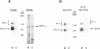Abstract
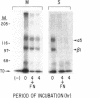
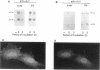
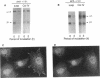
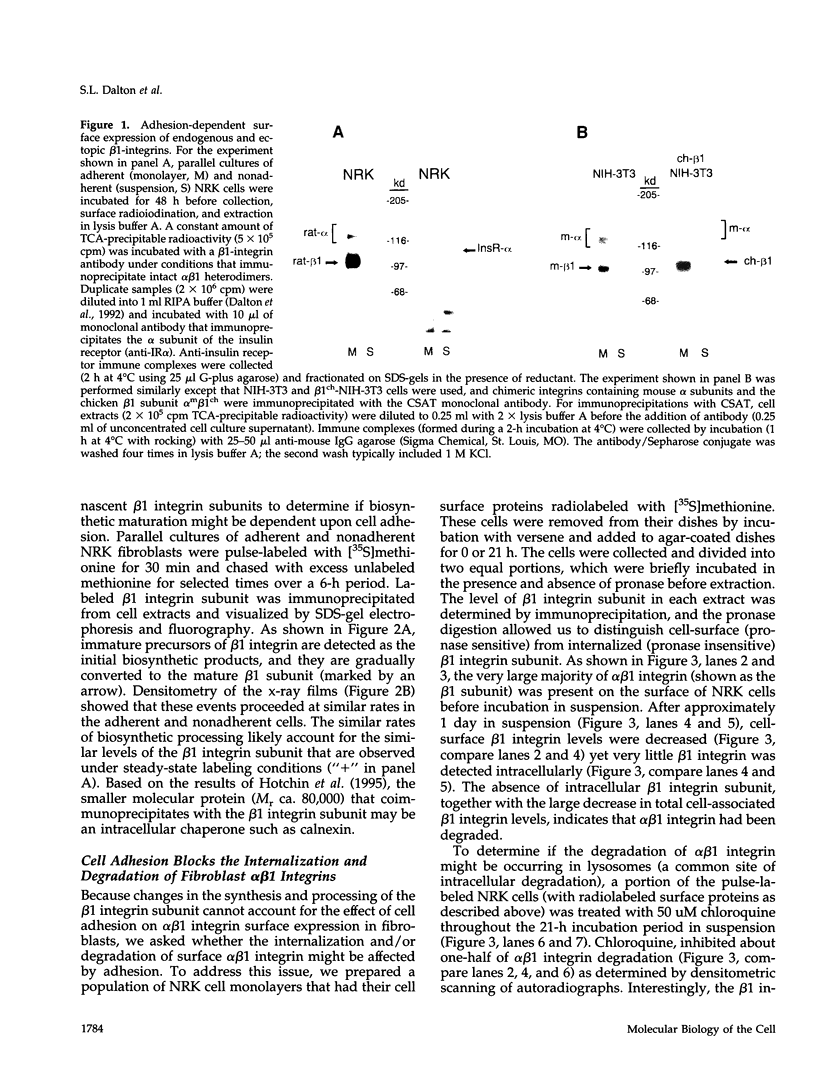
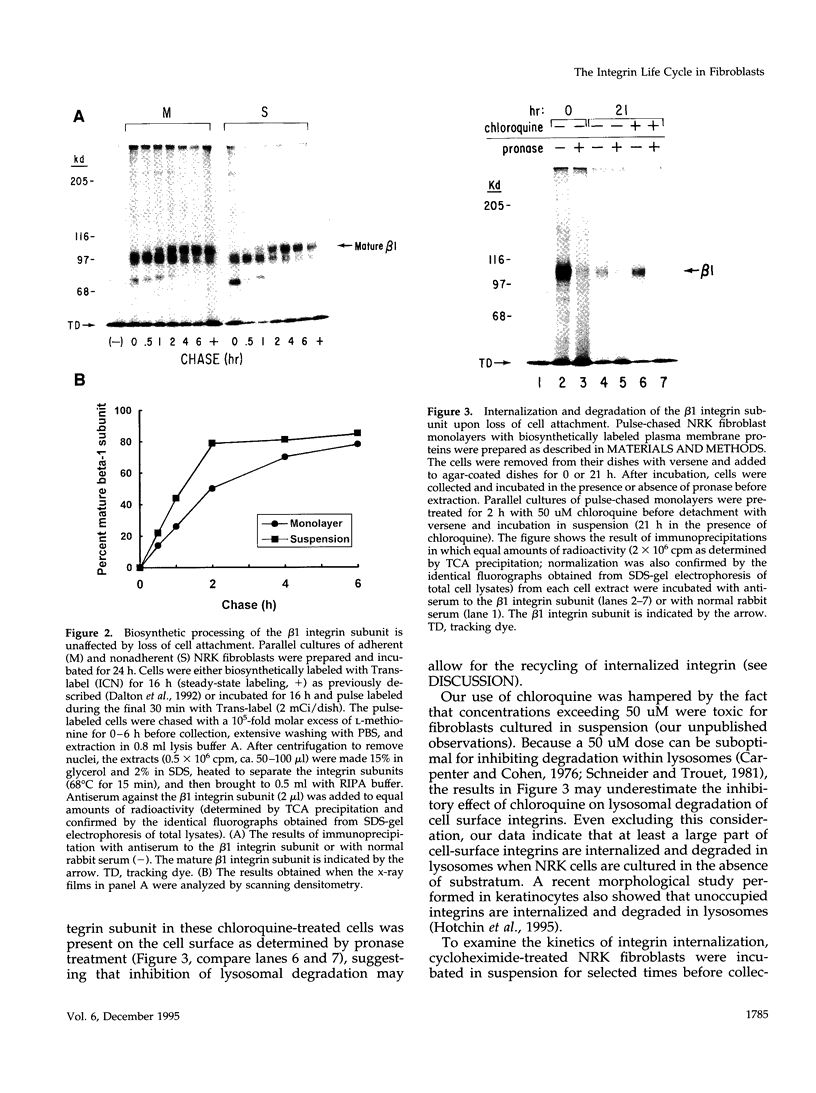
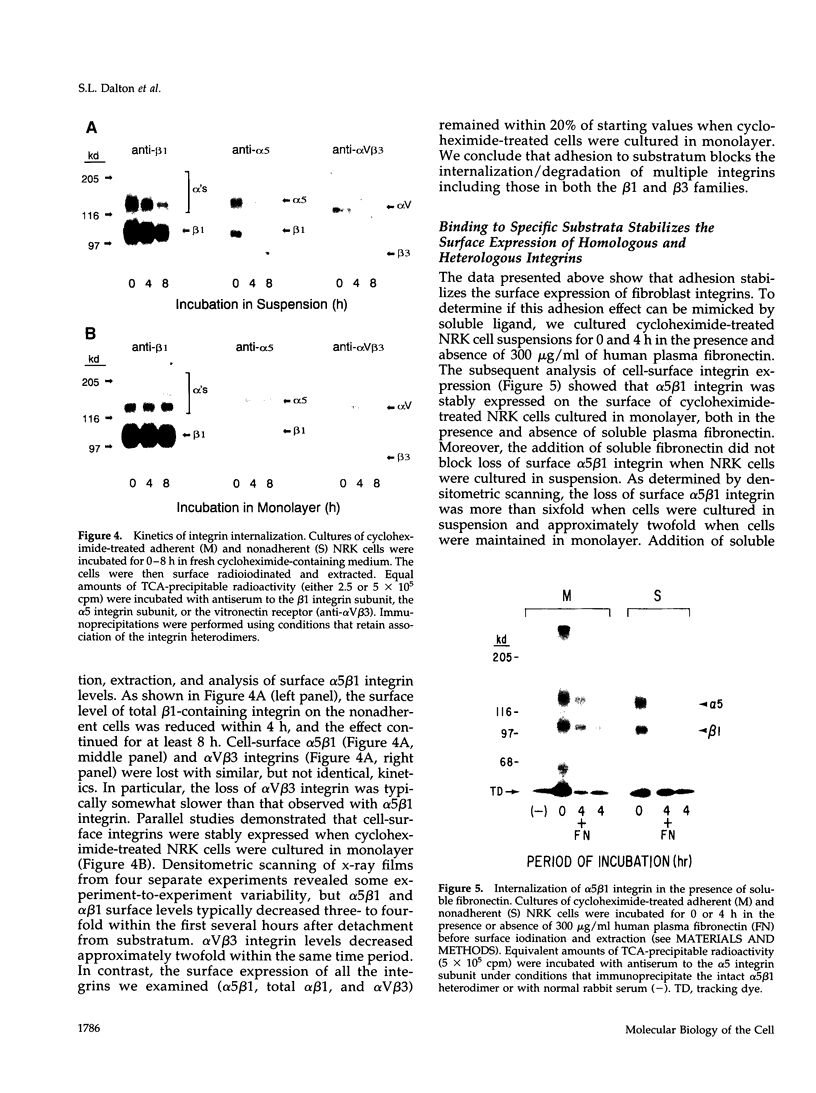
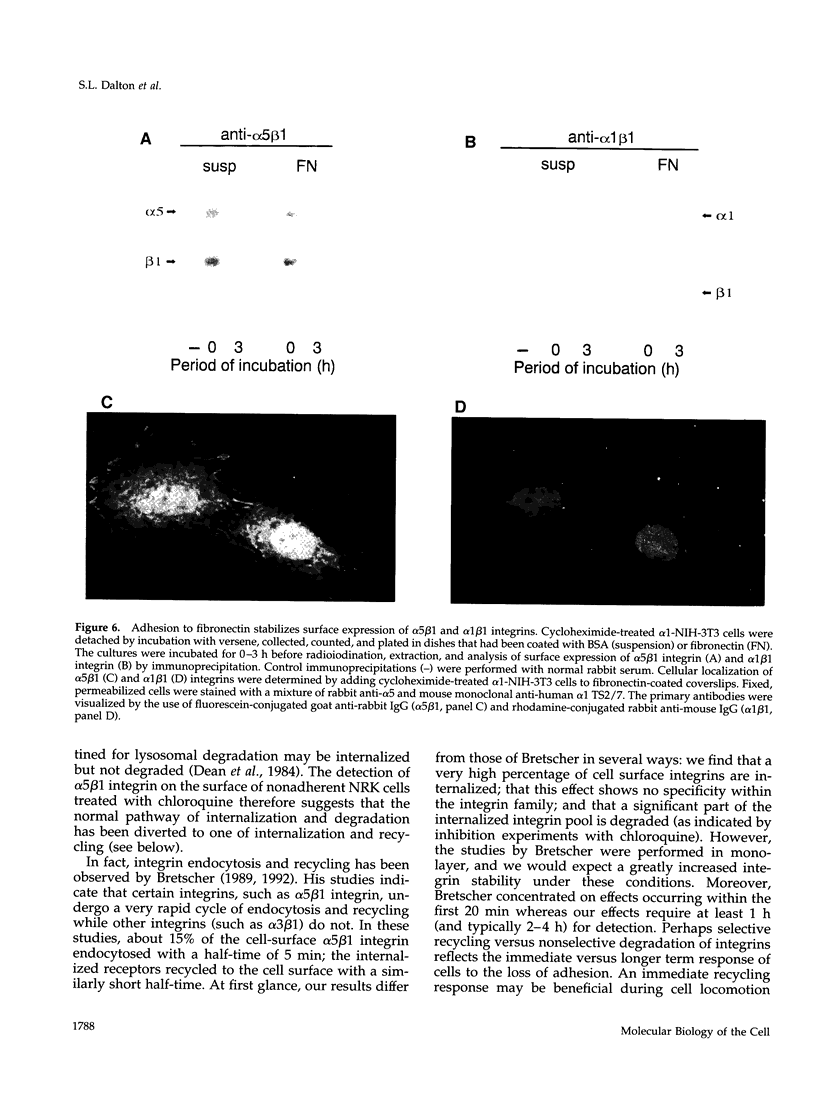
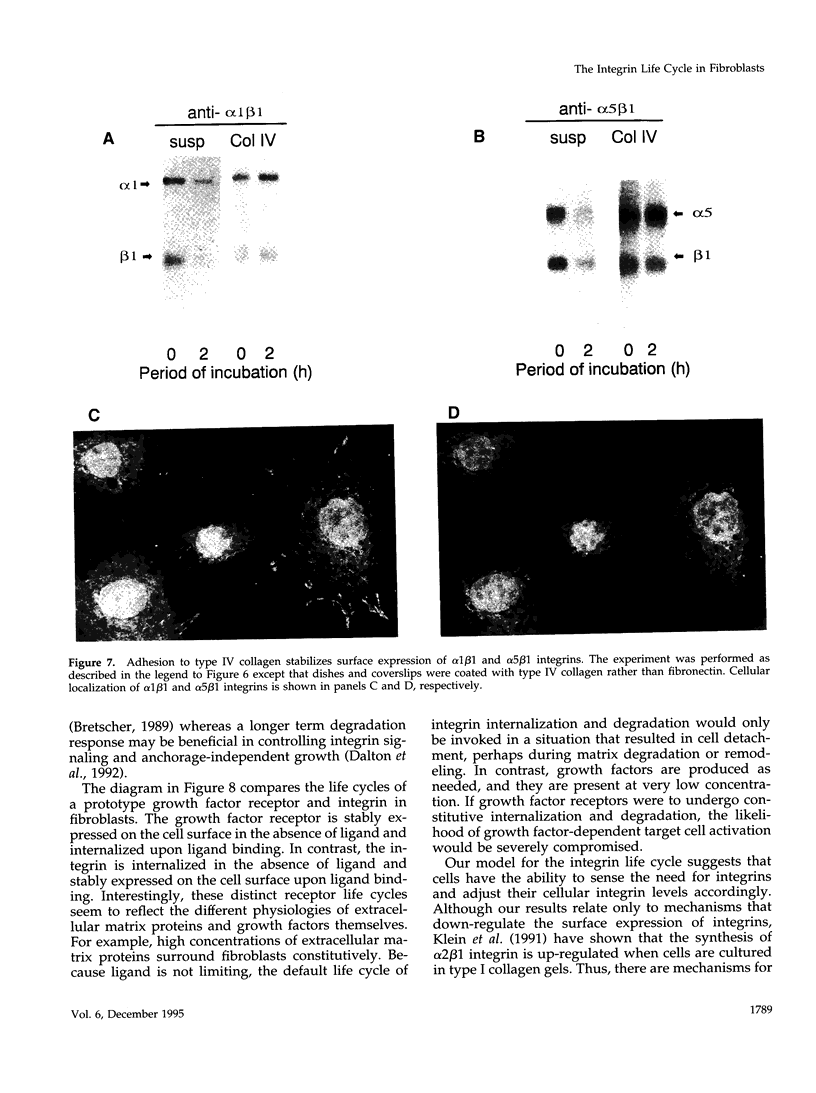
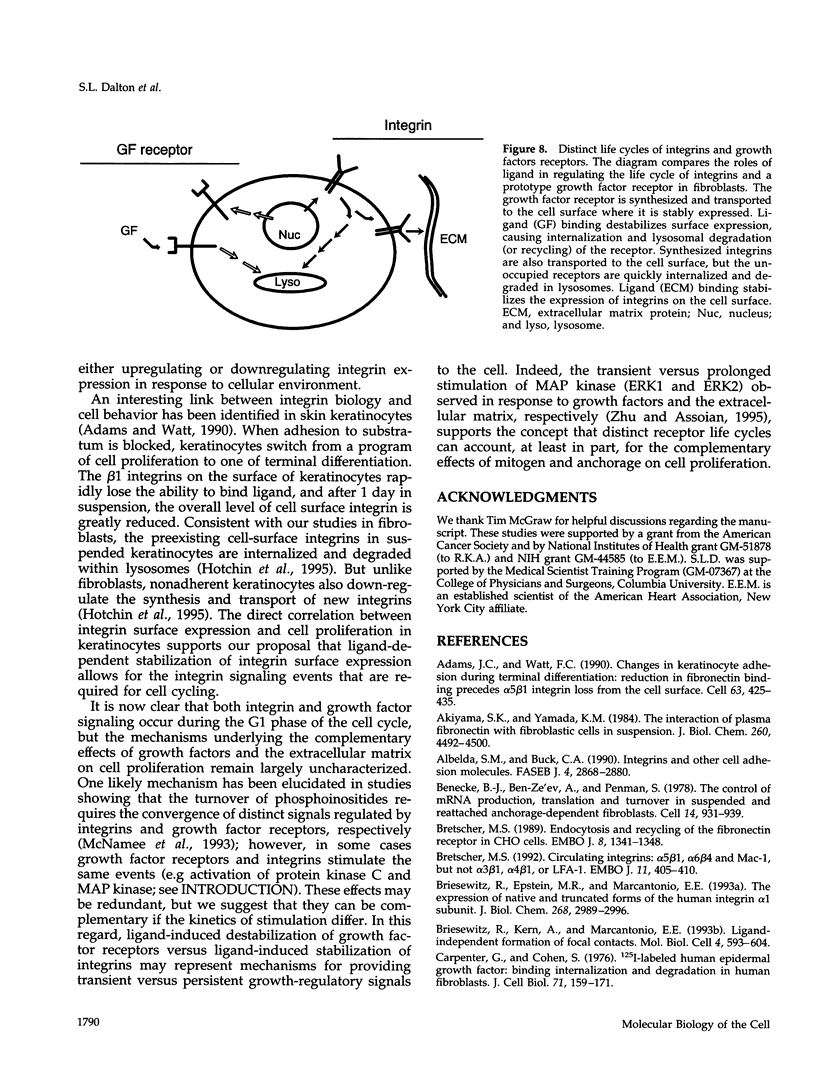
The expression of alpha 5 beta 1 integrin on the surface of fibroblasts requires adhesion to substratum. We have examined the basis for this adhesion-dependent surface expression by comparing the life cycle of integrins in parallel cultures of adherent and nonadherent cells. Results of biosynthetic labeling experiments in NRK fibroblasts showed that the synthesis and biosynthetic processing of the beta 1 integrin subunit proceed in the absence of cell attachment; however, when examining the behavior of preexisting cell surface integrins, we observed that the alpha beta 1 integrins are internalized and degraded when adhesion to substratum is blocked. A kinetic analysis of integrin internalization in cycloheximide-treated NRK cells showed that each of the fibroblast integrins we examined (in both the beta 1 and beta 3 families) are lost from the cell surface after detachment from substratum. Thus, the default integrin life cycle in fibroblasts involves continuous synthesis, processing, transport to the cell