F., Weingart R. Multiple conductance states of newly formed single gap junction channels between insect cells. Pflugers Arch. 1993 Apr;423(1-2):152–154. doi: 10.1007/BF00374973. [DOI] [PubMed] [Google Scholar]
- Chanson M., Chandross K. J., Rook M. B., Kessler J. A., Spray D. C. Gating characteristics of a steeply voltage-dependent gap junction channel in rat Schwann cells. J Gen Physiol. 1993 Nov;102(5):925–946. doi: 10.1085/jgp.102.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow D. S., Beyer E. C., Paul D. L., Kobe S. S., Lau A. F. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol. 1990 Apr;10(4):1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali B., Kessler J. A., Spray D. C. Expression of gap junction channels in communication-incompetent cells after stable transfection with cDNA encoding connexin 32. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1328–1331. doi: 10.1073/pnas.87.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfgang C., Eckert R., Lichtenberg-Fraté H., Butterweck A., Traub O., Klein R. A., Hülser D. F., Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995 May;129(3):805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman G. I., Spray D. C., Leinwand L. A. Molecular characterization and functional expression of the human cardiac gap junction channel. J Cell Biol. 1990 Aug;111(2):589–598. doi: 10.1083/jcb.111.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S. H., Corbin J. D. Progress in understanding the mechanism and function of cyclic GMP-dependent protein kinase. Adv Pharmacol. 1994;26:115–170. doi: 10.1016/s1054-3589(08)60053-8. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Masaracchia R. A., Feramisco J. R., Kemp B. E. Isolation of phosphorylated peptides and proteins on ion exchange papers. Anal Biochem. 1978 Jul 1;87(2):566–575. doi: 10.1016/0003-2697(78)90707-8. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger J. A., Bruzzone R., Jenkins N. A., Gilbert D. J., Copeland N. G., Paul D. L. Four novel members of the connexin family of gap junction proteins. Molecular cloning, expression, and chromosome mapping. J Biol Chem. 1992 Jan 25;267(3):2057–2064. [PubMed] [Google Scholar]
