Abstract
Regulation of NMDA receptor (NMDAR) activity by desensitization is important in physiological and pathological states; NMDAR desensitization contributes in shaping synaptic responses and may be protective by limiting calcium influx during sustained glutamate insults. We previously reported that glycine-independent desensitization decreases during hippocampal neuronal development, correlating with NMDAR synaptic localization and association with post-synaptic density 95 (PSD-95). PSD-95/Discs large/zona occludens (PDZ)-1,2 domains of PSD-95 bind to the C-terminus of NMDAR NR2 subunits. The role of PSD-95 in anchoring signaling proteins near NMDARs is well documented. To determine if PSD-95-induced changes in NMDAR desensitization occur because of direct binding to NR2 or due to recruitment of regulatory proteins, we tested the effects of various PSD-95 constructs on NMDAR currents in human embryonic kidney 293 (HEK293) cells and neurons. In HEK cells, wild-type PSD-95 significantly reduced wild-type NMDAR desensitization without altering currents of NMDARs containing NR2A-S1462A, a mutation that abolishes PSD-95 binding. The PSD-95 N-terminus truncated after the PDZ1-2 domains was sufficient for this effect in neurons with low endogenous PSD-95 levels; in NMDAR-expressing HEK cells, the effect persisted when PSD-95 multimerization was eliminated. Moreover, other PSD-95 family members with highly homologous PDZ1-2 domains significantly reduced NMDAR desensitization. In mature neurons, disruption of PSD-95/NMDAR interaction through protein kinase C (PKC) activation increased desensitization to levels found in immature neurons, and this effect was not due to PKC direct regulation of NMDAR activity. We conclude that direct binding of PSD-95 increases stability of NMDAR responses to agonist exposure in neuronal and non-neuronal cells.
Introduction
N-methyl-D-aspartic acid (NMDA) – type glutamate receptors are expressed at most CNS excitatory synapses and play key roles in synaptogenesis, induction of synaptic plasticity, and excitotoxic neuronal death. These receptors are tetrameric complexes of two NR1 with two NR2, or one NR2 and one regulatory NR3, subunits (Cull-Candy et al. 2001). NR2 subunits are encoded by four different genes – NR2A, B, C, D – that are tightly regulated temporally and spatially (Monyer et al. 1994), and these subunits determine differences in NMDAR pharmacological and physiological properties (Cull-Candy et al. 2001). Both NR2A and NR2B subunits are highly expressed in the mature forebrain where NMDARs containing NR1/NR2B predominate at non-synaptic sites in the neuronal plasma membrane and NR2A-containing NMDARs are enriched at the synapse (Barria and Malinow 2002; Stocca and Vicini 1998; Tovar and Westbrook 1999). Differences in subcellular distribution are not absolute, however, and determining effects of subunit composition on NMDAR localization and signaling in neurons is complicated by expression of tri-heteromeric NR1/NR2A/NR2B complexes (Luo et al. 1997; Thomas et al. 2006). Additionally, subcellular localization of NMDARs affects their functional regulation (Li et al. 2002; Li et al. 2003).
Desensitization of glutamate receptors -- the decay of current with sustained agonist exposure – protects neurons from excitotoxicity (Zorumski et al. 1990). NMDAR desensitization also shapes neuronal responses to repeated stimulation (Tong et al. 1995). Previously, we reported that NMDAR desensitization is regulated by receptor subcellular localization in hippocampal pyramidal neurons (Li et al. 2003). Interestingly, synaptic NMDARs showed dramatically less desensitization than non-synaptic NMDARs, despite enrichment of NR2A subunits at the synapse and extrasynaptic localization of NR2B-containing receptors. This result was unexpected since NR1/NR2A desensitizes more extensively than NR1/NR2B when expressed in non-neuronal cells (Dingledine et al. 1999; Krupp et al. 1996). NMDAR association with PSD-95 in hippocampal neurons also correlated with decreased desensitization (Li et al. 2003); however, it was not clear whether this effect was secondary to PSD-95’s role as a scaffolding protein, anchoring protein kinases and phosphatases in close proximity to NMDARs (Kim and Sheng 2004), or if its binding to NMDARs directly altered receptor desensitization.
Palmitoylation at two cysteines near the N-terminus of PSD-95 targets this protein to the synapse (Craven et al., 1999) where it participates in a complex of nearly 200 proteins associated with NMDARs (Collins et al. 2006; Kim and Sheng 2004). Expression of PSD-95 with NMDARs in non-neuronal cells results in receptor clustering (Kim and Sheng 1996), although PSD-95 is not required for NMDAR targeting or clustering at neuronal synapses (El-Husseini et al. 2000) In neurons, studies suggest a role for PSD-95 in regulating NMDAR surface expression (Lin et al. 2004; Roche et al. 2001) and stabilizing NR2B-containing NMDARs at synapses (Prybylowski et al. 2005), but evidence for a direct role in regulating NMDAR function is lacking. Here we show for the first time that direct binding of the PDZ1-2 domains of PSD-95 to NR2A- or NR2B-containing receptors reduces NMDAR desensitization. Additionally, PSD-95 binding to NMDARs and its effect on current desensitization is regulated by PKC activation.
Materials and Methods
Primary neuronal cultures and transfection
Hippocampal cultures were prepared from 17- to 18-day-old rat embryos as described previously. Cells were plated on poly-D-lysine-coated coverslips at a density of ~300–400 cells/mm2 and grown in B-27 supplemented Neurobasal medium in a humidified atmosphere with 5% CO2. Medium was refreshed twice every week by replacing half the volume. Neurons were transfected with 1.2ug of plasmid DNA per 24 well, using a calcium phosphate kit (Clonetech, Mountain View, CA). Neurons were transfected at 4 days in vitro and used for patch clamp recording 2–3 days after transfection.
HEK293 cell culture and transfection
HEK293 cells (CRL 1573; American Type Culture Collection, Rockville, MD) were maintained as we described previously . Cells were transfected using the calcium phosphate precipitation method with a total of 12 μg of plasmid DNA per 10 cm plate. Cells were transfected with a 1:1:2 ratio of cDNAs encoding NR1-1a, NR2 (A or B or NR2A-S1462A), and either green fluorescent protein (GFP), PSD-95-GFP, PSD-95 PDZ1-2-GFP, growth-associated protein 43 (GAP-43) first 12 amino acids (GAP12), GAP12 PDZ1-2-GFP, Prenylated PSD-95-GFP, SAP-102, or PSD-93. To minimize NMDAR-mediated death, cells were bathed in medium supplemented with 100 μM memantine following transfection until the time of recording.
