Abstract
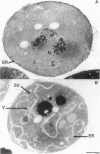
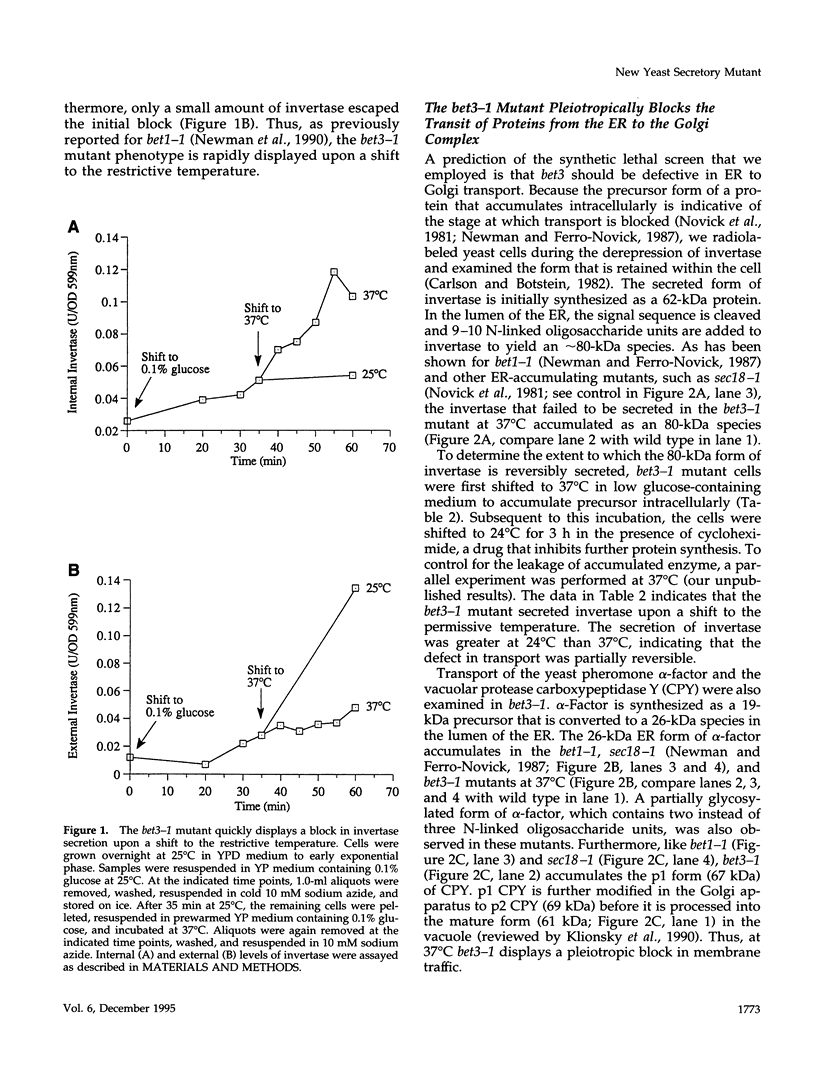
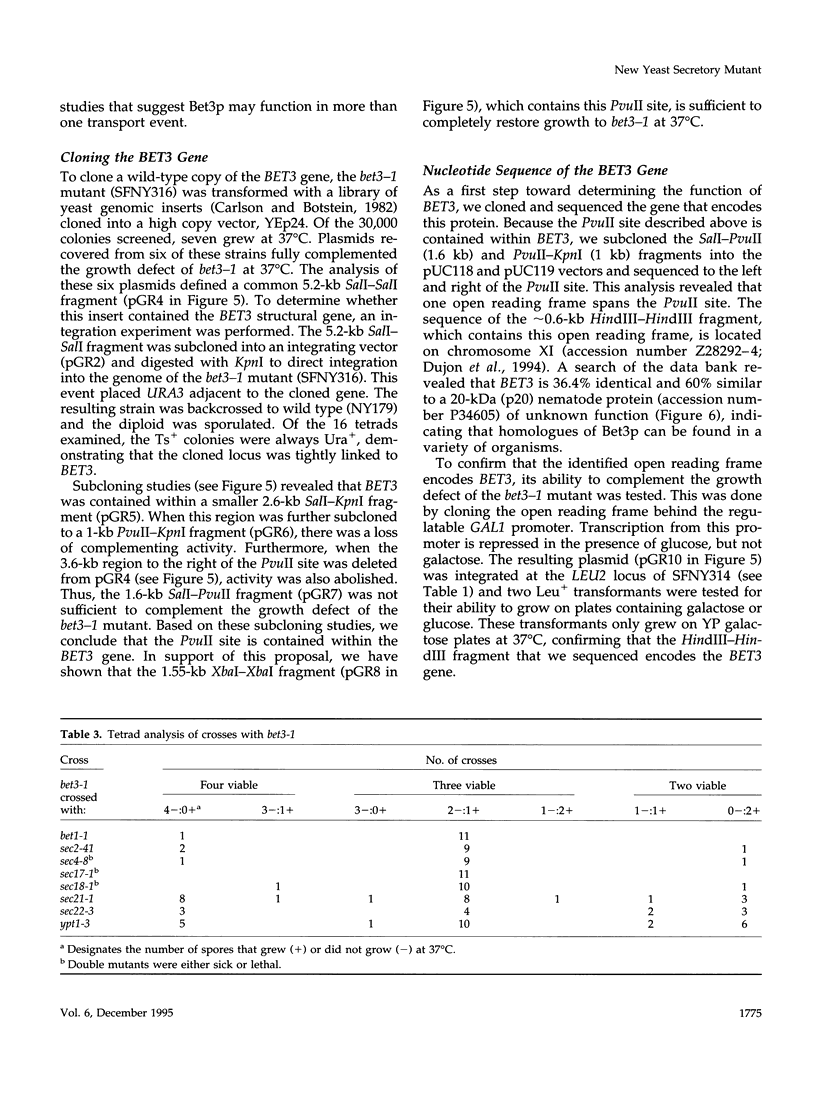
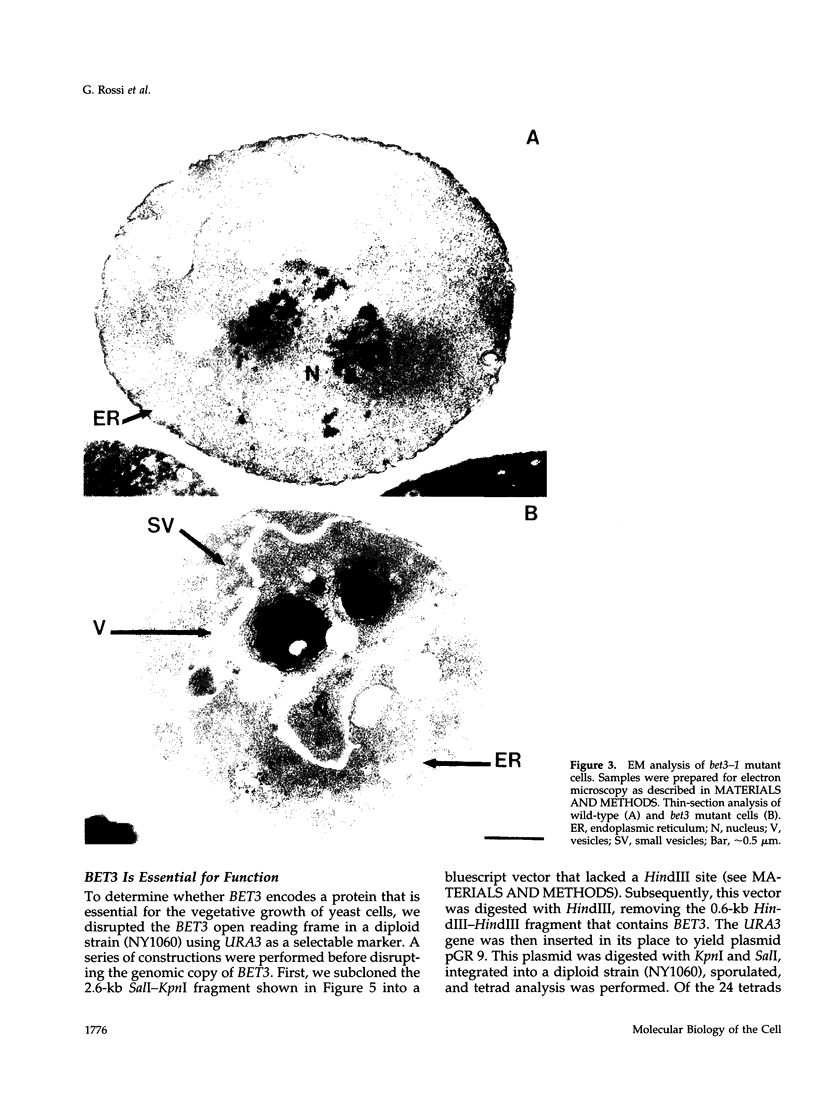
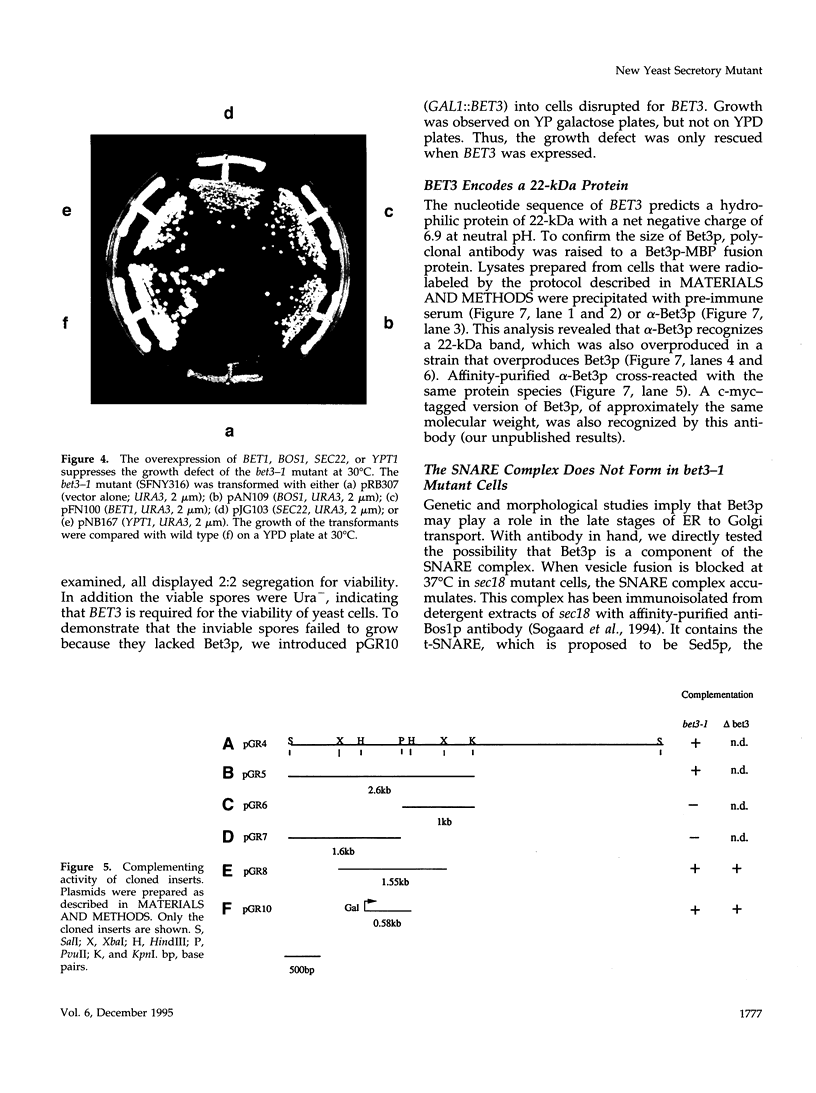
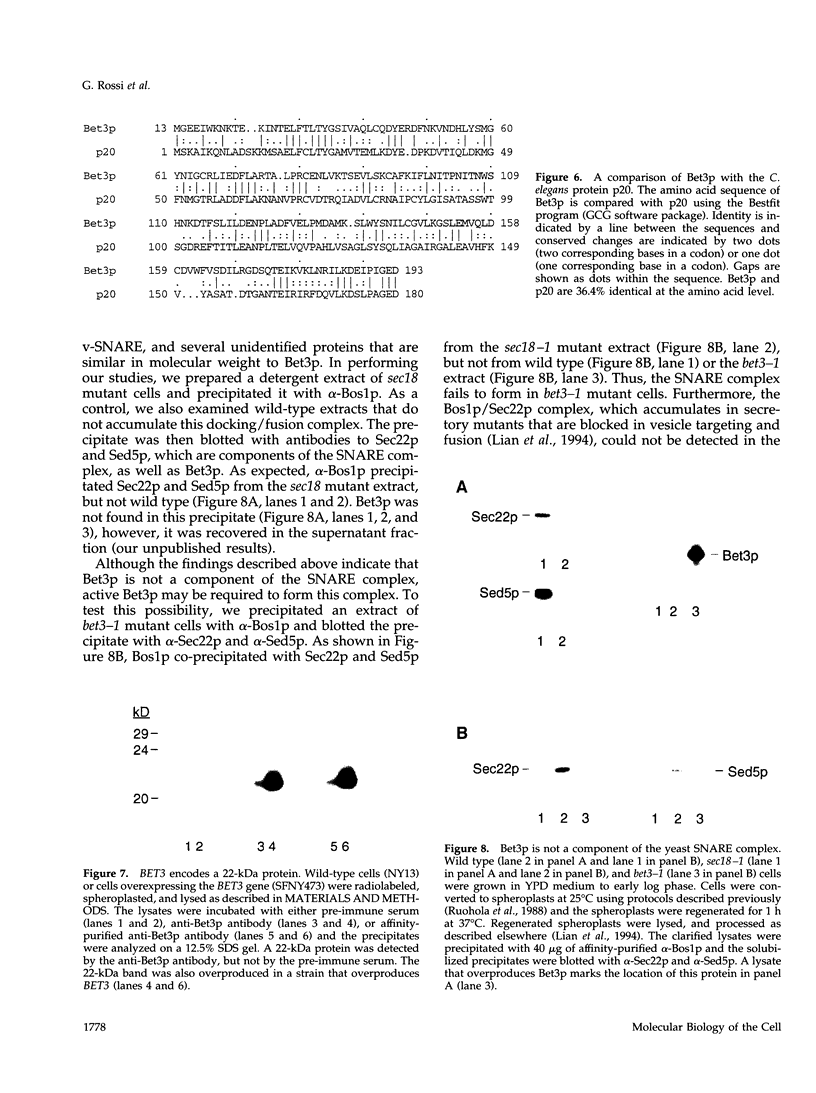
Here we report the identification of BET3, a new member of a group of interacting genes whose products have been implicated in the targeting and fusion of endoplasmic reticulum (ER) to Golgi transport vesicles with their acceptor compartment. A temperature-sensitive mutant in bet3-1 was isolated in a synthetic lethal screen designed to identify new genes whose products may interact with BET1, a type II integral membrane protein that is required for ER to Golgi transport. At 37 degrees C, bet3-1 fails to transport invertase, alpha-factor, and carboxypeptidase Y from the ER to the Golgi complex. As a consequence, this mutant accumulates dilated ER and small vesicles. The SNARE complex, a docking/fusion complex, fails to form in this mutant. Furthermore, BET3 encodes an essential 22-kDa hydrophilic protein that is conserved in evolution, which is not a component of this complex. These findings support the hypothesis that Bet3p may act before the assembly of the SNARE complex.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992 Jan 2;110(1):119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Griff I. C., Rothman J. E. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990 May 18;61(4):709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- Dascher C., Ossig R., Gallwitz D., Schmitt H. D. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991 Feb;11(2):872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B., Alexandraki D., André B., Ansorge W., Baladron V., Ballesta J. P., Banrevi A., Bolle P. A., Bolotin-Fukuhara M., Bossier P. Complete DNA sequence of yeast chromosome XI. Nature. 1994 Jun 2;369(6479):371–378. doi: 10.1038/369371a0. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S., Jahn R. Vesicle fusion from yeast to man. Nature. 1994 Jul 21;370(6486):191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S., Novick P. The role of GTP-binding proteins in transport along the exocytic pathway. Annu Rev Cell Biol. 1993;9:575–599. doi: 10.1146/annurev.cb.09.110193.003043. [DOI] [PubMed] [Google Scholar]
- Garrett M. D., Zahner J. E., Cheney C. M., Novick P. J. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 1994 Apr 1;13(7):1718–1728. doi: 10.1002/j.1460-2075.1994.tb06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A., Lampen J. O. Beta-D-fructofuranoside fructohydrolase from yeast. Methods Enzymol. 1975;42:504–511. doi: 10.1016/0076-6879(75)42159-0. [DOI] [PubMed] [Google Scholar]
- Graham T. R., Emr S. D. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991 Jul;114(2):207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griff I. C., Schekman R., Rothman J. E., Kaiser C. A. The yeast SEC17 gene product is functionally equivalent to mammalian alpha-SNAP protein. J Biol Chem. 1992 Jun 15;267(17):12106–12115. [PubMed] [Google Scholar]
- Groesch M. E., Ruohola H., Bacon R., Rossi G., Ferro-Novick S. Isolation of a functional vesicular intermediate that mediates ER to Golgi transport in yeast. J Cell Biol. 1990 Jul;111(1):45–53. doi: 10.1083/jcb.111.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi M., Kreis T., Schekman R. SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 1992 Dec 10;360(6404):603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990 Sep;54(3):266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J. P., Ferro-Novick S. Bos1p, an integral membrane protein of the endoplasmic reticulum to Golgi transport vesicles, is required for their fusion competence. Cell. 1993 May 21;73(4):735–745. doi: 10.1016/0092-8674(93)90253-m. [DOI] [PubMed] [Google Scholar]
- Lian J. P., Stone S., Jiang Y., Lyons P., Ferro-Novick S. Ypt1p implicated in v-SNARE activation. Nature. 1994 Dec 15;372(6507):698–701. doi: 10.1038/372698a0. [DOI] [PubMed] [Google Scholar]