- Hennemann H., Suchyna T., Lichtenberg-Fraté H., Jungbluth S., Dahl E., Schwarz J., Nicholson B. J., Willecke K. Molecular cloning and functional expression of mouse connexin40, a second gap junction gene preferentially expressed in lung. J Cell Biol. 1992 Jun;117(6):1299–1310. doi: 10.1083/jcb.117.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarchau T., Häusler C., Markert T., Pöhler D., Vanderkerckhove J., De Jonge H. R., Lohmann S. M., Walter U. Cloning, expression, and in situ localization of rat intestinal cGMP-dependent protein kinase II. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9426–9430. doi: 10.1073/pnas.91.20.9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadle R., Zhang J. T., Nicholson B. J. Tissue-specific distribution of differentially phosphorylated forms of Cx43. Mol Cell Biol. 1991 Jan;11(1):363–369. doi: 10.1128/mcb.11.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak B. R., Sáez J. C., Wilders R., Chanson M., Fishman G. I., Hertzberg E. L., Spray D. C., Jongsma H. J. Effects of cGMP-dependent phosphorylation on rat and human connexin43 gap junction channels. Pflugers Arch. 1995 Sep;430(5):770–778. doi: 10.1007/BF00386175. [DOI] [PubMed] [Google Scholar]
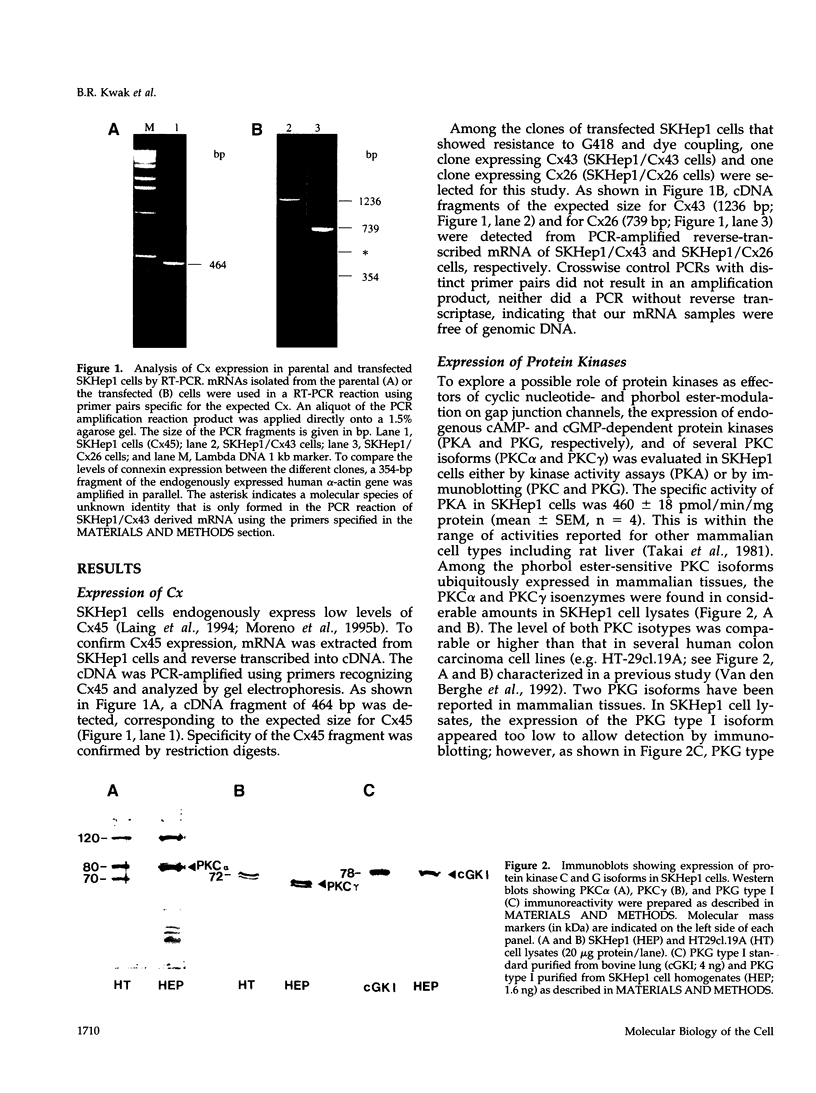
- Laing J. G., Westphale E. M., Engelmann G. L., Beyer E. C. Characterization of the gap junction protein, connexin45. J Membr Biol. 1994 Apr;139(1):31–40. doi: 10.1007/BF00232672. [DOI] [PubMed] [Google Scholar]
- Laird D. W., Puranam K. L., Revel J. P. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J. 1991 Jan 1;273(Pt 1):67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe P. D. Analyzing phorbol ester effects on gap junctional communication: a dramatic inhibition of assembly. J Cell Biol. 1994 Dec;127(6 Pt 2):1895–1905. doi: 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A. F., Hatch-Pigott V., Crow D. S. Evidence that heart connexin43 is a phosphoprotein. J Mol Cell Cardiol. 1991 Jun;23(6):659–663. doi: 10.1016/0022-2828(91)90975-r. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Tomasetto C., Paul D., Keyomarsi K., Sager R. Transcriptional downregulation of gap-junction proteins blocks junctional communication in human mammary tumor cell lines. J Cell Biol. 1992 Sep;118(5):1213–1221. doi: 10.1083/jcb.118.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
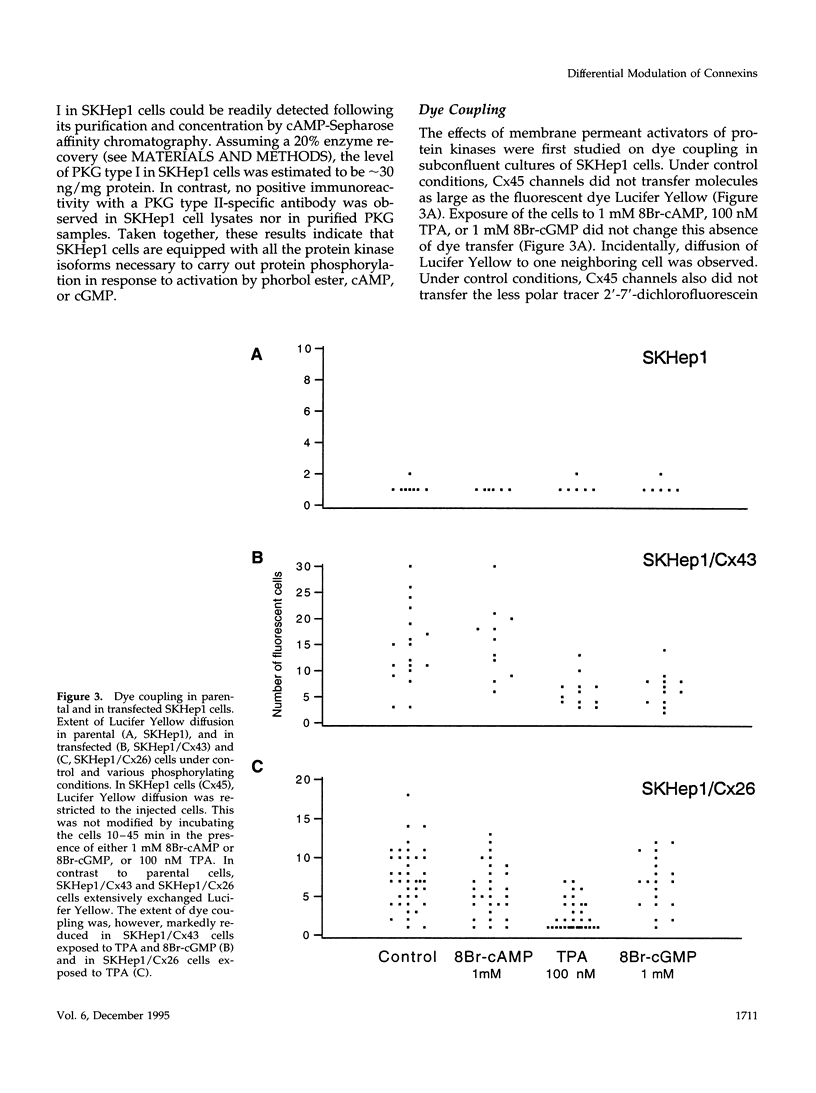