surface, and internalization/degradation. Interestingly, studies with NIH-3T3 cells expressing alpha 1 beta 1 integrin showed that the loss of cell-surface alpha 5 beta 1 integrin is blocked by adhesion of cells to dishes coated with type IV collagen (a ligand for alpha 1 beta 1 integrin) as well as fibronectin. Similarly, adhesion of these cells to dishes coated with type IV collagen stabilizes the surface expression of alpha 5 beta 1 as well as alpha 1 beta 1 integrin. We propose that the adhesion of fibroblasts to extracellular matrix protein alters the integrin life cycle and permits retention of these proteins at the cell surface where they can play important roles in transmitting adhesion-dependent signals.
Full text
PDF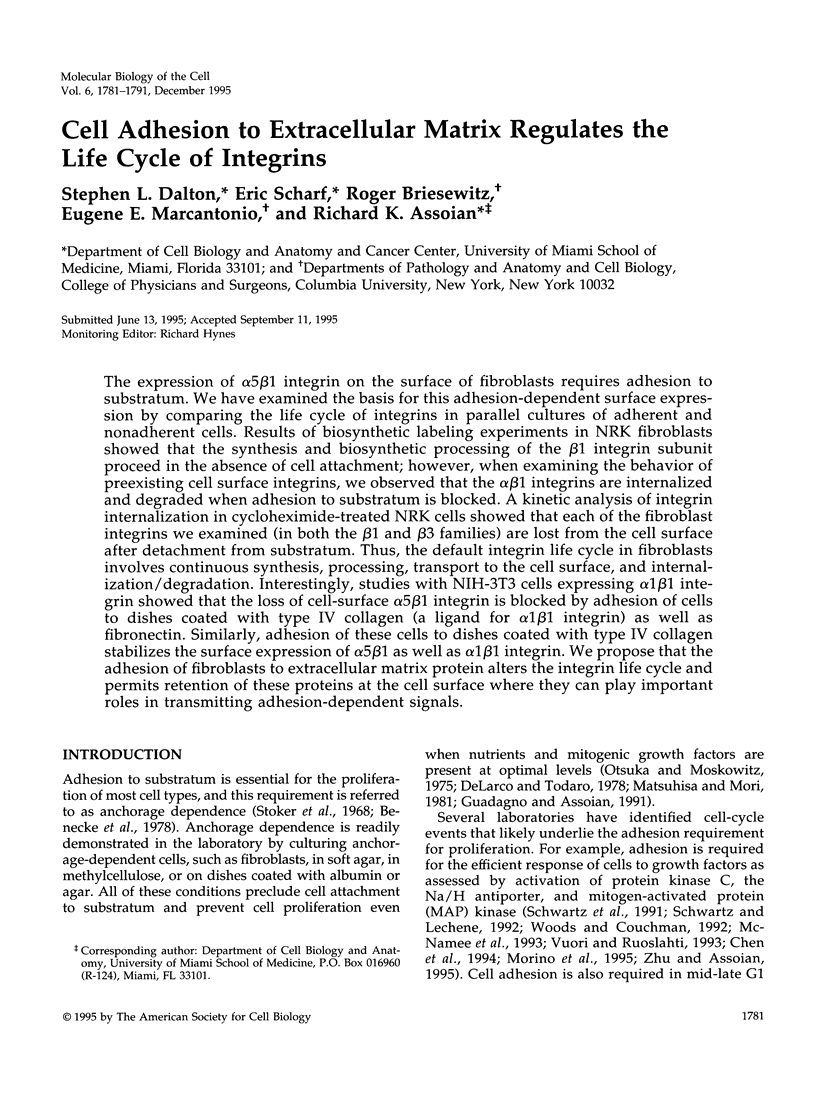




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990 Oct 19;63(2):425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Akiyama S. K., Yamada K. M. The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem. 1985 Apr 10;260(7):4492–4500. [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Benecke B. J., Ben-Ze'ev A., Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978 Aug;14(4):931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Circulating integrins: alpha 5 beta 1, alpha 6 beta 4 and Mac-1, but not alpha 3 beta 1, alpha 4 beta 1 or LFA-1. EMBO J. 1992 Feb;11(2):405–410. doi: 10.1002/j.1460-2075.1992.