Abstract
Wound biopsies are an essential diagnostic component in the management of chronic wounds. First, the possibility of malignancy or infection in the wound often requires sampling of the wound edge and its bed. Secondly, several practice guidelines recommend biopsying wounds that have not responded to treatment after 2–6 weeks. However, there has always been a concern that the biopsy may worsen the wound and delay overall healing. In this report, we investigated the safety and effects of wound biopsies on overall chronic wound healing rates (advance of the wound edge per week toward the center) before and after the biopsy was performed. In a cohort of 14 consecutive patients with chronic wounds of the lower extremity, we found that postbiopsy chronic wound healing rates (0.99±1.18 mm/week; mean±SD) were not decreased and were actually higher than prebiopsy chronic wound healing rates (0.49±0.85 mm/week; mean±SD, p < 0.05). In addition, we documented that healing of the biopsy sites up to the original wound edge occurred within 6 weeks in 11 of the 14 subjects. Therefore, we conclude that chronic wounds do not worsen after being biopsied and that wound biopsies are a safe procedure that does not delay overall healing of the chronic wound.
Chronic wounds are sometimes biopsied for diagnostic purposes and in experimental protocols when information regarding the wound bed or the wound edge is required. In addition, also in experimental protocols, wound biopsies are sometimes performed to grow cells in vitro from the nonhealing wound. This cellular analysis has led to a better understanding of the phenotypic cellular changes occurring in impaired healing.1–3 Our investigative group has been involved in performing wound biopsies for at least two decades. We initially noted that an obvious worsening of the wound did not occur after a biopsy of the edge was taken. Indeed, this led us to state that biopsying the wound is not deleterious to healing of the wound and that the biopsy site heals up to the original edge of the wound.4 However, lingering concerns remain about the safety of biopsying nonhealing wounds, and questions are often raised by clinicians about whether this procedure worsens the outcome of the wound. Moreover, there are biological implications regarding the notion that a biopsy site would heal in the context of a chronic wound that, otherwise, has not responded to treatment. Therefore, an important question is whether, indeed, one can document that the biopsy heals up to the original wound’s edge.
In this report, we analyzed our recent experience with a cohort of patients with nonhealing wounds which were biopsied at the wound edge and were followed after the biopsy was taken. In order to develop a more complete answer to our questions, we included patients on conventional therapy alone and those who were undergoing experimental treatment. It was our hypothesis that, in agreement with our previous but more anecdotal clinical experience, there is no deleterious effect of a biopsy to the overall healing of the wounds. The results shown in this report support this hypothesis. They also show that the biopsy site indeed heals up to the original wound edge, raising interesting questions about the biological and pathophysiological events involved.
METHODS
Data for this report came from recent research studies we have performed for the treatment of chronic wounds at our institution. All studies were approved by our Institutional Review Board (IRB). Although we have been involved in numerous clinical trials at our institution, we focused on research studies (n=4) with identical screening periods and protocols for biopsying the wound, and with the same inclusion and exclusion criteria. The inclusion criteria were the following: patients with wounds > 3 months in duration; a run-in phase of 2–3 weeks during which standard treatment was consistently applied; patients who had a biopsy of the wound’s edge at the end of the run-in period and had follow-up measurements and photographs of the wound and biopsy site available 2–3 weeks after the initial biopsy. The exclusion criteria were the following: patients with circumferential wounds or other situations where measurements of size were not possible or would be deemed unreliable; patients being treated with immunosuppressive drugs; patients with systemic or skin malignancies; patients with poor compliance with follow-up visits that would prevent reliable follow-up measurements. Data were collected and analyzed for each individual patient only once. The duration of the studies was between 5 and 12 months.