Drug treatments
Cultured hippocampal neurons were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), RO-320432 (RO), or 2-bromopalmitate (2-BP) by direct addition to the medium. Cultures were then returned to the incubator for time periods ranging from 10 min to 6 hours, as indicated in the Results section. At the end of the drug incubation period, cells were collected for biochemical experiments, or else the medium was replaced by external recording solution and cells were moved on glass cover slips to the recording chamber for electrophysiology, where recordings were made within five minutes of removal of the drug.
Electrophysiology
Cultured neurons were used for recording at 4–7 DIV (“immature”), or >13 DIV (“mature”). Recordings from HEK293 cells were made 12–24 hours following the end of transfection. Conventional whole-cell patch clamp recording was conducted as previously described . Electrodes were fabricated from borosilicate glass (Warner Instruments, Hamden, CT) using a Narashige (Tokyo, Japan) PP-83 electrode puller. Open tip resistance was 5–6 MΩ for electrodes containing (in mM): 115 Cs-methanesulfonate, 10 HEPES, 20 K2-creatine phosphate, 4 MgATP, 10 BAPTA, as well as 50 U/ml creatine phosphokinase, pH 7.26, 310 mOsm.
HEK293 cells were superfused with external recording solution, containing (in mM): 145 NaCL, 5.4 KCl, 0.2 CaCl2, 10 HEPES and 11 glucose, pH 7.3. For neurons, the external solution contained (in mM): 167 NaCl, 2.4 KCl, 10 HEPES, 10 glucose, 0.2 CaCl2, pH 7.3 (325 mOsm), and tetrodotoxin (TTX) (300 nM) and glycine (50 μM) were added just before use. Agonist (1 mM NMDA) was dissolved in the same solution used to bathe the cells and gravity-fed to the cells through one side of a theta-tube . All other drugs, dissolved in the external bathing solution, were included in both the control and agonist side of the theta-tube. Computer-controlled solenoid-driven valves were used to rapidly switch between the two solutions. Agonist was applied for 10 sec at 1-min intervals. For experiments in cultured hippocampal neurons, large, pyramidal-shaped neurons were selected for recording. In experiments with cultured HEK293 cells, smaller cells (<30 micron diameter, with capacitance of <30 pF) were preferentially selected for recording in order to optimize the rate of agonist exchange, keeping the 10–90% rise-time to peak less than 100 ms. In all recordings, the peak and steady-state current amplitudes were stable over periods of more than 15 minutes.
All recordings were made in voltage-clamp mode at a holding potential of −70 mV. Data were acquired using the Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA). Currents were filtered at 1 kHz and digitized at 10 kHz. pClamp 8.1 software (Axon Instruments) was used for data acquisition and analysis. Series resistance and cell capacitance were regularly monitored and recordings were abandoned if series resistance exceeded 20 MΩ.
Co-immunoprecipitation and western blot analysis
Batches of 14–17 DIV hippocampal neuronal cultures in 10-cm dishes were treated with drugs or vehicle as described in Results, then each dish was collected in 1 ml Harvest buffer containing 1 mM EGTA, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 20 μg/ml pepstatin A, and 20 μg/ml leupeptin in PBS. Cell suspensions were processed as described previously . Briefly, after centrifugation, pellets were lysed and solubilized in Harvest buffer containing 0.1% SDS and 0.8% Triton X-100 (0.5 ml final volume). One-tenth of the lysate was reserved for input loading, and the remainder was incubated with protein-A and protein-G beads then briefly centrifuged to remove nonspecifically bound proteins. The supernatant was incubated with 10 μg of rabbit polyclonal anti-NR2A antibody (Upstate Biotechnology) for 1 hr at 4°C, then Protein-A and protein-G beads were added for another 1-hr incubation period. Beads were washed three times with 50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, and 1% Triton X-100. Proteins were eluted from the beads and denatured by boiling in loading buffer for 5 min, then loaded to SDS-PAGE. Proteins from each group of different treatment conditions (vehicle, 2-BP, TPA, and TPA with 2-BP; or vehicle, RO, and TPA) were loaded to the same gel. After transfer, membranes were probed with antibodies against NR2A (the same as used for immunoprecipitation, at 1 μg/ml) and PSD-95 (mouse monocolonal; 5 μg/ml; Chemicon). Bands were visualized using Enhanced Chemiluminescence (Amersham) and densities were quantified by densitometric analysis . The PSD-95 to NR2A band-density ratio was calculated to determine the amount of PSD-95 co-immunoprecipitated with NR2A.
Materials
All chemicals were purchased from Sigma (St. Louis, MO). PSD-95 constructs were described previously . NR2A-S1462A and wild-type (rat) NR2A (on which the mutant NR2A was made) cDNAs were generous gifts from Dr. Robert Wenthold , and were previously described (Prybylowski et al., 2005). NR1-1a , NR2B and NR2A (ε1) cDNAs were described previously . NR2A (ε1) was used for all experiments in HEK cells except those comparing effects of PSD-95 on wild-type versus mutant S1462A NR2A, when the corresponding rat cDNA construct was used for wild-type NR2A. Tissue culture reagents were obtained from Invitrogen. Stock solutions of 100 mM NMDA and 100 mM glycine were each stored in individual aliquots for up to 6 weeks at −20° C. TPA was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 1mM and diluted to a final concentration of 100nM. A 200mM stock solution of 2-BP in ethanol was diluted to a final concentration of 100 μM. RO was dissolved in DMSO for a stock concentration of 2mM and used at 1μM. Stock solutions of TPA, 2-BP and RO were stored as aliquots at −20° C and thawed only once.
Data analysis
Results are presented as mean ± SE. Sets of different results were compared using one-way ANOVA followed by Bonferroni post-test or Student's t-test as appropriate, and significant differences were determined at the 95% confidence intervals unless otherwise indicated. Three to 10 responses of each cell were averaged for estimation of steady-state to peak current ratio (Iss/Ip).
Results
PSD-95 regulates NMDAR glycine-independent desensitization
NMDAR desensitization attenuates receptor activation during sustained exposure to agonists (Tong et al. 1995). Glycine-independent desensitization can be isolated in cultured hippocampal neurons by recording NMDAR currents in response to 10-sec applications of saturating concentrations of agonist – 1 mM NMDA with 50 μM glycine – in low external calcium and a high intracellular concentration of BAPTA (Mayer et al. 1989; Tong and Jahr 1994). Previously, we demonstrated a developmental decrease in this form of NMDAR desensitization recorded from cultured hippocampal neurons, which could be explained by receptor localization to the synapse; however, the reduction in desensitization also correlated with co-association of NMDARs with PSD-95 (Li et al. 2003).