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 1989 May;8(5):1341–1348. doi: 10.1002/j.1460-2075.1989.tb03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briesewitz R., Epstein M. R., Marcantonio E. E. Expression of native and truncated forms of the human integrin alpha 1 subunit. J Biol Chem. 1993 Feb 5;268(4):2989–2996. [PubMed] [Google Scholar]
- Briesewitz R., Kern A., Marcantonio E. E. Ligand-dependent and -independent integrin focal contact localization: the role of the alpha chain cytoplasmic domain. Mol Biol Cell. 1993 Jun;4(6):593–604. doi: 10.1091/mbc.4.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Kinch M. S., Lin T. H., Burridge K., Juliano R. L. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994 Oct 28;269(43):26602–26605. [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Dautry-Varsat A., Lodish H. F. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983 Aug 25;258(16):9681–9689. [PubMed] [Google Scholar]
- Dalton S. L., Marcantonio E. E., Assoian R. K. Cell attachment controls fibronectin and alpha 5 beta 1 integrin levels in fibroblasts. Implications for anchorage-dependent and -independent growth. J Biol Chem. 1992 Apr 25;267(12):8186–8191. [PubMed] [Google Scholar]
- Dean R. T., Jessup W., Roberts C. R. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem J. 1984 Jan 1;217(1):27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Guadagno T. M., Assoian R. K. G1/S control of anchorage-independent growth in the fibroblast cell cycle. J Cell Biol. 1991 Dec;115(5):1419–1425. doi: 10.1083/jcb.115.