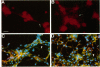
Abstract
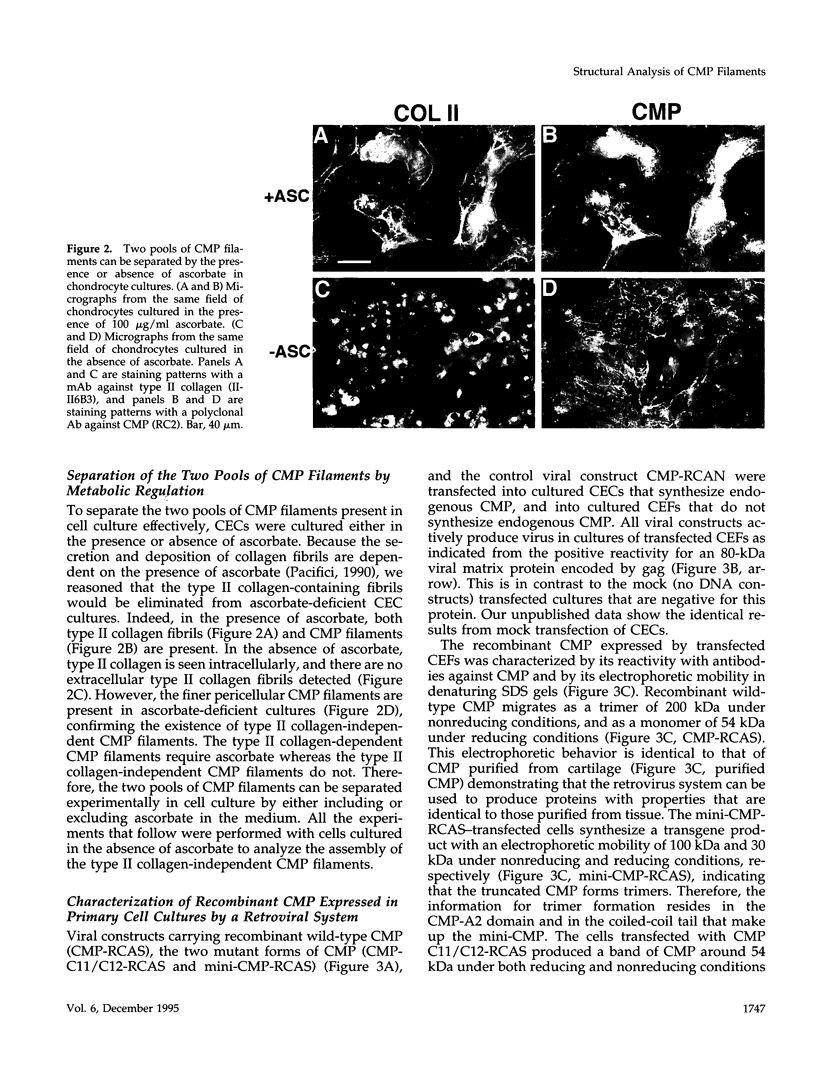
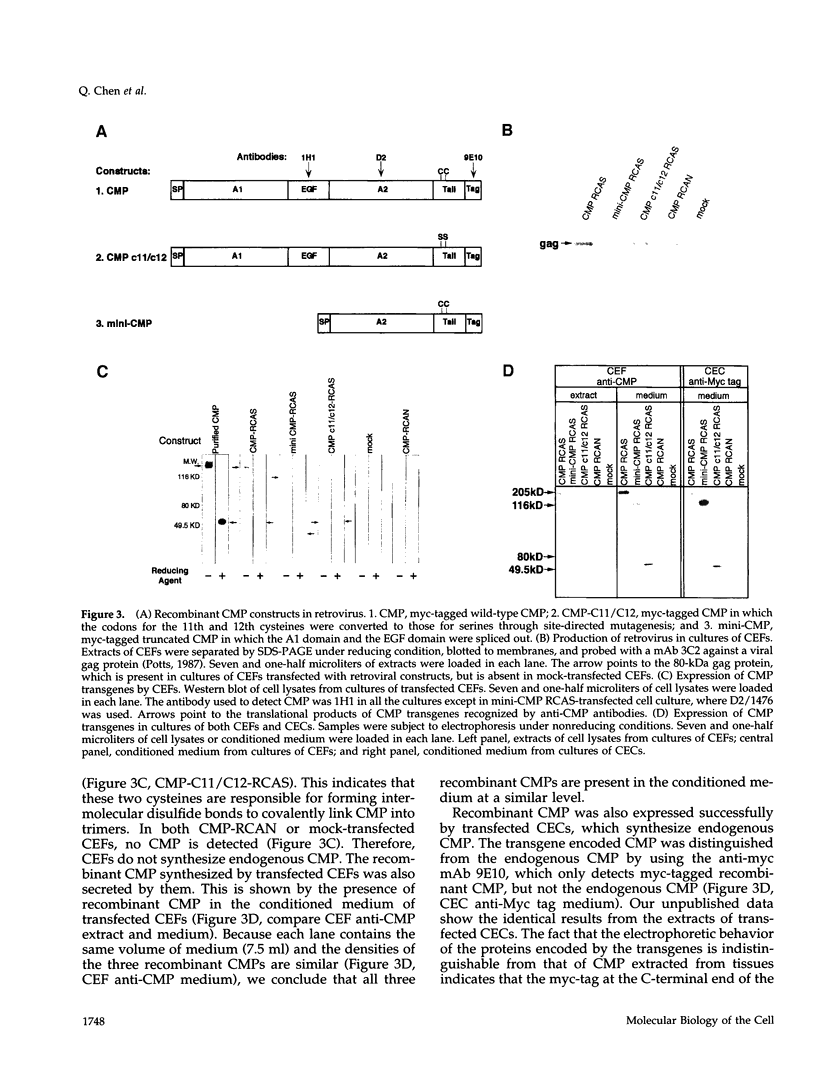
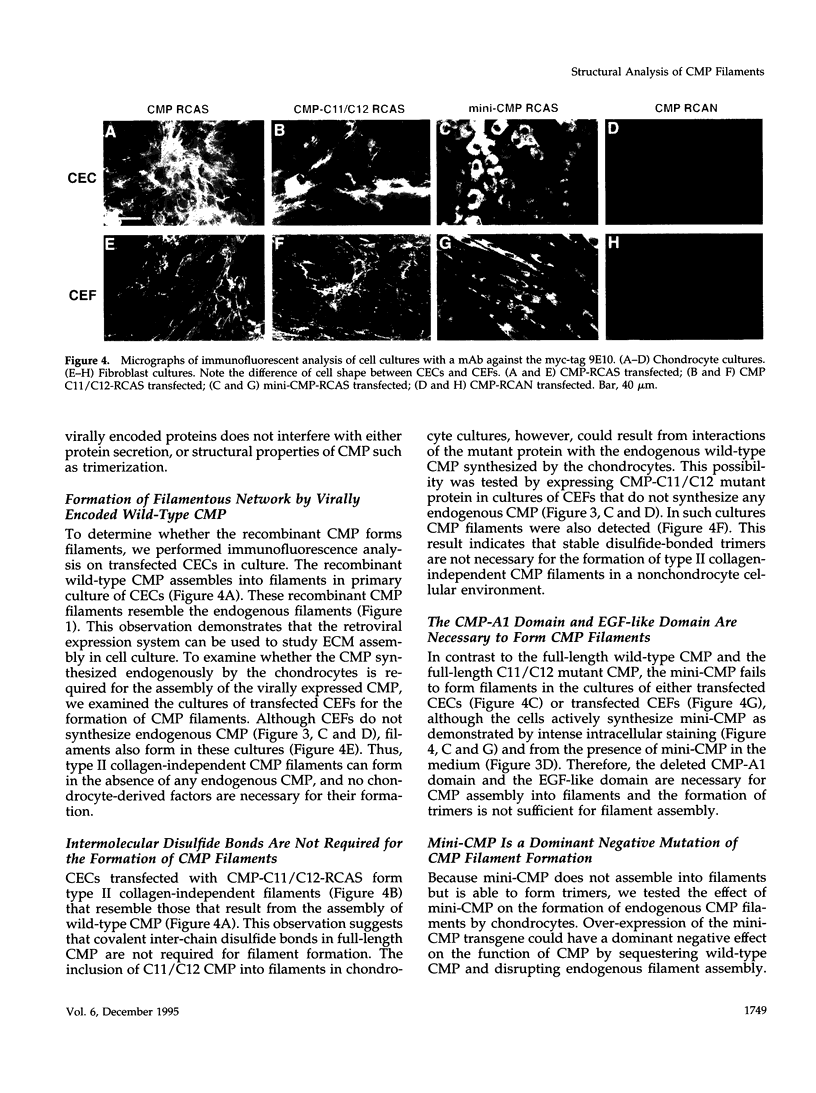
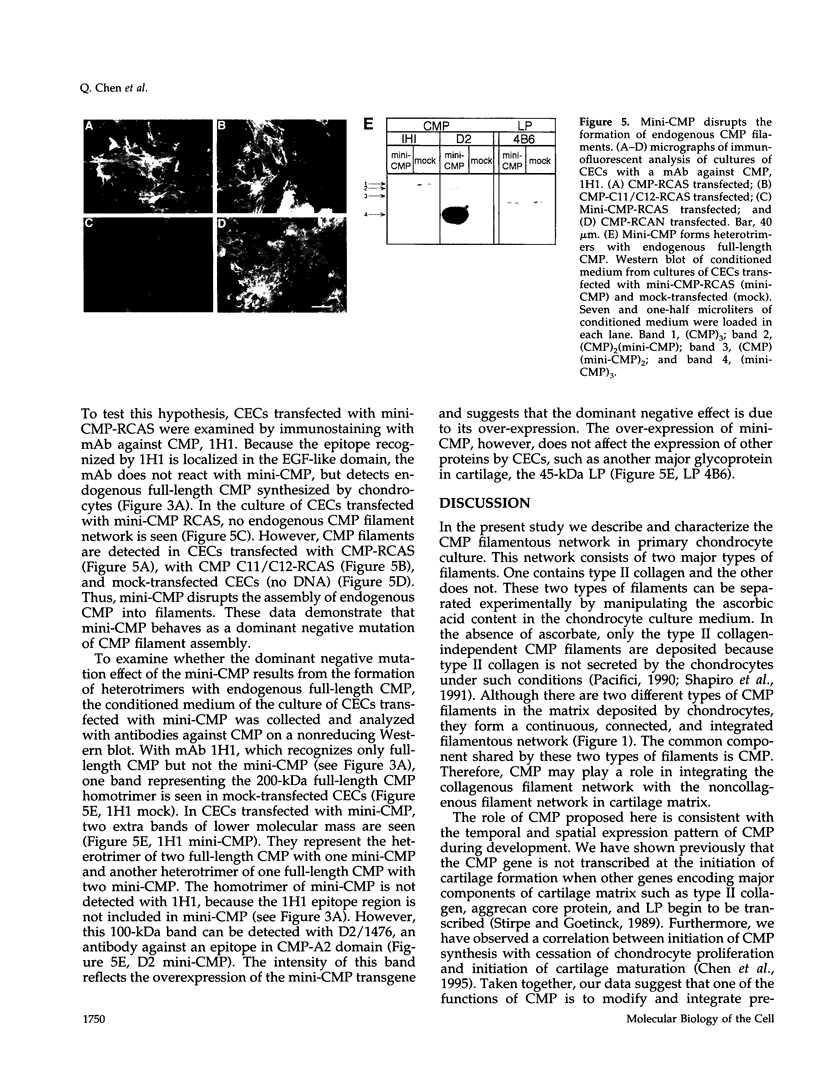
Cartilage matrix protein (CMP) is expressed specifically in mature cartilage and consists of two von Willebrand factor A domains (CMP-A1 and CMP-A2) that are separated by an epidermal growth factor-like domain, and a coiled-coil tail domain at the carboxyl terminal end. We have shown previously that CMP interacts with type II collagen-containing fibrils in cartilage. In this study, we describe a type II collagen-independent CMP filament and we analyze the structural requirement for the formation of this type of filament. Recombinant wild-type CMP and two mutant forms were expressed in chick primary cell cultures using a retrovirus expression system. In chondrocytes, the wild-type virally encoded CMP is able to form disulfide bonded trimers and to assemble into filaments. Filaments also form with CMP whose Cys455 and Cys457 in the tail domain were mutagenized to prevent interchain disulfide bond formation. Therefore, intermolecular disulfide bonds are not necessary for the assembly of CMP into filaments. Both the wild-type and the double cysteine mutant also form filaments in fibroblasts, indicating that chondrocyte-specific factors are not required for filament formation. A truncated form of CMP that consists only of the CMP-A2 domain and the tail domain can form trimers but fails to form filaments, indicating that the deleted CMP-A1 domain and/or the epidermal growth factor domain are necessary for filament assembly but not for trimer formation. Furthermore, the expression of the virally encoded truncated CMP in chondrocyte culture disrupts endogenous CMP filament formation. Together these data suggest a role for CMP in cartilage matrix assembly by forming filamentous networks that require participation and coordination of individual domains of CMP.