After completing the run-in phase, the patients underwent a full-thickness wedge biopsy of the wound’s edge (Figure 1). Continued conventional or initiation of experimental treatments took place after the biopsy was obtained. Eligible patients were followed weekly with clinical wound observation, wound area tracings, and photography. The area and perimeter of the wounds and areas of the biopsy sites were determined by digital planimetry.5,6 Measured dimensions of the wound were used as a scale. Area and perimeter were then determined by using Image J, a program available from the National Institutes of Health (NIH; website http://www.rsbweb.nih.gov/ij). Overall chronic wound healing rates were derived using Gilman’s formula.7 In this formula, the healing rate= {(A1−A2)/[(P1+P2)/2]}/(T2−T1), where A is the area in square millimeters (mm2), P the perimeter in millimeters (mm), and T the weeks. The result is a chronic wound healing rate in millimeters (mm) per week. In practical terms, this relatively simple equation gives an indication of how quickly the wound’s perimeter moves toward the center of the wound, and is not dependent on original wound size. Our group has reported a variation of the Gilman formula.8 However, we used the Gilman formula because it is more simple and has been more widely used.
Figure 1.
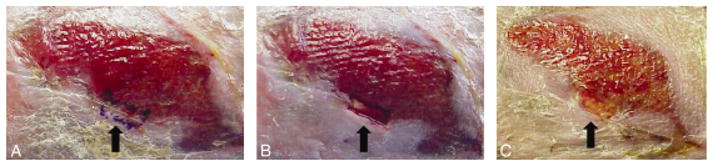
Example of a biopsy site. An example of a chronic ulcer before and after it is biopsied. As with other patients, this subject completed a 2–3 week run-in period during which standard care was provided. (A) Wound before biopsy, with wound area to be biopsied identified and marked by ink; (B) full thickness biopsy performed at the edge of the ulcer and appearing as a rectangular area; (C) chronic wound 3 days after it was biopsied. The black arrow refers to the site that was biopsied (Copyright V. Falanga 2009).
Statistical analysis of overall chronic wound healing rates with the Gilman’s formula was performed before and after a biopsy by using the Wilcoxon matched-pairs signed-ranks two-tailed test. Statistical calculations were made with the Graphpad Instat statistical software (Graphpad Software Inc., La Jolla, CA). The significance level used to determine the validity of the results was set at p < 0.05 (95% confidence interval). In addition, serial wound photographs and wound tracings were analyzed to determine when the biopsy sites healed. The patients were followed clinically for adverse events, including infection, before and after the biopsy was performed. Charts were reviewed to determine if the chronic wound healed.
RESULTS
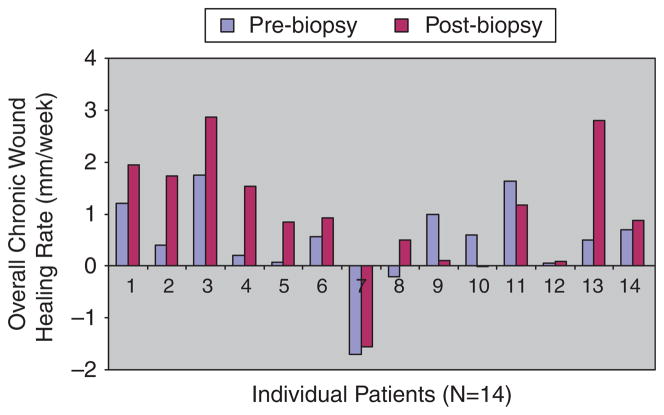
In this study aimed at evaluating data from clinical trials/experimental protocols that had similar or identical population of patients, wounds, and inclusion/exclusion criteria, the results were available for analysis in 14 patients. Overall, nine patients received an experimental treatment plus standard of care, whereas five patients had standard of care alone. These two groups of patients comprised eight men and six women with a mean age of 65 (range: 50–82 years). Baseline wounds had a mean area of 16.87 cm2 (range: 2.86–80.29 cm2). The mean of the biopsy areas was 0.55 cm2 (range: 0.26–1.23 cm2). There was no statistical significance between the two groups in terms of these demographic and wound-related parameters. Eleven patients had venous ulcers, two had diabetic neuropathic foot ulcers, and one had a nondiabetic neuropathic foot ulcer. Table 1 shows the mean, median, and range of overall chronic wound healing rates (mm/week) pre and postbiopsy, using the Gilman formula. As shown in Table 1, there was no evidence that the biopsy led to a deterioration of healing. Indeed, postbiopsy chronic wound healing rates were actually greater than those obtained prebiopsy. This is shown in Figure 2, where each bar represents the healing rate for each patient at pre and postbiopsy. Indeed, the Wilcoxon matched-pairs signed-ranks test showed that the mean prebiopsy overall chronic wound healing rates were smaller (slower) than the mean postbiopsy overall chronic healing rates (p < 0.05).