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno T. M., Ohtsubo M., Roberts J. M., Assoian R. K. A link between cyclin A expression and adhesion-dependent cell cycle progression. Science. 1993 Dec 3;262(5139):1572–1575. doi: 10.1126/science.8248807. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991 Nov;2(11):951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Calalb M. B., Harper M. C., Patel S. K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. K., Mooney D. J., Vacanti J. P., Ingber D. E. Integrin binding and cell spreading on extracellular matrix act at different points in the cell cycle to promote hepatocyte growth. Mol Biol Cell. 1994 Sep;5(9):967–975. doi: 10.1091/mbc.5.9.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Jacobson J. G., Strominger J. L. Biochemical characterization of VLA-1 and VLA-2. Cell surface heterodimers on activated T cells. J Biol Chem. 1985 Dec 5;260(28):15246–15252. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hotchin N. A., Gandarillas A., Watt F. M. Regulation of cell surface beta 1 integrin levels during keratinocyte terminal differentiation. J Cell Biol. 1995 Mar;128(6):1209–1219. doi: 10.1083/jcb.128.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Klein C. E., Dressel D., Steinmayer T., Mauch C., Eckes B., Krieg T., Bankert R. B., Weber L. Integrin alpha 2 beta 1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991 Dec;115(5):1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992 Nov 25;267(33):23439–23442. [PubMed] [Google Scholar]