Full text
PDF


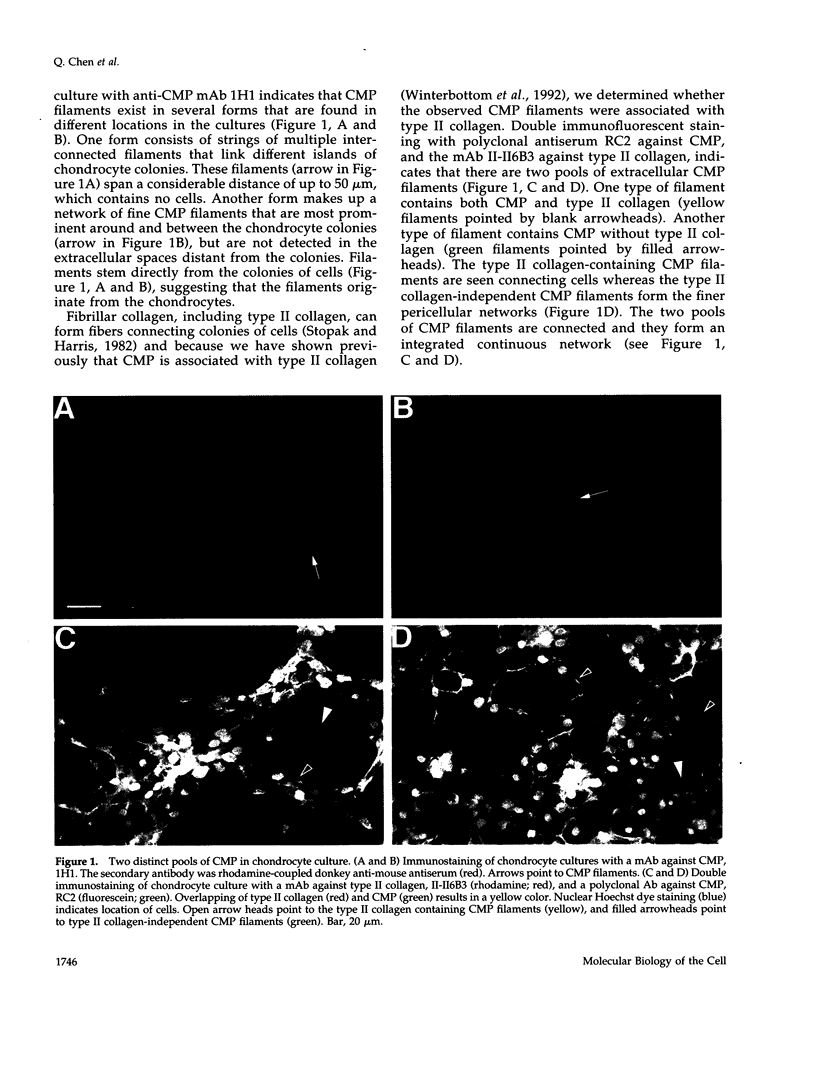

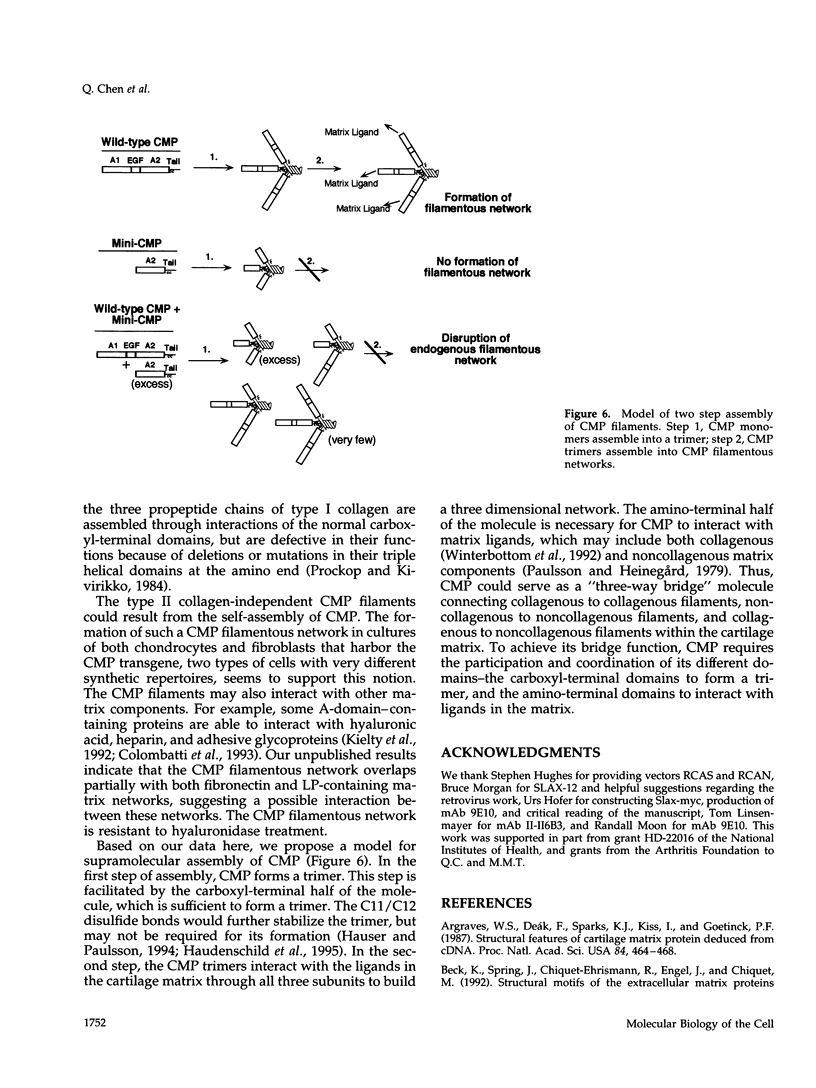

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argraves W. S., Deák F., Sparks K. J., Kiss I., Goetinck P. F. Structural features of cartilage matrix protein deduced from cDNA. Proc Natl Acad Sci U S A. 1987 Jan;84(2):464–468. doi: 10.1073/pnas.84.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binette F., Cravens J., Kahoussi B., Haudenschild D. R., Goetinck P. F. Link protein is ubiquitously expressed in non-cartilaginous tissues where it enhances and stabilizes the interaction of proteoglycans with hyaluronic acid. J Biol Chem. 1994 Jul 22;269(29):19116–19122. [PubMed] [Google Scholar]
- Colombatti A., Bonaldo P., Doliana R. Type A modules: interacting domains found in several non-fibrillar collagens and in other extracellular matrix proteins. Matrix. 1993 Jul;13(4):297–306. doi: 10.1016/s0934-8832(11)80025-9. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Engel J. Common structural motifs in proteins of the extracellular matrix. Curr Opin Cell Biol. 1991 Oct;3(5):779–785. doi: 10.1016/0955-0674(91)90050-9. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete D. M., Cepko C. L. Retroviral infection coupled with tissue transplantation limits gene transfer in the chicken embryo. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2350–2354. doi: 10.1073/pnas.90.6.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild D. R., Tondravi M. M., Hofer U., Chen Q., Goetinck P. F. The role of coiled-coil alpha-helices and disulfide bonds in the assembly and stabilization of cartilage matrix protein subunits. A mutational analysis. J Biol Chem. 1995 Sep 29;270(39):23150–23154. doi: 10.1074/jbc.270.39.23150. [DOI] [PubMed] [Google Scholar]
- Hauser N., Paulsson M. Native cartilage matrix protein (CMP). A compact trimer of subunits assembled via a coiled-coil alpha-helix. J Biol Chem. 1994 Oct 14;269(41):25747–25753. [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. N., Osborne-Lawrence S. L., Sinclair A. K., Eddy R. L., Jr, Byers M. G., Shows T. B., Duby A. D. Structure and chromosomal location of the human gene encoding cartilage matrix protein. J Biol Chem. 1990 Nov 15;265(32):19624–19631. [PubMed] [Google Scholar]