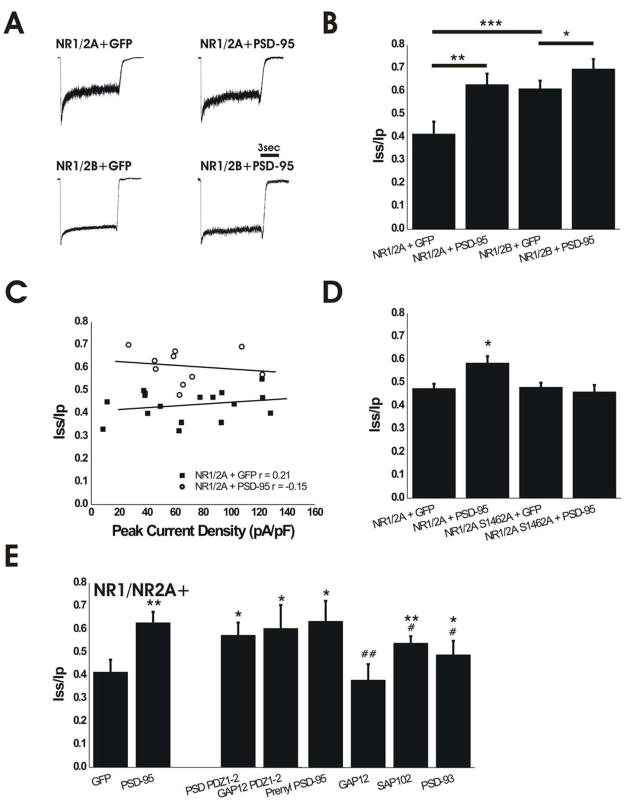
PSD-95 recruits many scaffolding and signaling molecules to the post-synaptic density directly through its various domains or indirectly through macromolecular MAGUK-AKAP complexes (Colledge et al. 2000; Kim and Sheng 2004; Lim et al. 2002; Sheng and Pak 2000). To assess whether the previously reported effect of PSD-95 on NMDAR desensitization (Li et al. 2003) occurred as a result of neuronal-specific mechanisms, we compared NMDAR currents from HEK293 cells transfected with either GFP (control) or PSD-95, in combination with different subtypes of NMDARs. HEK293 cells expressing GFP and NR1/NR2A showed significantly greater desensitization compared to cells co-expressing GFP and NR1/NR2B (Fig. 1A, B), as previously reported (Dingledine et al. 1999; Krupp et al. 1996). Strikingly, immature neurons that predominantly express NR1 and NR2B subunits exhibit more extensive NMDAR desensitization than recombinant NR1/NR2B in HEK293 cells (compare Figs. 2A,B with 1A,B), whereas mature hippocampal neurons that express largely NR2A-containing receptors (Li et al. 2002) show significantly less desensitization compared with either NMDARs in immature neurons or NR1/NR2A expressed in non-neuronal cells (compare Figs. 4A,B with 1A,B). Co-expression of PSD-95 significantly reduced the extent of NMDA-evoked current desensitization in both NR1/NR2A- and NR1/NR2B-expressing HEK293 cells (Fig. 1A,B). In contrast, expression of PSD-95 did not alter the mean NMDA-evoked peak current density (69 ± 9 pA/pF, n=17 for NR1/NR2A + GFP and 63 ± 8 pA/pF, n=10 for NR1/NR2A + PSD-95; P>0.05 by unpaired t-test). Moreover, the extent of desensitization showed no correlation with peak current density for individual cells as illustrated in Fig. 1C, suggesting that the number of active, surface NMDARs does not influence desensitization of whole-cell NMDAR current. Notably, the steady-state to peak ratio for NMDAR current in PSD-95-expressing cells was similar for NR1/NR2A and NR1/NR2B, and also resembled the ratio found in mature cultured hippocampal neurons (compare Figs. 1A,B with 4A,B).
Figure 1.
Expression of PSD-95 Regulates Glycine-Independent Desensitization of Recombinant NMDARs. A. Representative traces of NMDAR currents in HEK cells expressing NMDAR subunits with GFP or PSD-95-GFP. Currents were normalized for comparison of desensitization. B. Expression of PSD-95 significantly reduced the extent of NMDA-evoked current desensitization in both NR1/NR2A- and NR1/NR2B-expressing cells. NR1/2A + GFP, n=23; NR1/2A + PSD-95-GFP, n=15; NR1/2B + GFP, n=23; NR1/2B + PSD-95, n=13; *P<0.05, **P<0.01, ***P<0.001; one-way ANOVA followed by Bonferroni post-test. C. Iss/Ip shows no correlation with peak current density in NR1/NR2A-expressing HEK cells co-transfected with either GFP (closed squares, n=17) or wild-type PSD-95 (open circles, n=10). D. Mutation of the PDZ ligand in (Prybylowski et al.) NR2A eliminates effect of PSD-95 on NMDAR desensitization. NR1/NR2A + GFP, n=10; NR1/NR2A + PSD-95, n=15; NR1/NR2A-S1462A + GFP, n=8; NR1/NR2A-S1462A + PSD-95, n=21. *P<0.05 compared to all other conditions; one-way ANOVA followed by Bonferroni post-test. E. PDZ1-2 domains are sufficient to alter NMDAR desensitization; multimerization of PSD-95 and protein targeting to the membrane are not required. NR1/2A +: GFP, n=23 (same data shown in B); PSD-95-GFP, n=15 (same data shown in B); PSD PDZ1-2-GFP, n=11; GAP12 PDZ1-2, n=7; Prenyl PSD-95, n=8; GAP12, n=8; SAP-102, n=12; PSD-93, n=10. *P<0.05 and **P<0.01 by One-way ANOVA followed by Bonferroni post-test compared to NR1/2A + GFP; #P<0.05 and ##P<0.01 by one-way ANOVA followed by Bonferroni post-test compared to NR1/2A + PSD-95.
