Manganese is an essential metal in all forms of life.[1] It participates as a co-factor in diverse classes of enzymes and the photosynthetic machinery[2] and is used widely as a versatile tool for biological studies. For example, high spin Mn2+ is an excellent MRI relaxation agent that has been used in clinical diagnosis and is of widespread interest as a tool in neurobiological research.[3] However, chronic over-exposure can result in movement disorders and mental disturbances and other brain-related toxicities.[4] Fluorescent probes would be useful for detection and quantification of Mn2+, as this method offers high efficiency, high sensitivity and easy operation[5] among available methods of detection.[6] However, development of an effective fluorescent probe for Mn2+ faces several challenges: 1) Unlike diamagnetic metal ions such as Zn2+, paramagnetic Mn2+ can quench fluorescence. Although chelation-induced fluorescence quenching (CHEQ) is the most commonly used method of paramagnetic metal ion detection,[7] “on-fluorescence” probes for Mn2+ are preferred. 2) Mn2+ selectivity over abundant cellular metal ions is required, especially Ca2+ (up to high μM).[8] Mn2+ and Ca2+ share many common properties, underscored by the fact that Mn2+ can enter cells using some of the same transport systems as Ca2+.[9] 3) Mn2+ probes must be compatible with biological environments, including water solubility, biological inertness, long-wavelength excitation and emission profiles to minimize sample damage and native cellular autofluorescence.[10] 4) To visualize Mn2+ in living cells or tissues, membrane permeability is important.[10]
Several commercially available chelating dyes produce strong fluorescence enhancement upon binding Mn2+.[11] However, the fluorescence of available dyes such as calcium green is also enhanced in the presence of Ca2+. BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N,N-tetraacetic acid) is a known Ca2+-selective ligand that serves as the chelating moiety of calcium green.[12] We undertook to modify the BAPTA unit in such a way as to achieve adequate Mn/Ca selectivity.
Optimizing stereoelectronic complementarity between host and guest to achieve efficient complexation is a long-held axiom of supramolecular and coordination chemistry. However, while optimizing a receptor for a substrate leads to strong binding, it may not result in good selectivity over competing substrates. In the case of metal ion complexation, selection of ligand donor atom can sometimes be used to advantage. For example, choosing soft donor atoms may improve selectivity for relatively soft metal ions in competition with hard ones. Both Mn2+ and Ca2+, however, are generally considered to be hard and show maximal stability with hard oxygen donors. Thus, superficial considerations would lead away from hard/soft donor atom considerations as a strategy for achieving Mn2+ selectivity.
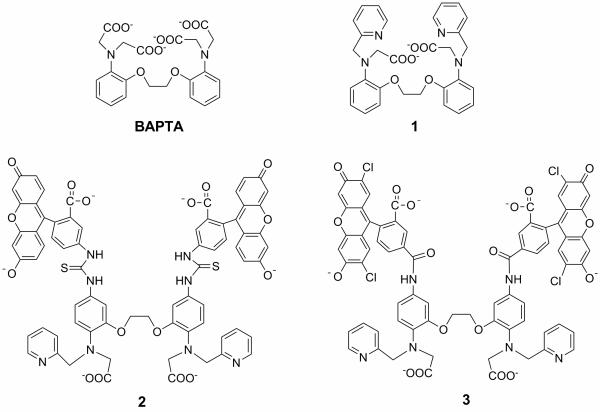
However, although both Mn2+ and Ca2+ are classified as “hard” metals and therefore form stronger complexes with oxygen donors, Mn2+ appears to be more tolerant of softer atom donors than Ca2+.[13,14] More recently, the relative softness of Mn2+ compared with Mg2+ has been debated as the basis for Mn2+ rescue of activity in dialkyl-thiophosphate RNAzymes[15-17]. Our hypothesis was thus to replace two or more carboxylate groups in BAPTA with softer ligating moieties. Since Mn2+ is a hard metal ion, weaker binding might be expected from such a change, but since Ca2+ is an even harder metal ion, the effect on Ca2+ should be more pronounced, resulting in a net increase in selectivity. We chose nitrogen atom donors from pyridine, which is a common binding group in transition metal ion ligands considered to be borderline but softer than oxygen.[14] To evaluate the feasibility of our strategy, a prototype ligand 1 was synthesized, which has one carboxylate group of each dicarboxymethylamino moiety of BAPTA replaced by a pyridine (Scheme 1). The chemical synthesis of ligands 1-3 is included as supporting information.
Scheme 1.
Structures of ligands and probes.