- Kielty C. M., Whittaker S. P., Grant M. E., Shuttleworth C. A. Type VI collagen microfibrils: evidence for a structural association with hyaluronan. J Cell Biol. 1992 Aug;118(4):979–990. doi: 10.1083/jcb.118.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss I., Deák F., Holloway R. G., Jr, Delius H., Mebust K. A., Frimberger E., Argraves W. S., Tsonis P. A., Winterbottom N., Goetinck P. F. Structure of the gene for cartilage matrix protein, a modular protein of the extracellular matrix. Exon/intron organization, unusual splice sites, and relation to alpha chains of beta 2 integrins, von Willebrand factor, complement factors B and C2, and epidermal growth factor. J Biol Chem. 1989 May 15;264(14):8126–8134. [PubMed] [Google Scholar]
- Linsenmayer T. F., Hendrix M. J. Monoclonal antibodies to connective tissue macromolecules: type II collagen. Biochem Biophys Res Commun. 1980 Jan 29;92(2):440–446. doi: 10.1016/0006-291x(80)90352-6. [DOI] [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. A., Izpisúa-Belmonte J. C., Duboule D., Tabin C. J. Targeted misexpression of Hox-4.6 in the avian limb bud causes apparent homeotic transformations. Nature. 1992 Jul 16;358(6383):236–239. doi: 10.1038/358236a0. [DOI] [PubMed] [Google Scholar]
- Pacifici M. Independent secretion of proteoglycans and collagens in chick chondrocyte cultures during acute ascorbic acid treatment. Biochem J. 1990 Nov 15;272(1):193–199. doi: 10.1042/bj2720193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Matrix proteins bound to associatively prepared proteoglycans from bovine cartilage. Biochem J. 1979 Dec 1;183(3):539–545. doi: 10.1042/bj1830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Purification and structural characterization of a cartilage matrix protein. Biochem J. 1981 Aug 1;197(2):367–375. doi: 10.1042/bj1970367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Radioimmunoassay of the 148-kilodalton cartilage protein. Distribution of the protein among bovine tissues. Biochem J. 1982 Nov 1;207(2):207–213. doi: 10.1042/bj2070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos C. J., Hughes S. H. Replication-competent retrovirus vectors for the transfer and expression of gene cassettes in avian cells. J Virol. 