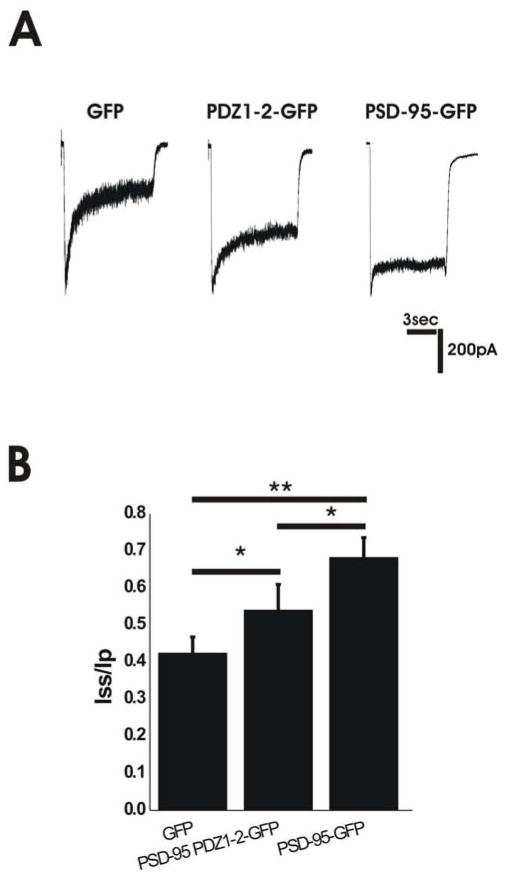
Figure 2.
Overepression of PSD-95 in Immature Neurons Reduces Glycine-Independent Desensitization. A. Representative traces of NMDAR responses in immature neurons overexpressing GFP, PSD-95 PDZ1-2-GFP or PSD-95-GFP. B. Overexpression of PSD-95 PDZ1-2-GFP or PSD-95-GFP in immature hippocampal neurons results in decreased desensitization compared to GFP-transfected controls. GFP n=7; PSD-95 PDZ1-2-GFP, n=6; PSD-95-GFP, n=12; *P<0.05, **P<0.01 by one-way ANOVA followed by Bonferroni post-test.
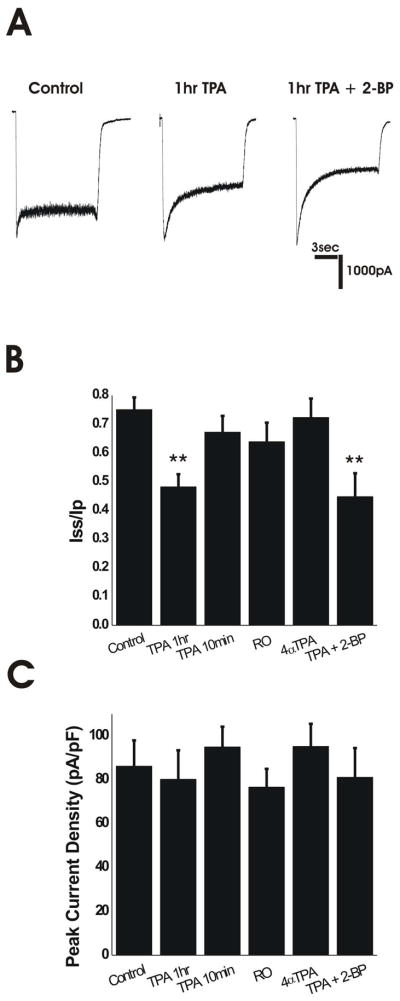
Figure 4.
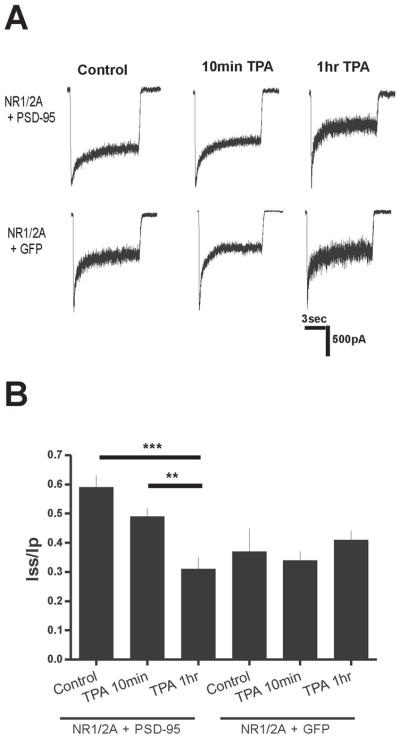
PKC Activation Alters Desensitization in Mature Neurons. A. Representative traces of mature neurons under control conditions, treated with TPA for 1 hour, and treated with TPA for one hour following 6-hr 2-BP treatment. B. 1-hr TPA treatment reduced Iss/Ip of mature (>14 DIV) neurons. Shorter 10-min TPA treatments, 10-min incubation with a PKC inhibitor (RO), or 1-hr treatment with an inactive phorbol ester (4 αTPA) did not alter desensitization of mature neurons. Combined TPA and 2-BP treatments did not further reduce Iss/Ip. Control, n=23; 1-hr TPA, n=9; 10-min TPA, n=7; 10-min RO, n=9; 60-min 4α TPA, n=8; 2-BP + TPA, n=9. ** P<0.01 compared to all other conditions by one-way ANOVA followed by Bonferroni post-test. C. Change in desensitization not due to increased peak current. Mature neurons displayed no changes in NMDAR current density with any treatment. Control, n=12; 60-min TPA, n=9; 10-min TPA, n=7; 10-min RO, n=9; 60-min 4 α TPA, n=8; 2-BP + TPA, n=9. P>0.05 by one-way ANOVA followed by Bonferroni post-test.
Previous studies have shown that nanomolar concentrations of zinc accelerate NMDAR desensitization in transfected HEK cells (Chen et al. 1997; Erreger et al. 2005; Erreger and Traynelis 2008; Zheng et al. 2001), and that PSD-95 expression in Xenopus oocytes reduces the effect of zinc on NR1/NR2A channel function (Yamada et al. 2002) However, when we recorded NMDAR currents from NR1/NR2A-transfected HEK cells under similar conditions to those used in Fig. 1A,B except for the addition of 1 μM TPEN, which chelates any contaminating zinc in the recording solution (Paoletti et al. 1997) we also found a significant difference in steady-state to peak ratio between cells co-transfected with PSD-95 compared with GFP alone. The Iss/Ip for current evoked by 1 mM glutamate in the presence of 100 μM glycine, nominal zero calcium, and 1 μM TPEN in the bathing solution (and all other conditions the same as described for data in Fig. 1) was 61 ± 4% and 22 ± 6% for PSD-95- and GFP-expressing cells, respectively (n=7 and 6 different cells, respectively; P<0.001 by unpaired t-test). The more profound desensitization observed in the absence of PSD-95 in these experiments was typical of the current responses evoked by saturating glutamate compared to NMDA in NR1/NR2A-transfected HEK293 cells. The lack of apparent zinc effect on NMDAR desensitization in our experiments may be a result of minimal zinc contamination in the recording solutions. Calcium salts could be a major source of zinc contamination, and our recording solutions contained nominal zero, or very low, added Ca2+.