UV titrations were carried out by addition of MnCl2 to MOPS buffered aqueous solution (pH=7.2) of 1 (SI). In the absence of Mn2+, the spectrum of 1 showed a maximum at 256 nm with a shoulder at 286 nm, similar to BAPTA. Mn2+ complexation caused significant hypsochromic shifts towards a limiting spectrum with a small maximum at 278 nm surrounded by shoulders. Absorbance at 256 nm was plotted as a function of Mn2+ concentration and the minimum level of absorbance was reached upon addition of 1 equivalent of Mn2+, suggesting 1:1 metal-ligand complex. The same analysis was applied to determine 1:1 complexation of BAPTA to Mn2+. The binding constants were obtained by titration in pH- and Mn2+- buffered aqueous media. The plot of absorbance as a function of free Mn2+ produced a sigmoidal curve. Nonlinear fitting analysis[18] gave association constants (logK) of 8.62 for ligand 1 and 9.14 for BAPTA (Table 1). These results indicate that substitution of two carboxylate groups of BAPTA with two pyridines impairs binding affinity to Mn2+ to a surprisingly small extent.
Table 1.
Mn2+ and Ca2+ affinities and spectroscopic properties of ligands.
| logK |
selectivity log(KMn/KCa) |
λ
max |
ϕ
f |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ligands | Mn2+ | Ca2+ | Mg2+ | Zn2+ | Free ligand |
Mn2+ complex |
Ex | Em | Free ligand |
Mn2+ Complex |
|
| BAPTA | 9.14 | 6.89 | 1.69 | 9.41 | 2.25 | 290(4.8)[a] | 278 (4.5) | -- | -- | -- | -- |
| 1 | 8.62 | 3.79 | 0.76 | 9.19 | 4.83 | 286 (6.3) | 283 (4.1) | -- | -- | -- | -- |
| 2 | 7.01 | 2.96 | 0.42 | 6.16 | 4.05 | 493 (84) | 493 (100) | 493 | 519 | 0.10 | 0.37 |
| 3 | 8.00 | 3.33 | 0.93 | 7.09 | 4.68 | 507 (80) | 507 (92) | 505 | 530 | 0.13 | 0.49 |
In parentheses is the corresponding molar absorption coefficient ε in units of 103 L mol−1 cm−1.
Calcium binding to ligand 1 was investigated in a similar manner. A hypsochromic shift was again observed, but even excess Ca2+ did not saturate the UV absorption indicating weak affinity. The association constant of 1-Ca2+ was determined to be log(Ka) = 3.79, about 4.83 log units weaker than for Mn2+. The log(Ka) of BAPTA-Ca2+ was 6.89,[11] only 2.25 log units lower than that of BAPTA-Mn2+ complex. Therefore, ligand 1 indeed shows much higher selectivity for Mn2+ over Ca2+ as compared to BAPTA. The significant improvement in Mn2+/Ca2+ selectivity validates our “selective poisoning” strategy.
To realize the goal of a fluorescent Mn2+ probe, compound 1 was further functionalized to include a chromophore similar to that present in calcium green. Thus, amino groups installed para-to the N-atoms of both aniline moieties were coupled with fluorescein 5-isothiocyanate in high yield followed by hydrolysis afforded fluorescent probe 2. Probe 3, most similar in structure to calcium green-2, was prepared containing chloro-substituents and an amide linkage between chelating unit and fluorophore.
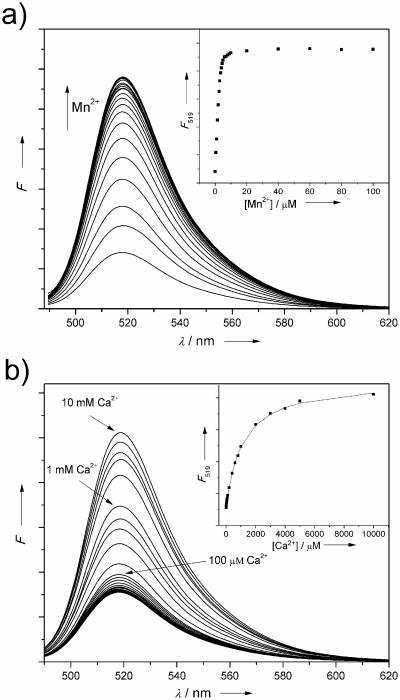
The binding properties of fluorescent probe 2 were first investigated by titration with Mn2+ (Figure 1a). When excited at 493 nm, 2 showed an emission maximum at 519 nm. Upon addition of Mn2+, an enhanced fluorescence was observed until saturation after one equivalent. The quantum yields of the free probe and Mn2+-bound complex were determined to be 0.10 and 0.37, respectively. The association constant with Mn2+ was logK = 7.01. Similar to the prototype ligand 1, only a large excess of Ca2+ ion (mM) caused fluorescence enhancement indicating weak association of 2 to Ca2+ (logK = 2.96). In the cellular environment, calcium concentration is generally lower than 100 μM. Therefore, probe 2 has the potential to detect Mn2+ in the presence of calcium ion interference in biological systems. Screening for selectivity against other metal ions (SI) Na+, Mg2+, Ba2+ and K+ showed no effect on fluorescence intensity of 2. However, transition metal ions, Ni2+ and Cu2+ quench the fluorescence, while Fe2+, Co2+, Zn2+, Cd2+ and Hg2+ may interfere with Mn2+ binding. Among these, Zn2+ (Table 1) is probably the most significant concern for likely applications. Dissociation constants determined for Co2+, Ni2+, and Cd2+ for BAPTA (9.13, 10.51, 13.38) and 1 (9.22, 10.23, 13.58) together with data for Mn2+ and Zn2+ (Table 1) indicate that the two ligands follow the Irving-Williams series very similarly, and that the discrimination against Ca2+ is much greater than the effect observed on soft metals.