Table 1.
Measurement of overall chronic healing rates pre and postwound biopsy
| Healing rate prebiopsy (mm/week) | Healing rate postbiopsy (mm/week) | |
|---|---|---|
| Mean | 0.49 | 0.99 |
| Median | 0.54 | 0.90 |
| Range | (−01.7 to +1.75) | (−01.56 to +2.86) |
| SD/SEM | 0.85/0.23 | 1.18/0.31 |
SD, standard deviation; SEM, standard error of the mean.
Figure 2.
Overall chronic healing rates of patients. Fourteen patients were included in the study. Each pair of bars corresponds to healing rates of each patient before (2–3 weeks) and after (2–3 weeks) the biopsy was performed, respectively. From left to right along the x-axis, the first five patients were on standard care alone, whereas patients 6–14 along the x-axis were on standard care plus an experimental treatment.
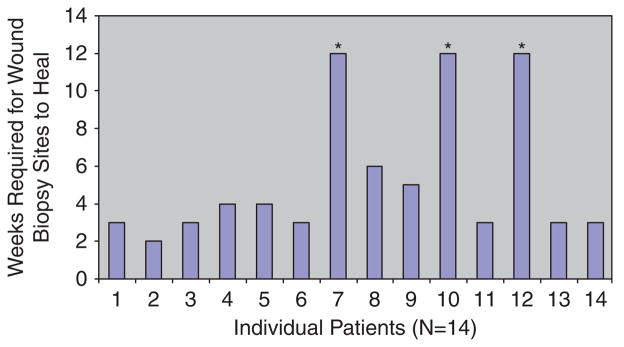
In 11 (79%) of the 14 patients, the biopsy sites healed completely by week 6. In three patients, however, the biopsy sites remained unhealed at week 12. This occurrence may theoretically reflect continued impaired healing per se or may be because of the intervention and biopsy. However, there were other concomitant adverse events that may have played a role. Figure 3 shows the number of weeks required for each patient’s biopsy site to heal. The mean and median time required for biopsy sites to heal was 3 and 4 weeks, respectively. As noted above, there were concomitant or associated adverse events that may have influenced healing of the biopsy site. Overall, there were three adverse events in a total of three patients with venous ulcers: two of these events were wound infections and one was the development of a new wound adjacent to the original ulcer and next to the biopsy site. Notably, the same three patients whose biopsy sites did not heal were the same three patients with these adverse events. In the two patients with postbiopsy infections, this occurrence developed 1 and 3 weeks after the biopsy, respectively. Interestingly, the patient developing an infection 1 week after a wound biopsy had already developed an infection at the same site a few weeks earlier, and that infection had been judged clinically to have resolved 1 week before the biopsy.
Figure 3.
Biopsy site healing. Overall, 11 (79%) of 14 patients healed by 6 weeks. The asterisk marks the patients who did not heal by week 12.
Figures 4 shows two representative examples of how the biopsy sites healed. The biopsies were full-thickness and encompassed both the wound edge and the wound bed. The figure illustrates in a representative way how biopsying the wound does not lead to overall deterioration of the wound.