The data shown in Fig. 1A,B demonstrate that the role of PSD-95 in regulating NMDAR desensitization is independent of neuronal- and synapse-specific proteins. Therefore, we hypothesized that direct binding of PSD-95 to NMDARs is required to alter glycine-independent desensitization. NMDARs bind PSD-95 through the C-terminal PDZ-binding motif (ESDV) of the NR2 subunit (Kornau et al. 1995). To test our hypothesis, we compared NMDAR desensitization in NR1-transfected HEK cells co-expressing wild-type NR2A (using the rat cDNA) versus NR2A-S1462A (containing a mutation that eliminates binding to PDZ domains, made on the wild-type rat cDNA construct) with or without PSD-95. Consistent with our hypothesis, PSD-95 had no effect on desensitization of NMDARs composed of NR1/NR2A-S1462A (Fig. 1D). Importantly, these mutant receptors showed similar desensitization to wild-type NR1/NR2A in the absence of PSD-95, and wild-type NR1/NR2A desensitization was significantly reduced by co-transfection with PSD-95 (Fig. 1D).
PDZ1-2 domains of PSD-95 are sufficient to alter NMDAR desensitization
PSD-95 contains three N-terminal PDZ domains, a central Src homology 3 (SH3) domain, and a C-terminal guanylate kinase-like (Aoki et al.) domain, which can each recruit various proteins to the membrane (Cho et al. 1992; Kim et al. 1995; Kornau et al. 1995). PDZ domains 1 and 2 are required for binding the NR2 C-terminus (Kornau et al. 1995). Deletion mutagenesis studies established that the N-terminal region of PSD-95, containing palmitoylated Cys-3 and Cys-5, regulates both membrane targeting and multimerization (Christopherson et al. 2003; Craven et al. 1999; Hsueh and Sheng 1999).
In order to isolate which domains of PSD-95 are required for regulating NMDAR desensitization, we utilized PSD-95 constructs with deletions of specific domains and/or with mutations that eliminated PSD-95 multimerization. In HEK293 cells expressing NR1/NR2A, co-transfection of PSD-95 truncated after domain PDZ2 (PSD-95 PDZ1-2-GFP) resulted in significantly reduced NMDA-evoked current desensitization compared to cells co-expressing only GFP, and an Iss/Ip ratio similar to that found with full-length wild-type PSD-95 (Fig. 1E). Although the PSD-95 PDZ1-2 construct lacks many of the domains involved in recruiting kinases and phosphatases to the membrane, it can multimerize, allowing proteins such as GluR1 to co-cluster via free PDZ domains (Schnell et al. 2002). To assess the role of PSD-95 multimerization in the regulation of NMDAR desensitization we used two different PSD-95 mutants that targeted to the membrane but remained in monomeric form: a variant of the truncated PSD-95 PDZ1-2-GFP construct, in which the N-terminal 13 amino acids were replaced with the first 12 amino acids of GAP-43 (GAP12 PDZ1-2-GFP); and a variant of the C3,5S mutant of full-length PSD-95, in which the prenylation motif of paralemmin was added to the C-terminus (Prenyl-PSD-95-GFP). The Iss/Ip of NMDA-evoked currents recorded from HEK293 cells expressing these multimerization-deficient constructs was not significantly different from that observed in cells expressing full-length wild-type PSD-95-GFP, indicating that multimerization does not play a role in regulating glycine-independent NMDAR desensitization (Fig. 1E). Although mutating the N-terminus of PSD-95 should block multimerization, these constructs were GFP-tagged and GFP may form oligomers (Jain et al. 2001). However, we transfected the same constructs without the GFP tag and found no significant difference in the Iss/Ip (data not shown). As an additional control, we recorded NMDAR current from cells expressing the first 12 amino acids of GAP-43 fused to GFP only (GAP12-GFP) together with NR1/NR2A. This construct had no effect on NMDAR desensitization (Fig. 1E; Iss/Ip was not significantly different from that found in cells co-expressing GFP and NR1/NR2A), confirming that it is the PDZ domains of the GAP12 PDZ1-2-GFP protein that are responsible for altering NMDAR current desensitization. Together, these data strongly support the idea that PSD-95 regulation of NMDAR desensitization occurs through direct binding of PDZ1-2 domains to the NMDAR and does not require domains involved in scaffolding other proteins in proximity to NMDARs.
Two closely related family members of PSD-95, synapse-associated protein 102 (SAP-102) and postsynaptic density protein 93 (PSD-93), which both contain PDZ1-2 domains that are highly homologous to those in PSD-95 (Kim and Sheng 2004), are also abundantly expressed in hippocampal neurons and may, in some cases, compensate for effects mediated by PSD-95 (Elias et al. 2006). We found that both SAP-102 and PSD-93 significantly reduced NR1/NR2A desensitization in transfected HEK293 cells (Fig. 1E) albeit to a smaller extent than PSD-95; this may be, in part, a result of lower affinity of these family members for NR2A (Sans et al. 2000). Notably, SAP-102 had no effect on desensitization of NR1/NR2B in transfected HEK cells (Iss/Ip was 61 ± 4%, n=23 for NR1/NR2B+GFP and 60 ± 3%, n=7 for NR1/NR2B+SAP-102; P>0.05 by unpaired t-test), and both NR1/NR2B and SAP-102 are highly expressed in immature hippocampal neurons prior to upregulation of NR2A and PSD-95 (Sans et al. 2000). SAP102 may be a less effective interactor with surface NMDARs as well because of lack of anchoring at the plasma membrane.
To determine whether expression of PSD-95 PDZ1-2-GFP, including only the N-terminal region through PDZ1 and PDZ2, in immature cultured hippocampal neurons is sufficient to reduce NMDA-evoked current desensitization to levels found in mature neurons, we transfected GFP, PSD-95-GFP, or PSD-95 PDZ1-2-GFP plasmids into neurons at 4 DIV and compared NMDA-evoked currents recorded at 6 DIV. Expression of PSD-95-GFP in immature neurons resulted in a decrease in NMDA-evoked current desensitization compared to GFP-transfected controls and an Iss/Ip similar to that observed in mature neurons (compare Figs. 2A,B with 4A,B). Notably, there was no difference in peak current density between neurons transfected with GFP and PSD-95 (51 ± 7 pA/pF, n=7 and 46 ± 9 pA/pF, n=12 for GFP- and PSD-95-transfected neurons, respectively). Transfection of PSD-95 PDZ1-2-GFP also significantly reduced NMDAR desensitization compared to GFP-transfected neurons, although the effect was smaller than that observed for full-length PSD-95-GFP (Fig. 2A,B). From these data, our observations in HEK293 cells, and our previously published results (Li et al. 2003), we conclude that binding of PSD-95 PDZ1-2 domains to NMDARs plays a critical role in reducing receptor desensitization over the course of neuronal development.