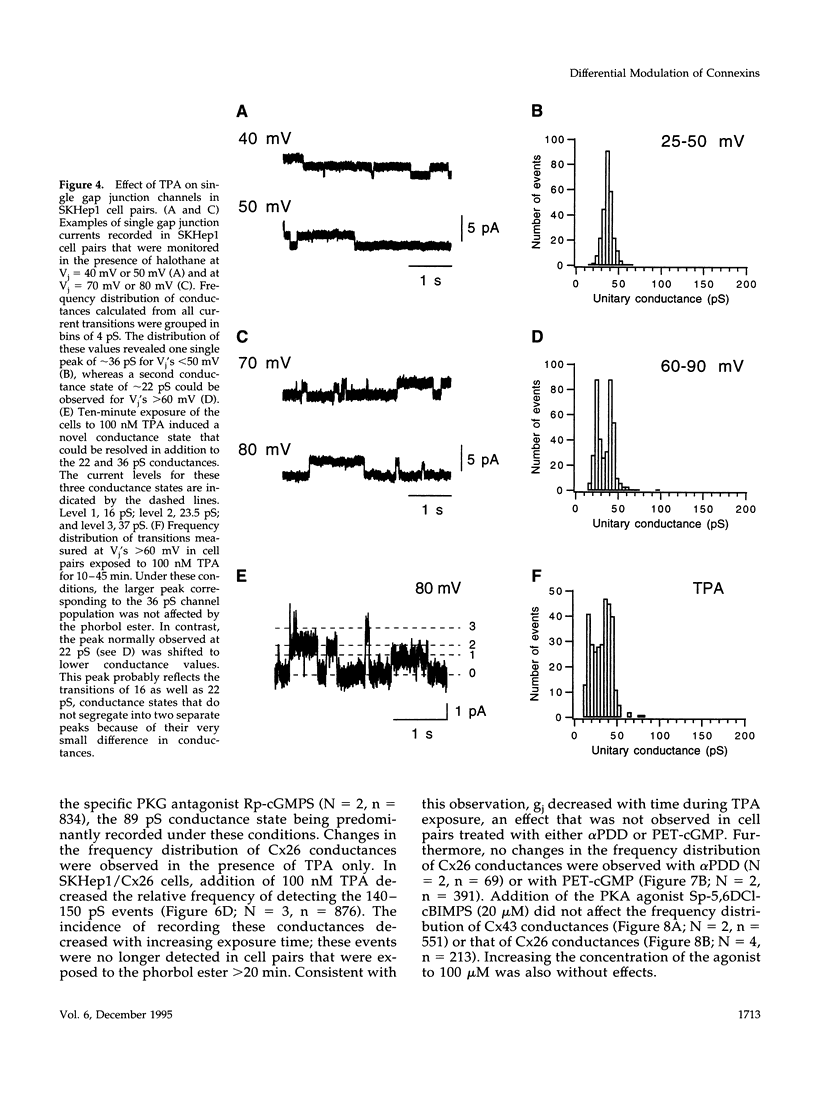
- Moreno A. P., Laing J. G., Beyer E. C., Spray D. C. Properties of gap junction channels formed of connexin 45 endogenously expressed in human hepatoma (SKHep1) cells. Am J Physiol. 1995 Feb;268(2 Pt 1):C356–C365. doi: 10.1152/ajpcell.1995.268.2.C356. [DOI] [PubMed] [Google Scholar]
- Moreno A. P., Sáez J. C., Fishman G. I., Spray D. C. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ Res. 1994 Jun;74(6):1050–1057. doi: 10.1161/01.res.74.6.1050. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Beyer E. C., Goodenough D. A. Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol. 1990 Jun;116(2):163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Cunningham B. A., Edelman G. M., Goodenough D. A. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990 Nov;111(5 Pt 1):2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Single-channel currents of an intercellular junction. 1985 Sep 26-Oct 2Nature. 317(6035):331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Oh S. Y., Grupen C. G., Murray A. W. Phorbol ester induces phosphorylation and down-regulation of connexin 43 in WB cells. Biochim Biophys Acta. 1991 Sep 3;1094(2):243–245. doi: 10.1016/0167-4889(91)90016-q. [DOI] [PubMed] [Google Scholar]
- Rook M. B., Jongsma H. J., van Ginneken A. C. Properties of single gap junctional channels between isolated neonatal rat heart cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H770–H782. doi: 10.1152/ajpheart.1988.255.4.H770. [DOI] [PubMed] [Google Scholar]
- Rubin C. S. A kinase anchor proteins and the intracellular targeting of signals carried by cyclic AMP. Biochim Biophys Acta. 1994 Dec 30;1224(3):467–479. [PubMed] [Google Scholar]
- Saez J. C., Spray D. C., Nairn A. C., Hertzberg E., Greengard P., Bennett M. V. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Lohmann S. M., Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993 Aug 18;1178(2):153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Stauffer K. A. The gap junction proteins beta 1-connexin (connexin-32) and beta 2-connexin (connexin-26) can form heteromeric hemichannels. J Biol Chem. 1995 Mar 24;270(12):6768–6772. [PubMed] [Google Scholar]
