Abstract
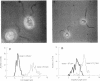
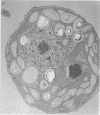
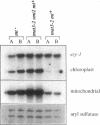
An intriguing feature of early zygote development in Chlamydomonas reinhardtii is the active elimination of chloroplast DNA from the mating-type minus parent due presumably to the action of a zygote-specific nuclease. Meiotic progeny thus inherit chloroplast DNA almost exclusively from the mating-type plus parent. The plus-linked nuclear mutation mat3 prevents this selective destruction of minus chloroplast DNA and generates progeny that display a biparental inheritance pattern. Here we show that the mat3 mutation creates additional phenotypes not previously described: the cells are much smaller than wild type and they possess substantially reduced amounts of both mitochondrial and chloroplast DNA. We propose that the primary defect of the mat3 mutation is a disruption of cell-size control and that the inhibition of the uniparental transmission of chloroplast genomes is a secondary consequence of the reduced amount of chloroplast DNA in the mat3 parent.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armbrust E. V., Ferris P. J., Goodenough U. W. A mating type-linked gene cluster expressed in Chlamydomonas zygotes participates in the uniparental inheritance of the chloroplast genome. Cell. 1993 Sep 10;74(5):801–811. doi: 10.1016/0092-8674(93)90460-8. [DOI] [PubMed] [Google Scholar]
- Bolen P. L., Grant D. M., Swinton D., Boynton J. E., Gillham N. W. Extensive methylation of chloroplast DNA by a nuclear gene mutation does not affect chloroplast gene transmission in chlamydomonas. Cell. 1982 Feb;28(2):335–343. doi: 10.1016/0092-8674(82)90351-8. [DOI] [PubMed] [Google Scholar]
- Bråten T. Autoradiographic evidence for the rapid disintegration of one chloroplast in the zygote of the green alga Ulva mutabilis. J Cell Sci. 1973 Mar;12(2):385–389. doi: 10.1242/jcs.12.2.385. [DOI] [PubMed] [Google Scholar]
- Bråten T. The ultrastructure of fertilization and zygote formation in the green alga Ulva mutabilis Foyn. J Cell Sci. 1971 Nov;9(3):621–635. doi: 10.1242/jcs.9.3.621. [DOI] [PubMed] [Google Scholar]
- Charlesworth B. Evolutionary genetics. The nature and origin of mating types. Curr Biol. 1994 Aug 1;4(8):739–741. doi: 10.1016/s0960-9822(00)00165-2. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmides L. M., Tooby J. Cytoplasmic inheritance and intragenomic conflict. J Theor Biol. 1981 Mar 7;89(1):83–129. doi: 10.1016/0022-5193(81)90181-8. [DOI] [PubMed] [Google Scholar]
- D'Urso G., Nurse P. Checkpoints in the cell cycle of fission yeast. Curr Opin Genet Dev. 1995 Feb;5(1):12–16. doi: 10.1016/s0959-437x(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Feng T. Y., Chiang K. S. The persistence of maternal inheritance in Chlamydomonas despite hypomethylation of chloroplast DNA induced by inhibitors. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3438–3442. doi: 10.1073/pnas.81.11.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris P. J., Goodenough U. W. The mating-type locus of Chlamydomonas reinhardtii contains highly rearranged DNA sequences. Cell. 1994 Mar 25;76(6):1135–1145. doi: 10.1016/0092-8674(94)90389-1. [DOI] [PubMed] [Google Scholar]
- Gillham N. W., Boynton J. E., Harris E. H. Specific elimination of mitochondrial DNA from Chlamydomonas by intercalating dyes. Curr Genet. 1987;12(1):41–47. doi: 10.1007/BF00420726. [DOI] [PubMed] [Google Scholar]
- Gillham N. W., Boynton J. E., Johnson A. M., Burkhart B. D. Mating type linked mutations which disrupt the uniparental transmission of chloroplast genes in chlamydomonas. Genetics. 1987 Apr;115(4):677–684. doi: 10.1093/genetics/115.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillham N. W., Boynton J. E., Lee R. W. Segregation and recombination of non-Mendellan genes in Chlamydomonas. Genetics. 1974 Sep;78(1):439–457. doi: 10.1093/genetics/78.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U. W., Detmers P. A., Hwang C. Activation for cell fusion in Chlamydomonas: analysis of wild-type gametes and nonfusing mutants. J Cell Biol. 1982 Feb;92(2):378–386. doi: 10.1083/jcb.92.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U., Wharton D., Josefsson A., Wilson A. C. Paternal inheritance of mitochondrial DNA in mice. Nature. 1991 Jul 18;352(6332):255–257. doi: 10.1038/352255a0. [DOI] [PubMed] [Google Scholar]
- Harris E. H., Burkhart B. D., Gillham N. W., Boynton J. E. Antibiotic resistance mutations in the chloroplast 16S and 23S rRNA genes of Chlamydomonas reinhardtii: correlation of genetic and physical maps of the chloroplast genome. Genetics. 1989 Oct;123(2):281–292. doi: 10.1093/genetics/123.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeh W. R., Blakley K. H., Brown W. M. Heteroplasmy suggests limited biparental inheritance of Mytilus mitochondrial DNA. Science. 1991 Mar 22;251(5000):1488–1490. doi: 10.1126/science.1672472. [DOI] [PubMed] [Google Scholar]