- Louvard D., Reggio H., Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982 Jan;92(1):92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V., Orci L., Glick B. S., Block M. R., Rothman J. E. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 1988 Jul 15;54(2):221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- Newman A. P., Ferro-Novick S. Characterization of new mutants in the early part of the yeast secretory pathway isolated by a [3H]mannose suicide selection. J Cell Biol. 1987 Oct;105(4):1587–1594. doi: 10.1083/jcb.105.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A. P., Groesch M. E., Ferro-Novick S. Bos1p, a membrane protein required for ER to Golgi transport in yeast, co-purifies with the carrier vesicles and with Bet1p and the ER membrane. EMBO J. 1992 Oct;11(10):3609–3617. doi: 10.1002/j.1460-2075.1992.tb05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A. P., Shim J., Ferro-Novick S. BET1, BOS1, and SEC22 are members of a group of interacting yeast genes required for transport from the endoplasmic reticulum to the Golgi complex. Mol Cell Biol. 1990 Jul;10(7):3405–3414. doi: 10.1128/mcb.10.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Rossi G., Yu J. A., Newman A. P., Ferro-Novick S. Dependence of Ypt1 and Sec4 membrane attachment on Bet2. Nature. 1991 May 9;351(6322):158–161. doi: 10.1038/351158a0. [DOI] [PubMed] [Google Scholar]
- Ruohola H., Kabcenell A. K., Ferro-Novick S. Reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex in yeast: the acceptor Golgi compartment is defective in the sec23 mutant. J Cell Biol. 1988 Oct;107(4):1465–1476. doi: 10.1083/jcb.107.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J., Newman A. P., Ferro-Novick S. The BOS1 gene encodes an essential 27-kD putative membrane protein that is required for vesicular transport from the ER to the Golgi complex in yeast. J Cell Biol. 1991 Apr;113(1):55–64. doi: 10.1083/jcb.113.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J. N., Higgins D. R. Recovery of plasmids from yeast into Escherichia coli: shuttle vectors. Methods Enzymol. 1991;194:319–329. doi: 10.1016/0076-6879(91)94024-7. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Søgaard M., Tani K., Ye R. R., Geromanos S., Tempst P., Kirchhausen T., Rothman J. E., Söllner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994 Sep 23;78(6):937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Warren G. Cell biology. Bridging the gap. Nature. 1993 Mar 25;362(6418):297–298. doi: 10.1038/362297a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Wilcox C. A., Flynn G. C., Chen E., Kuang W. J., Henzel W. J., Block M. R., Ullrich A., Rothman J. E. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989 Jun 1;339(6223):355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]