- Steinberg T. H., Civitelli R., Geist S. T., Robertson A. J., Hick E., Veenstra R. D., Wang H. Z., Warlow P. M., Westphale E. M., Laing J. G. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J. 1994 Feb 15;13(4):744–750. doi: 10.1002/j.1460-2075.1994.tb06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez J. C., Berthoud V. M., Moreno A. P., Spray D. C. Gap junctions. Multiplicity of controls in differentiated and undifferentiated cells and possible functional implications. Adv Second Messenger Phosphoprotein Res. 1993;27:163–198. [PubMed] [Google Scholar]
- Sáez J. C., Nairn A. C., Czernik A. J., Spray D. C., Hertzberg E. L., Greengard P., Bennett M. V. Phosphorylation of connexin 32, a hepatocyte gap-junction protein, by cAMP-dependent protein kinase, protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Eur J Biochem. 1990 Sep 11;192(2):263–273. doi: 10.1111/j.1432-1033.1990.tb19223.x. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kawahara Y., Minakuchi R., Sano K., Kikkawa U., Mori T., Yu B., Kaibuchi K., Nishizuka Y. Calcium and phosphatidylinositol turnover as signalling for transmembrane control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1981;14:301–313. [PubMed] [Google Scholar]
- Takeda A., Hashimoto E., Yamamura H., Shimazu T. Phosphorylation of liver gap junction protein by protein kinase C. FEBS Lett. 1987 Jan 5;210(2):169–172. doi: 10.1016/0014-5793(87)81330-3. [DOI] [PubMed] [Google Scholar]
- Takeda A., Saheki S., Shimazu T., Takeuchi N. Phosphorylation of the 27-kDa gap junction protein by protein kinase C in vitro and in rat hepatocytes. J Biochem. 1989 Oct;106(4):723–727. doi: 10.1093/oxfordjournals.jbchem.a122923. [DOI] [PubMed] [Google Scholar]
- Takens-Kwak B. R., Jongsma H. J. Cardiac gap junctions: three distinct single channel conductances and their modulation by phosphorylating treatments. Pflugers Arch. 1992 Nov;422(2):198–200. doi: 10.1007/BF00370421. [DOI] [PubMed] [Google Scholar]
- Traub O., Look J., Dermietzel R., Brümmer F., Hülser D., Willecke K. Comparative characterization of the 21-kD and 26-kD gap junction proteins in murine liver and cultured hepatocytes. J Cell Biol. 1989 Mar;108(3):1039–1051. doi: 10.1083/jcb.108.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub O., Look J., Paul D., Willecke K. Cyclic adenosine monophosphate stimulates biosynthesis and phosphorylation of the 26 kDa gap junction protein in cultured mouse hepatocytes. Eur J Cell Biol. 1987 Feb;43(1):48–54. [PubMed] [Google Scholar]