Figure 4.
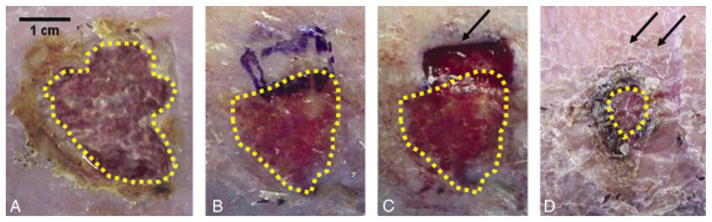
Biopsy sites heal. A representative example of a chronic wound after a 2–3 weeks run-in period, after which it was biopsied and followed for another 2–3 weeks. The single arrow refers to the wound site that was biopsied. The double arrows represent the healed biopsy site. The dotted yellow line outlines the area of the chronic wound at baseline (prebiopsy), and at the later time points after the biopsy was performed: (A) Wound at baseline before starting the run-in period; (B) Wound after completion of the run-in period immediately before performing a biopsy of the wound edge; (C) Appearance of the wound immediately after biopsy was performed; (D) Appearance of the wounds 2 weeks later, showing rapid healing of the biopsy site (Copyright V. Falanga 2009).
Overall, five (36%) of 14 patients healed within 13 weeks with a mean overall healing rate of 1.86 mm/week (range: 0.92–2.8 mm/week). By the end of 12 months, nine (64%) of the 14 subjects were healed, with a mean healing rate of 1.14 mm/week (range: −0.015 to 1.95 mm/week). In all, five patients did not heal after a year, and those five subjects had a mean healing rate 0.01 mm/week (range −1.5 to 0.84).
At the time of the biopsy, three patients had more than one lower extremity ulcer. In one patient, the biopsied wound healed a week before the nonbiopsied ulcer. In another patient both the biopsied and nonbiopsied ulcer remained open after a year. The biopsied ulcer in the third patient healed at week 10 and the nonbiopsied ulcer healed at week 3.
DISCUSSION
We report the effect of biopsying the wound edge on the healing of chronic wounds. We show that chronic wounds do not worsen overall after a biopsy of the wound edge and wound bed was performed. Indeed, perhaps antiintuitively, our data indicate that the overall chronic wound healing rate improved after the biopsy.
We show that, in the majority of the patients, the biopsy site heals up to the original wound edge. The biological underpinning for this occurrence is unclear. There is a paucity of studies investigating the effects of biopsies on the healing of chronic wounds. However, recent research on the molecular pathogenesis of nonhealing wounds has shown that there are phenotypic differences in the cells populating the wound edge and the surrounding skin. Keratinocytes at the edge of the wound show deregulation in cell differentiation and cell migration, resulting in a hyper-proliferative epidermal edge that fails to reepithelialize the wound bed. However, cells in the periwound area retain the capacity to differentiate, migrate, and respond appropriately to wound healing signals.9–14 In other studies, phenotypic changes have been found between dermal fibroblasts cultured from chronic vs. acute wounds. Dermal fibroblasts cultured from the edge of chronic wounds have decreased responsiveness to transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor in terms of collagen production and proliferation.2,3 The mechanism for decreased responsiveness to TGF-β1 may be the result of down-regulation of TGF-β Type II receptor expression and under phosphorylation of key signaling proteins, such as Smad2/3 and p48/44 MAPK.1 The expression of a downstream TGF-β inducible protein, βig-H3, has been found to be decreased in chronic wound fibroblasts and in the dermis of chronic nonhealing wounds. Thus, taking a biopsy of the wound edge may remove part of the nonhealing edge and abnormal cell populations, and thus produce an acute injury capable of resetting the healing process. This sequence of events may lead to healing in a more timely manner.