PKC uncouples PSD-95 from NMDARs and increases receptor desensitization in mature hippocampal neurons
The above results suggested that NMDAR desensitization in mature neurons might be altered by signaling proteins that regulate the association of NMDARs with PSD-95. Treatment of mature hippocampal neurons with phorbol esters to activate PKC increases diffuse staining of NMDAR clusters throughout dendrites, indicating a shift from synaptic to non-synaptic localization; under these conditions, PSD-95 staining remained punctate and clustered at the synapse suggesting that NMDARs and PSD-95 become uncoupled after PKC activation (Fong et al. 2002).
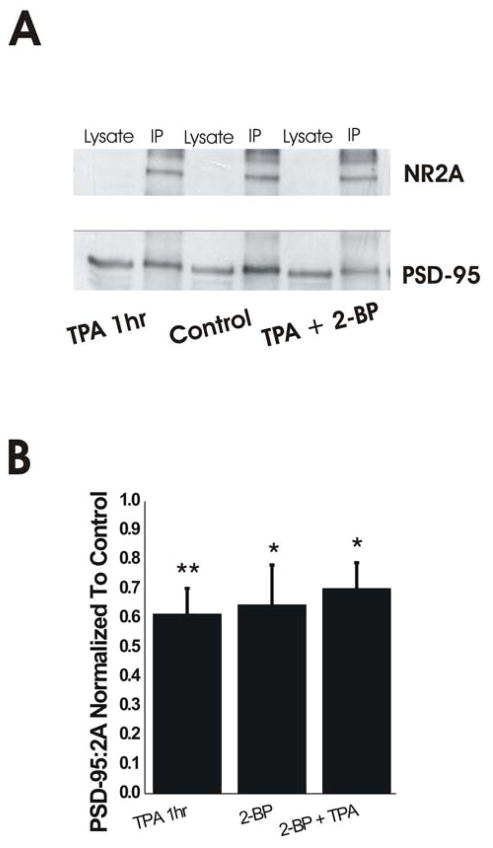
To confirm that treatment with phorbol esters results in dissociation of NMDARs from PSD-95, we treated neurons with 100 nM TPA for 10, 30 or 60 minutes and examined the interaction between NMDARs and PSD-95 by co-immunoprecipitation with an anti-NR2A antibody. TPA significantly decreased the amount of PSD-95 co-immunoprecipitated with NR2A compared with control after a 60-min treatment (PSD-95/NR2A ratio scaled to control was 77 ± 13%, 67 ± 15%, and 51 ± 13% after 10-, 30- and 60-min TPA treatments, respectively; n=5 independent experiments with all four conditions done in parallel; P <0.01 for control vs. 60-min TPA). Although phorbol esters activate PKC over the course of minutes, prolonged treatment can reduce PKC activity in many cell types (Wagey et al. 2001). To confirm that the effect of a 60-min TPA treatment was not a result of down-regulation of PKC activity, we treated neurons with 1 μM RO, which inhibits PKC activity by binding to the catalytic region ATP binding cassette (Birchall et al. 1994). Consistent with our hypothesis that NR2A and PSD-95 co-association was reduced because of PKC activation by TPA, treatment with RO for 10 minutes had no effect on the co-immunoprecipitation of NR2A and PSD-95 (PSD-95/NR2A ratio scaled to control was 98 ± 19% and 64 ± 12% after 10-min RO and 60-min TPA treatments, respectively; n=5 independent experiments with all three conditions done in parallel; P<0.05 for control vs. 60-min TPA).
Previous studies have shown that inhibition of PSD-95 palmitoylation by treatment with 2-BP shifts PSD-95 away from synapses and reduces co-localization with NMDARs (El-Husseini Ael et al. 2002). Indeed, 6-hr treatment with 100 μM 2-BP caused a significant reduction in co-immunoprecipitation of PSD-95 with NR2A (Fig. 3B). Co-treatment of neurons with 2-BP (6 hours) and TPA (60 min) resulted in a reduction in the association of PSD-95 with NR2A that was not significantly different than the decrease found for either treatment alone (Fig. 3A,B). Together, these results indicate that PKC activation by TPA results in a partial uncoupling of NR2A and PSD-95 that is similar, and not additive, to that produced by 2-BP.
Figure 3.
PKC Activation Uncouples NR2A from PSD-95 in Mature Neurons. A. Representative blot of co-immunoprecipitation. PSD-95 band density was significantly reduced in IP lanes with TPA alone or TPA plus 2-BP treatments compared to control. B. 1-hr TPA treatment of mature neurons significantly uncoupled PSD-95 from NR2A similarly to 6-hr 2-BP treatments. Combined 1-hr TPA and 6-hr 2-BP treatment did not produce additive reduction in NR2A/PSD-95 association. n=8 independent experiments with all four conditions done in parallel; *P<0.05, **P<0.01 by one-way ANOVA followed by Bonferroni post-test.
To determine whether PKC-induced dissociation of PSD-95 from NMDARs could alter desensitization, we recorded NMDA-evoked current from cultured mature hippocampal neurons after a 60-min treatment with 100 nM TPA or control solution. TPA treatment caused a marked increase in desensitization to levels similar to those found in immature neurons (compare Fig. 4A,B with Fig. 2A,B), whereas shorter treatments with TPA (10 min) or inhibition of PKC with RO – both treatments that did not affect coupling between PSD-95 and NR2A – did not alter NMDAR desensitization (Fig. 4B). As a control, incubation with an inactive phorbol ester, 4α-TPA (100 nM), for 1 hour did not alter NMDAR current desensitization (Fig. 4B). Consistent with the similar reduction in co-association of NR2A and PSD-95 produced by treatment with TPA or 2-BP or the two together, these treatments also produced a similar, non-additive, reduction in NMDAR current Iss/Ip (Fig. 4A,B). Both treatments result in dissociation of NMDARs from PSD-95: TPA treatment results in extrasynaptic localization of NMDARs while PSD-95 distribution remains unaltered at synaptic sites (Fong et al. 2002); 2-BP treatment results in removal of PSD-95 from synaptic sites while NMDAR distribution is unaltered at the synapse (El-Husseini Ael et al. 2002). The increase in desensitization that results from either treatment suggests binding of PSD-95 to NMDARs is critical in regulating this process.