- Vaandrager A. B., De Jonge H. R. Effect of cyclic GMP on intestinal transport. Adv Pharmacol. 1994;26:253–283. doi: 10.1016/s1054-3589(08)60057-5. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., Wang H. Z., Beyer E. C., Brink P. R. Selective dye and ionic permeability of gap junction channels formed by connexin45. Circ Res. 1994 Sep;75(3):483–490. doi: 10.1161/01.res.75.3.483. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., Wang H. Z., Beyer E. C., Ramanan S. V., Brink P. R. Connexin37 forms high conductance gap junction channels with subconductance state activity and selective dye and ionic permeabilities. Biophys J. 1994 Jun;66(6):1915–1928. doi: 10.1016/S0006-3495(94)80985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra R. D., Wang H. Z., Westphale E. M., Beyer E. C. Multiple connexins confer distinct regulatory and conductance properties of gap junctions in developing heart. Circ Res. 1992 Nov;71(5):1277–1283. doi: 10.1161/01.res.71.5.1277. [DOI] [PubMed] [Google Scholar]
- Walter U., Miller P., Wilson F., Menkes D., Greengard P. Immunological distinction between guanosine 3':5'-monophosphate-dependent and adenosine 3':5'-monophosphate-dependent protein kinases. J Biol Chem. 1980 Apr 25;255(8):3757–3762. [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989 Dec;109(6 Pt 2):3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Caveney S., Kidder G. M., Naus C. C. Transfection of C6 glioma cells with connexin 43 cDNA: analysis of expression, intercellular coupling, and cell proliferation. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1883–1887. doi: 10.1073/pnas.88.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge H. R. Cyclic GMP-dependent protein kinase in intestinal brushborders. Adv Cyclic Nucleotide Res. 1981;14:315–333. [PubMed] [Google Scholar]
- van den Berghe N., Vaandrager A. B., Bot A. G., Parker P. J., de Jonge H. R. Dual role for protein kinase C alpha as a regulator of ion secretion in the HT29cl.19A human colonic cell line. Biochem J. 1992 Jul 15;285(Pt 2):673–679. doi: 10.1042/bj2850673. [DOI] [PMC free article] [PubMed] [Google Scholar]