- Marcantonio E. E., Hynes R. O. Antibodies to the conserved cytoplasmic domain of the integrin beta 1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988 May;106(5):1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhisa T., Mori Y. An anchorage-dependent locus in the cell cycle for the growth of 3T3 cells. Exp Cell Res. 1981 Oct;135(2):393–398. doi: 10.1016/0014-4827(81)90176-2. [DOI] [PubMed] [Google Scholar]
- McNamee H. P., Ingber D. E., Schwartz M. A. Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J Cell Biol. 1993 May;121(3):673–678. doi: 10.1083/jcb.121.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis A. J., Hoyle M., Mann D. M., Brennan M. J. Incorporation of cellular and plasma fibronectins into smooth muscle cell extracellular matrix in vitro. Proc Natl Acad Sci U S A. 1985 May;82(9):2746–2750. doi: 10.1073/pnas.82.9.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino N., Mimura T., Hamasaki K., Tobe K., Ueki K., Kikuchi K., Takehara K., Kadowaki T., Yazaki Y., Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44erk-1 and p42erk-2. J Biol Chem. 1995 Jan 6;270(1):269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- Neff N. T., Lowrey C., Decker C., Tovar A., Damsky C., Buck C., Horwitz A. F. A monoclonal antibody detaches embryonic skeletal muscle from extracellular matrices. J Cell Biol. 1982 Nov;95(2 Pt 1):654–666. doi: 10.1083/jcb.95.