There are no studies focused primarily on the safety of performing wound biopsies in chronic wounds. However, several practice guidelines have recommended performing wound biopsies for histological diagnosis or microbiologic testing in wounds that have not improved within 2–6 weeks of appropriate management.15–22 The Food and Drug Administration (FDA) recommends performing biopsies of the wound, when needed clinically, as an objective tool to exclude neoplastic, immune-mediated or primary infectious disease. In addition, they suggest performing wound biopsies to diagnose wound infections and to guide treatment.23 The majority of our patients did not experience any adverse events on follow-up. Infections did occur in two subjects, as described in this report. However, we attribute this complication to the disease process itself. Thus, in one of these two patients infection occurred 3 weeks after the biopsy was performed. In the other case, the patient had a recent history of infection at the same site. In the future, we may use this information to guide us when to best perform wound biopsies. For example, based on our results, one might recommend that diagnostic or experimental biopsies be performed several weeks after resolution of the infection.
Wound healing rates at 3 and 4 weeks have been used as predictors of ultimate wound closure. A recent study published on healing rates of both venous and diabetic ulcers determined that a healing rate of 1.5 mm/week was predictive of wound closure at 12 weeks.7 Our findings are consistent with that report. Therefore, our data indicate that healing continues to occur after biopsies of a wound are performed.
In conclusion, our report shows that wound edge biopsies do not lead to overall deterioration of the chronic wound. Interestingly, the biopsy site heals up to the original wound edge. It is quite possible that, once stabilized (standard therapy with a run-in period), chronic wounds reach a size that corresponds to the underlying pathophysiological defects and are, therefore, as large as they can be, based on those intrinsic abnormalities. Certainly, more studies with a larger sample size and a more homogenous patient population are needed. However, the data presented in this report are consistent with our long clinical experience that biopsies are a safe procedure in patients with otherwise impaired healing. These findings should prove useful for clinicians and investigators.
Acknowledgments
This work was supported by National Institutes Grant P20RR018757 and its Imaging Core. We thank Dr. Yajni Warnapala for assistance in the statistical analysis and the determination of overall chronic wound healing rates.
References
- 1.Kim BC, Kim HT, Park SH, Cha JS, Yufit T, Kim SJ, Falanga V. Fibroblasts from chronic wounds show altered TGF-beta-signaling and decreased TGF-beta Type II receptor expression. J Cell Physiol. 2003;195:331–6. doi: 10.1002/jcp.10301. [DOI] [PubMed] [Google Scholar]
- 2.Agren MS, Steenfos HH, Dabelsteen S, Hansen JB, Dabelsteen E. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcerage dependent. J Invest Dermatol. 1999;112:463–9. doi: 10.1046/j.1523-1747.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 3.Hasan A, Murata H, Falabella A, Ochoa S, Zhou L, Badiavas E, Falanga V. Dermal fibroblasts from venous ulcers are unresponsive to the action of transforming growth factor-beta 1. J Dermatol Sci. 1997;16:59–66. doi: 10.1016/s0923-1811(97)00622-1. [DOI] [PubMed] [Google Scholar]
- 4.Falanga V, Eaglstein WH. Management of venous ulcers. Am Fam Phys. 1986;33:274–81. [PubMed] [Google Scholar]