Previous work indicated that PKC activation increases NMDAR peak current and surface expression in Xenopus oocytes (Lan et al. 2001). However, we found no change in peak NMDAR current density after a 60-min treatment with 100nM TPA (Fig. 4C), consistent with a previous study using a biochemical approach to show that NMDAR surface expression in cultured hippocampal neurons was unchanged following an identical TPA treatment (Fong et al. 2002). TPA has also been shown to enhance NMDAR currents in hippocampal slices (Chen and Huang 1992) and cultures (Xiong et al. 1998). To further test whether TPA directly alters channel gating under our experimental conditions, we determined whether 60-min TPA had any effect on NMDAR current in immature hippocampal neurons that express low levels of PSD-95. TPA did not significantly change the NMDAR Iss/Ip, peak current or current density compared with the control treatment (control vs. 60-min TPA showed: Iss/Ip of 34 ± 9% vs. 29 ± 11% and peak current density of 31 ± 10 pA/pF vs. 37 ± 13 pA/pF; n=15 for control n=7 for 60-min TPA; recordings were made from neurons at 4–5 DIV).
PKC increases NMDAR desensitization by uncoupling PSD-95 from recombinant NMDA receptors expressed in HEK cells
The effect of prolonged treatment with phorbol esters, resulting in dissociation of NMDARs from PSD-95, on recombinant NMDAR desensitization was also assessed in HEK cells expressing NR1 and NR2A along with either GFP or PSD-95-GFP. The cells were treated with TPA (100 nM) for 10 or 60 minutes, followed by the assessment of NMDAR desensitization. TPA significantly decreased the steady-state-to-peak ratio in cells expressing PSD-95 after a 60-min treatment, compared with control (Iss/Ip was 31 ± 4%, n=8 for 60-min treatment with TPA; and 59 ± 4%, n=8 for control cells, treated with DMSO; P<0.001 by one-way ANOVA followed by Bonferroni post-test). The 10-min treatment with TPA slightly increased NMDAR desensitization, though not significantly, compared to control (Iss/Ip was 49 ± 3%, n=8; P>0.05 by one-way ANOVA). The treatment with TPA had no significant effect on desensitization in HEK cells expressing NR1/NR2A and GFP (Iss/Ip was 41 ± 3%, n=4 for 60- min TPA treatment, 34 ± 3%, n=6 for 10-min TPA treatment, and 37 ± 7%, n=4 for control cells, treated with DMSO; P>0.05 by one-way ANOVA).
Discussion
Role of PSD-95 direct binding to NMDARs on current desensitization
Our findings indicate direct binding of PSD-95 PDZ1-2 domains to the NMDAR NR2A/B C-terminus reduces glycine-independent desensitization in HEK293 cells and neurons. This is somewhat surprising, since NMDAR desensitization gating is largely determined by amino acids in the lurcher motif of the channel vestibule (Hu and Zheng 2005). However, there is precedence for changes in NMDAR C-terminal domains affecting function of distant domains: src-mediated phosphorylation of NR2A C-terminal residues relieves zinc inhibition, which is determined by the amino-terminal domain (ATD) (Zheng et al. 1998).
It is important to note that we measured desensitization by calculating the ratio of steady-state to peak NMDA-evoked current during a 10s NMDA application. For technical reasons (cells were attached to the coverslip, limiting the rate of agonist application and thereby slowing the rise time to peak) we were unable to accurately measure the rate of onset of desensitization and therefore could not determine whether PSD-95 binding affected this rate specifically. Since entry to the desensitized state occurs through only one (C1) of three possible agonist-bound closed states (Auerbach and Zhou 2005), it is also possible that PSD-95 binding enhances the rate of exit and/or reduces the rate of entry to this particular closed state, favoring occupation of alternate agonist-bound closed states (C2 and C3), and/or alters the dwell time in open states.
Expression in HEK293 cells of wild-type PSD-95 or multimerization-deficient truncates containing just the N-terminus and PDZ1-2 domains is sufficient to reduce NMDAR current desensitization to levels similar to mature hippocampal neurons, indicating other neuronal-specific proteins and signaling pathways are not required. However, neuronal-specific mechanisms also contribute to regulating NMDAR desensitization. Hippocampal neurons expressing predominantly NR2B-containing receptors and low levels of PSD-95 in early development show more extensive NMDAR desensitization than NR1/NR2B-transfected HEK293 cells. Neuronal NR1/NR2B desensitization in the absence of PSD-95 binding can be enhanced by NR2B Ser1303 phosphorylation (Sessoms-Sikes et al. 2005) and reduced by tyrosine phosphatase inhibition (Li et al. 2003), and occurrence of these processes may be cell-type specific. Conversely, mature neurons exhibit minimal desensitization while expressing high levels of NR2A-containing receptors, which desensitize extensively in HEK293 cells. This may be explained by direct interaction with PSD-95: NMDARs in mature neurons are largely synaptic where they interact with PSD-95, while PSD-95 co-expression with NR1/NR2A in HEK293 cells is sufficient to reduce desensitization.
Previous work indicated PSD-95 expression enhances NMDAR surface expression (Lin et al. 2004). In contrast, another study reported that PSD-95 over-expression in immature cerebellar granule cells reduced whole-cell NMDAR current density (Losi et al. 2003) .We found similar NMDA-evoked peak current density in HEK cells co-expressing NR1/NR2A with GFP or PSD-95; also, there was no correlation between peak current density and Iss/Ip in individual cells. Moreover, in hippocampal neurons NMDAR peak current density was unaltered following PSD-95 over-expression or dissociation of NMDARs and PSD-95. We conclude that regulation of NMDAR desensitization by PSD-95 is not related to changes in receptor surface expression. Previously, PSD-95 was shown to stabilize NR2B-containing receptors at the synapse, whereas trafficking of NR2A- or NR2B-containing receptors to the cell surface occurred independently of PSD-95 (Prybylowski et al. 2005).. Together with our results, these studies suggest the larger role for PSD-95 regulation of neuronal NMDARs is in maintaining synaptic receptors and stabilizing their response to glutamate, rather than modulating surface delivery.