Figure 1.
Fluorescence titration of 2 (5 μM, λexc=493 nm) with MnCl2 and CaCl2 (50 mM HEPES, 0.1 M KNO3, pH 7.2): a) 0 to 200 μM Mn2+; b) 0 to 10 mM Ca2+. Inserts: λem=519 nm.
Compound 3 showed a longer λexc (505 nm) and λem (530 nm) due to the two incorporated chlorine atoms on the fluorophore (SI). The Mn2+ complex showed enhanced fluorescence. Probe 3 maintained high selectivity for Mn2+ over Ca2+ with logK of 8.00 for Mn2+ and 3.33 for Ca2+. The quantum yield of free probe 3 was 0.13, while that for the Mn2+ complex was 0.49. Probe 3 shared similar binding properties to probe 2 to other metal ions (SI). Probe 2 was responsive over physiologically relevant pH 6.8 to 8.2. Interestingly, probe 3 functions well in basic conditions: From pH 8.3 to pH 12.2, the high pH limit of our measurement, the fluorescence showed nearly full response. To our knowledge, probes 2 and 3 are the first selective “on-fluorescence” Mn2+ probes reported.
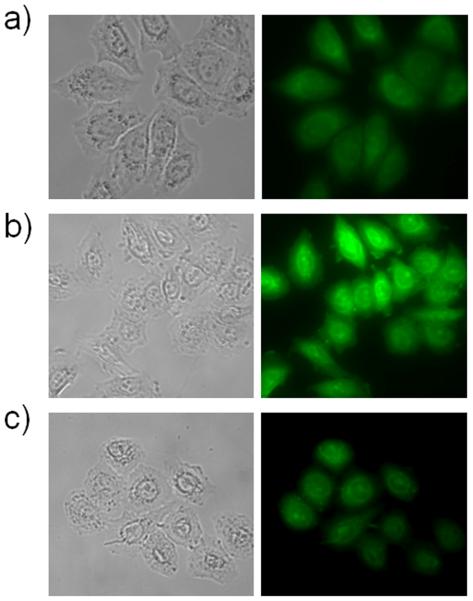
To confirm that the probes would function in a biological environment, intracellular Mn2+-sensing was performed with the Hela cell line. For cell membrane permeability, the ester precursor of 3 (SI) was used for in vitro Mn2+ detection, as it has been reported that permeable ester probes can be hydrolyzed in the intracellular environment.[19] Hela cells were first incubated with Mn2+, followed by fluorescent probe treatment. A 2.4-fold enhanced fluorescent signal was observed in the presence of the probe and Mn2+ (Fig. 2), consistent with the measurements performed in solution.
Figure 2.
Detection of Mn2+ in Hela cells. a) 5 μM 3 ethyl ester; b) 200 μM added MnCl2; c) Mn2+ supplemented cells treated with 2 mM TPEN for 5 min at room temperature.
In conclusion, ligand 1 was rationally designed from BAPTA using a “soft atom poisoning” strategy to differentiate binding affinities to Mn2+ and Ca2+. Binding preferences were tuned by substitution of carboxylate groups of BAPTA with pyridines, resulting in much stronger Mn2+ selectivity over Ca2+. Fluorescent probes based on ligand 1 were synthesized. Solution and in vitro properties demonstrated that these sensing compounds have good selectivity towards Mn2+ with “on” fluorescence response.