- 5.Jessup RL. What is the best method for assessing the rate of wound healing? A comparison of 3 mathematical formulas. Adv Skin Wound Care. 2006;19:138–47. doi: 10.1097/00129334-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Moore K, McCallion R, Searle RJ, Stacey MC, Harding KG. Prediction and monitoring the therapeutic response of chronic dermal wounds. Int Wound J. 2006;3:89–96. doi: 10.1111/j.1742-4801.2006.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardinal M, Eisenbud DE, Phillips T, Harding K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen. 2008;16:19–22. doi: 10.1111/j.1524-475X.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- 8.Tallman P, Muscare E, Carson P, Eaglstein WH, Falanga V. Initial rate of healing predicts complete healing of venous ulcers. Arch Dermatol. 1997;133:1231–4. [PubMed] [Google Scholar]
- 9.Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I, Golinko M, Rosenberg H, Tomic-Canic M. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13:30–9. doi: 10.2119/2006-00054.Brem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pastar I, Stojadinovic O, Tomic-Canic M. Role of keratinocytes in healing of chronic wounds. Surg Technol Int. 2008;17:105–12. [PubMed] [Google Scholar]
- 11.Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, Merchant A, Galiano RD, Tomic-Canic M. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167:59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stojadinovic O, Pastar I, Vukelic S, Mahoney MG, Brennan D, Krzyzanowska A, Golinko M, Brem H, Tomic-Canic M. Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J Cell Mol Med. 2008;12:2675–90. doi: 10.1111/j.1582-4934.2008.00321.x. [Epub March 28, 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usui ML, Mansbridge JN, Carter WG, Fujita M, Olerud JE. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J Histochem Cytochem. 2008;56:687–96. doi: 10.1369/jhc.2008.951194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charles CA, Tomic-Canic M, Vincek V, Nassiri M, Stojadinovic O, Eaglstein WH, Kirsner RJ. A gene signature of nonhealing venous ulcers: potential diagnostic markers. J Am Acad Dermatol. 2008;59:758–71. doi: 10.1016/j.jaad.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackroyd JS, Young AE. Leg ulcers that do not heal. Br Med J (Clin Res Ed) 1983;286:207–8. doi: 10.1136/bmj.286.6360.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combemale P, Bousquet M, Kanitakis J, Bernard P. Malignant transformation of leg ulcers: a retrospective study of 85 cases. J Eur Acad Dermatol Venereol. 2007;21:935–41. doi: 10.1111/j.1468-3083.2006.02118.x. [DOI] [PubMed] [Google Scholar]
- 17.Conde-Taboada A, De la Torre C, Florez A, Garcia-Doval I, Cruces M. Chronic leg ulcers and basal cell carcinoma. J Eur Acad Dermatol Venereol. 2006;20:359. doi: 10.1111/j.1468-3083.2006.01442.x. [DOI] [PubMed] [Google Scholar]
- 18.Harris B, Eaglstein WH, Falanga V. Basal cell carcinoma arising in venous ulcers and mimicking granulation tissue. J Dermatol Surg Oncol. 1993;19:150–2. doi: 10.1111/j.1524-4725.1993.tb03445.x. [DOI] [PubMed] [Google Scholar]
- 19.Pellizzer G, Strazzabosco M, Presi S, Furlan F, Lora L, Benedetti P, Bonato M, Erle G, de Lalla F. Deep tissue biopsy vs. superficial swab culture monitoring in the microbiological assessment of limb-threatening diabetic foot infection. Diabet Med. 2001;18:822–7. doi: 10.1046/j.1464-5491.2001.00584.x. [DOI] [PubMed] [Google Scholar]
- 20.Robson MC, Cooper DM, Aslam R, Gould LJ, Harding KG, Margolis DJ, Ochs DE, Serena TE, Snyder RJ, Steed DL, Thomas DR, Wiersma-Bryant L. Guidelines for the treatment of venous ulcers. Wound Repair Regen. 2006;14:649–62. doi: 10.1111/j.1524-475X.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 21.Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, Johnson A, Moosa H, Robson M, Serena T, Sheehan P, Veves A, Wiersma-Bryant L. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen. 2006;14:680–92. doi: 10.1111/j.1524-475X.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang D, Morrison BD, Vandongen YK, Singh A, Stacey MC. Malignancy in chronic leg ulcers. Med J Aust. 1996;164:718–20. doi: 10.5694/j.1326-5377.1996.tb122269.x. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Guidance for Industry Chronic Cutaneous Ulcer and Burn Wounds-Developing Products for Treatment. 2006 June; Available at: http://www.fda.gov/cber/gdlns/ulcburn.htm.