- Hosler J. P., Wurtz E. A., Harris E. H., Gillham N. W., Boynton J. E. Relationship between Gene Dosage and Gene Expression in the Chloroplast of Chlamydomonas reinhardtii. Plant Physiol. 1989 Oct;91(2):648–655. doi: 10.1104/pp.91.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda H., Hayashi J., Takahama S., Taya C., Lindahl K. F., Yonekawa H. Elimination of paternal mitochondrial DNA in intraspecific crosses during early mouse embryogenesis. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4542–4546. doi: 10.1073/pnas.92.10.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo R., Satta Y., Matsuura E. T., Ishiwa H., Takahata N., Chigusa S. I. Incomplete maternal transmission of mitochondrial DNA in Drosophila. Genetics. 1990 Nov;126(3):657–663. doi: 10.1093/genetics/126.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa T. Mechanisms of maternal inheritance of chloroplast DNA: an active digestion hypothesis. Microbiol Sci. 1985 Sep;2(9):267–270. [PubMed] [Google Scholar]
- LEVINE R. P., EBERSOLD W. T. The genetics and cytology of Chlamydomonas. Annu Rev Microbiol. 1960;14:197–216. doi: 10.1146/annurev.mi.14.100160.001213. [DOI] [PubMed] [Google Scholar]
- Martin N. C., Goodenough U. W. Gametic differentiation in Chlamydomonas reinhardtii. I. Production of gametes and their fine structure. J Cell Biol. 1975 Dec;67(3):587–605. doi: 10.1083/jcb.67.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne R. F., Mathieu D. Transmission of chloroplast genes in triploid and tetraploid zygospores of Chlamydomonas reinhardtii: Roles of mating-type gene dosage and gametic chloroplast DNA content. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4780–4783. doi: 10.1073/pnas.80.15.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meland S., Johansen S., Johansen T., Haugli K., Haugli F. Rapid disappearance of one parental mitochondrial genotype after isogamous mating in the myxomycete Physarum polycephalum. Curr Genet. 1991 Jan;19(1):55–59. doi: 10.1007/BF00362088. [DOI] [PubMed] [Google Scholar]
- Munaut C., Dombrowicz D., Matagne R. F. Detection of chloroplast DNA by using fluorescent monoclonal anti-bromodeoxyuridine antibody and analysis of its fate during zygote formation in Chlamydomonas reinhardtii. Curr Genet. 1990 Oct;18(3):259–263. doi: 10.1007/BF00318390. [DOI] [PubMed] [Google Scholar]
- Nash R., Tokiwa G., Anand S., Erickson K., Futcher A. B. The WHI1+ gene of Saccharomyces cerevisiae tethers cell division to cell size and is a cyclin homolog. EMBO J. 1988 Dec 20;7(13):4335–4346. doi: 10.1002/j.1460-2075.1988.tb03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke K. A., Leech R. M. A Genetic Analysis of Chloroplast Division and Expansion in Arabidopsis thaliana. Plant Physiol. 1994 Jan;104(1):201–207. doi: 10.1104/pp.104.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke K. A., Leech R. M. Chloroplast Division and Expansion Is Radically Altered by Nuclear Mutations in Arabidopsis thaliana. Plant Physiol. 1992 Jul;99(3):1005–1008. doi: 10.1104/pp.99.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke K. A., Rutherford S. M., Robertson E. J., Leech R. M. arc6, A Fertile Arabidopsis Mutant with Only Two Mesophyll Cell Chloroplasts. Plant Physiol. 1994 Nov;106(3):1169–1177. doi: 10.1104/pp.106.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIS H., PLAUT W. Ultrastructure of DNA-containing areas in the chloroplast of Chlamydomonas. J Cell Biol. 1962 Jun;13:383–391. doi: 10.1083/jcb.13.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987 May 22;49(4):559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Sager R., Lane D. Molecular basis of maternal inheritance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2410–2413. doi: 10.1073/pnas.69.9.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R. MENDELIAN AND NON-MENDELIAN INHERITANCE OF STREPTOMYCIN RESISTANCE IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1954 May;40(5):356–363. doi: 10.1073/pnas.40.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Ramanis Z. Biparental inheritance of nonchromosomal genes induced by ultraviolet irradiation. Proc Natl Acad Sci U S A. 1967 Sep;58(3):931–937. doi: 10.1073/pnas.58.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears B. B., VanWinkle-Swift K. The salvage/turnover/repair (STOR) model for uniparental inheritance in Chlamydomonas: DNA as a source of sustenance. J Hered. 1994 Sep-Oct;85(5):366–376. doi: 10.1093/oxfordjournals.jhered.a111481. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Sueoka N. MITOTIC REPLICATION OF DEOXYRIBONUCLEIC ACID IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. P., Beerman N., Griffith O. M. A small-scale five-hour procedure for isolating multiple samples of CsCl-purified DNA: application to isolations from mammalian, insect, higher plant, algal, yeast, and bacterial sources. Anal Biochem. 1986 Feb 1;152(2):376–385. doi: 10.1016/0003-2697(86)90423-9. [DOI] [PubMed] [Google Scholar]
- Wurtz E. A., Boynton J. E., Gillham N. W. Perturbation of chloroplast DNA amounts and chloroplast gene transmission in Chlamydomonas reinhardtii by 5-fluorodeoxyuridine. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4552–4556. doi: 10.1073/pnas.74.10.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hostos E. L., Schilling J., Grossman A. R. Structure and expression of the gene encoding the periplasmic arylsulfatase of Chlamydomonas reinhardtii. Mol Gen Genet. 1989 Aug;218(2):229–239. doi: 10.1007/BF00331273. [DOI] [PubMed] [Google Scholar]