1991 Jul;65(7):3728–3737. doi: 10.1128/jvi.65.7.3728-3737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts W. M., Olsen M., Boettiger D., Vogt V. M. Epitope mapping of monoclonal antibodies to gag protein p19 of avian sarcoma and leukaemia viruses. J Gen Virol. 1987 Dec;68(Pt 12):3177–3182. doi: 10.1099/0022-1317-68-12-3177. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Riddle R. D., Johnson R. L., Laufer E., Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993 Dec 31;75(7):1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Schmid T. M., Conrad H. E. A unique low molecular weight collagen secreted by cultured chick embryo chondrocytes. J Biol Chem. 1982 Oct 25;257(20):12444–12450. [PubMed] [Google Scholar]
- Shapiro I. M., Leboy P. S., Tokuoka T., Forbes E., DeBolt K., Adams S. L., Pacifici M. Ascorbic acid regulates multiple metabolic activities of cartilage cells. Am J Clin Nutr. 1991 Dec;54(6 Suppl):1209S–1213S. doi: 10.1093/ajcn/54.6.1209s. [DOI] [PubMed] [Google Scholar]
- Stirpe N. S., Goetinck P. F. Gene regulation during cartilage differentiation: temporal and spatial expression of link protein and cartilage matrix protein in the developing limb. Development. 1989 Sep;107(1):23–33. doi: 10.1242/dev.107.1.23. [DOI] [PubMed] [Google Scholar]
- Stopak D., Harris A. K. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev Biol. 1982 Apr;90(2):383–398. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]
- Tondravi M. M., Winterbottom N., Haudenschild D. R., Goetinck P. F. Cartilage matrix protein binds to collagen and plays a role in collagen fibrillogenesis. Prog Clin Biol Res. 1993;383B:515–522. [PubMed] [Google Scholar]
- Winterbottom N., Tondravi M. M., Harrington T. L., Klier F. G., Vertel B. M., Goetinck P. F. Cartilage matrix protein is a component of the collagen fibril of cartilage. Dev Dyn. 1992 Mar;193(3):266–276. doi: 10.1002/aja.1001930307. [DOI] [PubMed] [Google Scholar]