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto C. T., Song W., Bomsel M., Mostov K. E. Rapid internalization of the polymeric immunoglobulin receptor requires phosphorylated serine 726. J Biol Chem. 1994 Jun 3;269(22):15676–15682. [PubMed] [Google Scholar]
- Otsuka H., Moskowitz M. Arrest of 3T3 cells in G1 phase in suspension culture. J Cell Physiol. 1975 Dec;87(2):213–219. doi: 10.1002/jcp.1040870209. [DOI] [PubMed] [Google Scholar]
- Robinson M. S., Kreis T. E. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992 Apr 3;69(1):129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Roman J., LaChance R. M., Broekelmann T. J., Kennedy C. J., Wayner E. A., Carter W. G., McDonald J. A. The fibronectin receptor is organized by extracellular matrix fibronectin: implications for oncogenic transformation and for cell recognition of fibronectin matrices. J Cell Biol. 1989 Jun;108(6):2529–2543. doi: 10.1083/jcb.108.6.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider Y. J., Trouet A. Effect of chloroquine and methylamine on endocytosis of fluorescein-labelled controlled IgG and of anti-(plasma membrane) IgG by cultured fibroblasts. Eur J Biochem. 1981 Aug;118(1):33–38. doi: 10.1111/j.1432-1033.1981.tb05482.x. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Lechene C. Adhesion is required for protein kinase C-dependent activation of the Na+/H+ antiporter by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6138–6141. doi: 10.1073/pnas.89.13.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A., Lechene C., Ingber D. E. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin alpha 5 beta 1, independent of cell shape. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowska J., Guan J. L., Marcantonio E. E., Trevithick J. E., Buck C. A., Hynes R. O. Expression of normal and mutant avian integrin subunits in rodent cells. J Cell Biol. 1989 Aug;109(2):853–861. doi: 10.1083/jcb.109.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W., Geuze H. J., Strous G. J. Sorting of endocytosed transferrin and asialoglycoprotein occurs immediately after internalization in HepG2 cells. J Cell Biol. 1987 May;104(5):1261–1268. doi: 10.1083/jcb.104.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. Activation of protein kinase C precedes alpha 5 beta 1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993 Oct 15;268(29):21459–21462. [PubMed] [Google Scholar]
- Woods A., Couchman J. R. Protein kinase C involvement in focal adhesion formation. J Cell Sci. 1992 Feb;101(Pt 2):277–290. doi: 10.1242/jcs.101.2.277. [DOI] [PubMed] [Google Scholar]
- Zhu X., Assoian R. K. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995 Mar;6(3):273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]