Supplementary Material
Footnotes
We are grateful to the NIH (GM070602) and the NSF (CHE-0848234) for research support. J.L. was supported in part by a Margaret and Herman Sokol Fellowship.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Emsley J. Nature's Building Blocks: An A-Z Guide to the Elements. Oxford University Press; Oxford: 2001. pp. 249–253. [Google Scholar]
- 2.Dismukes GC, Willigen RT. Manganese: The Oxygen-Evolving Complex & Models. Encyclopedia of Inorganic Chemistry; 2005. [Google Scholar]
- 3.Lee JH, Koretsky AP. Curr. Pharm. Biotechnol. 2004;5:529–537. doi: 10.2174/1389201043376607. [DOI] [PubMed] [Google Scholar]
- 4.Roth JA. Biol. Res. 2006;39:45–57. doi: 10.4067/s0716-97602006000100006. [DOI] [PubMed] [Google Scholar]
- 5.a) Mason WT. Fluorescent and Luminescent Probes for Biological Activity. Academic Press; San Diego: 1999. [Google Scholar]; b) Que EL, Domaille DW, Chang CJ. Chem. Rev. 2008;108:1517–1549. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]; c) Domaille DW, Que EL, Chang CJ. Nature Chem. Biol. 2008;4:168–175. doi: 10.1038/nchembio.69. [DOI] [PubMed] [Google Scholar]; d) Dai Z, Canary JW. New J. Chem. 2007;31:1708–1718. [Google Scholar]
- 6.a) Smit MH, Rechnitz GA. Anal. Chem. 1992;64:245–249. doi: 10.1021/ac00027a002. [DOI] [PubMed] [Google Scholar]; b) Vinas P, Pardo-Martinez M, Hernandez-Cordoba M. J. Agri. Food Chem. 2000;48:5789–5794. doi: 10.1021/jf000479e. [DOI] [PubMed] [Google Scholar]; c) Schnell S, Ratering S, Jansen KH. Environ. Sci. Technol. 1998;32:1530–1537. [Google Scholar]; d) Motomizu S, Oshima M, Kuwabara M, Obata Y. Analyst. 1994;119:1787–1792. [Google Scholar]
- 7.a) Zeng HH, Thompson RB, Maliwal BP, Fones GR, Moffett JW, Fierke CA. Anal. Chem. 2003;75:6807–6812. doi: 10.1021/ac0345401. [DOI] [PubMed] [Google Scholar]; b) Yoon JY, Ohler NE, Vance DH, Aumiller WD, Czarnik AW. Tetrahedron Lett. 1997;38:3845–3848. [Google Scholar]; c) Kramer R. Angew. Chem. Int. Ed. 1998;37:772–773. doi: 10.1002/(SICI)1521-3773(19980403)37:6<772::AID-ANIE772>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]; d) Bernardo MA, Pina F, Escuder B, Garcia-Espana E, Godino-Salido ML, Latorre J, Luis SV, Ramirez JA, Soriano C. J. Chem. Soc. Dalton Trans. 1999:915–921. [Google Scholar]
- 8.Bronner F, Coburn JW. Disorders of Mineral Metabolism. II. Academic Press; New York: 1982. [Google Scholar]
- 9.a) Drapeau P, Nachshen DA. J. Physiol. 1984;348:493–510. doi: 10.1113/jphysiol.1984.sp015121. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Narita K, Kawasaki F, Kita H. Brain Res. 1990;510:289–295. doi: 10.1016/0006-8993(90)91379-u. [DOI] [PubMed] [Google Scholar]; c) Hunter DR, Haworth RA, Berkoff HA. J. Mol. Cell. Cardiol. 1981;13:823–832. doi: 10.1016/0022-2828(81)90239-x. [DOI] [PubMed] [Google Scholar]
- 10.Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ. J. Am. Chem. Soc. 2006;128:10–11. doi: 10.1021/ja055064u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haugland RP. Handbook of Fluorescent Probes and Research Products. Ninth ed. Molecular Probes; Eugene: 2002. Chapter 20. [Google Scholar]
- 12.Tsien RY. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 13.Dai ZD, Khosla KN, Canary JW. Supramol. Chem. 2009;21:296–300. [Google Scholar]
- 14.Pearson RG. J. Chem. Educ. 1968;45:581–586. [Google Scholar]
- 15.Chen Y, Li XQ, Gegenheimer P. Biochemistry. 1997;36:2425–2438. doi: 10.1021/bi9620464. [DOI] [PubMed] [Google Scholar]
- 16.Sigel RKO, Song B, Sigel H. J. Am. Chem. Soc. 1997;119:744–755. [Google Scholar]
- 17.Da Costa CP, Okruszek A, Sigel H. Chembiochem. 2003;4:593–602. doi: 10.1002/cbic.200200551. [DOI] [PubMed] [Google Scholar]
- 18.Fery-Forgues S, Le Bris MT, Guette JP, Valeur B. J. Phys. Chem. 1988;92:6233–6237. [Google Scholar]
- 19.Woodroofe CC, Masalha R, Barnes KR, Frederickson CJ, Lippard SJ. Chem. Biol. 2004;11:1659–1666. doi: 10.1016/j.chembiol.2004.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.