In immature neurons, we observed a significant reduction in NMDAR desensitization with expression of PSD-95 PDZ1-2-GFP compared to GFP-transfected controls, but the effect was attenuated compared with expression of full-length PSD-95, whereas similar effects were seen for both constructs expressed in HEK cells. The attenuated effect in neurons may be explained by inefficient synaptic targeting or clustering of PSD-95 PDZ1-2-GFP, resulting in less effective interaction with synaptically-localized NMDARs; synaptic targeting of PSD-95 is determined in part by the C-terminal 13–25 amino acids (Craven et al. 1999) and the PDZ3 domain interaction with postsynaptic protein CRIPT, which links PSD-95 to the microtubule cytoskeleton (Passafaro et al. 1999).
Effects of PSD-95/NMDAR interaction on neuronal NMDAR function
Previous studies investigated the role of PSD-95 in modulating neuronal NMDAR current. Consistent with our results, in mice expressing truncated C-terminus NR2A (NR2AΔC/ΔC, which is unable to bind PSD-95), whole-cell recordings from hippocampal neurons revealed enhanced NMDAR current desensitization (Steigerwald et al. 2000). Interestingly, dramatic reductions in hippocampal CA1 long-term potentiation (LTP) were reported in these mice (Sprengel et al. 1998; Steigerwald et al. 2000).
Increased desensitization should slow the late component of the NMDAR EPSC decay time course and potentially reduce EPSC amplitude (Lester and Jahr 1992). Both changes were observed in the NR2AΔC/ΔC mice, while the former was also observed in hippocampal slices from a PSD-95 knock-out mouse (Beique et al. 2006); however, effects of the mutations on synaptic NMDAR subunit composition (Beique et al. 2006) or peri-synaptic localization (Steigerwald et al. 2000) make EPSC changes difficult to interpret. Hippocampal slices from a different PSD-95 knock-out mouse, or in which PSD-95 was acutely knocked-down using short hairpin RNAs (shRNA), showed no change in NMDAR-mediated EPSC amplitude, but decay rate was not examined (Elias et al. 2006; Nakagawa et al. 2004).The authors suggested other PSD-95 family members (e.g., SAP-102, PSD-93) could compensate for PSD-95 function. As we have shown that SAP-102 and PSD-93 also regulate NR2A-containing NMDAR desensitization, such compensation may contribute to normalizing EPSC characteristics at a subset of synapses. Although acute disruption of PSD-95/NMDAR interactions, utilizing peptides that competitively bind to PSD-95 PDZ1-2 domains, in hippocampal slices did not alter NMDAR-mediated EPSC amplitude (Lim et al. 2003), the marginal efficacy of these peptides in uncoupling NMDAR/PSD-95 synaptic complexes may contribute to lack of effect on NMDAR EPSCs.
Phorbol ester-induced PKC activation and regulation of neuronal NMDAR function
Although previous studies have identified sites of PKC phosphorylation on NR1 ((Tingley et al. 1997), we found that PKC activity can regulate neuronal NMDAR desensitization by uncoupling PSD-95 from NMDARs. A previous study using different techniques also showed dissociation of PSD-95 and NMDARs following 60-min TPA treatment in hippocampal neurons, without changes in NMDAR surface expression (Fong et al. 2002). Several lines of evidence indicate the effect of 60-min TPA treatment on NMDAR desensitization is mediated by disrupting PSD-95/NMDAR binding rather than by direct phosphorylation of NMDARs or associated proteins. Brief (10-min) TPA treatments that did not uncouple NMDARs from PSD-95 in mature neurons also had no effect on desensitization, and 60-min TPA did not alter NMDAR desensitization in immature neurons expressing low PSD-95 levels or in mature neurons pre-treated with 2-BP to disrupt NMDAR/PSD-95 interaction. Moreover, in transfected HEK cells NR1/NR2A desensitization was significantly increased by 60-min (but not 10-min) TPA treatment in cells co-expressing PSD-95 but not GFP, matching the results in neurons. Interestingly, a previous study showed that in HEK293 cells expressing NR1/NR2A or NR1/NR2B in the absence of PSD-95, acute PKC activation also enhanced glycine-independent desensitization, and this effect persisted after deletion of the C-terminal tail of NR1 or NR2A (Jackson et al. 2006). Differences in the time course of phorbol ester-mediated effects may result in PKC regulation of NMDAR desensitization by distinct mechanisms.
Phorbol esters have been shown to remodel the postsynapse by dispersing NMDARs away from synaptic sites, while leaving other synaptic components, like PSD-95, in the PSD. (Fong et al. 2002). PKC phosphorylates NR1, NR2A and NR2B subunits in vitro, but there is no evidence that this phosphorylation directly disrupts NMDAR interaction with PSD-95. Fong et al. (2002) have speculated that dispesrsal of the receptors occurs because of PKC’s effect on the binding of NR1 to other scaffolding proteins such as actinin, spectrin or the AKAP yotiao, which also facilitate NMDAR/PSD-95 interaction by anchoring these receptors in synapses, in close proximity to PSD-95.
A recent study reported that an increase in peak NMDAR current mediated by PKC in Xenopus oocytes is occluded by PSD-95 co-expression and depends on phosphorylation of NR2A Serine-1462 (Liao et al. 2000; Lin et al. 2006). These results suggest that lack of NMDAR peak current increase in mature neurons following phorbol ester treatment may be explained by high levels of PSD-95/NMDAR co-association (Li et al. 2003). However, when we uncoupled PSD-95 from NMDARs with 2-BP and subsequently activated PKC, NMDAR peak current density was unchanged. Moreover, TPA does not alter NMDAR current density in immature neurons with low PSD-95 levels. While the outcomes of NMDAR regulation by PKC may be different in neurons and Xenopus oocytes, our data are in agreement with the major finding of Lin and colleagues (Lin et al. 2006), that PKC and PSD-95 converge in modulating NMDAR function.
PSD-95/NMDAR association may regulate synaptic plasticity and excitotoxicity
NMDAR desensitization limits calcium influx during repeated synaptic stimulation that can induce plasticity, or with prolonged exposure to glutamate such as may occur during ischemia. Here, we have demonstrated a novel mechanism by which NMDAR function can be regulated that may impact these processes. PSD-95 binding to NR2 subunits to reduce NMDAR desensitization may contribute to the shift in stimulation frequencies required to induce NMDAR-dependent synaptic plasticity that occur during brain development (Philpot et al. 2003), with consequences for new learning. As well, the effect of NMDAR/PSD-95 binding on the integrated calcium current in response to sustained glutamate insults may alter neuronal sensitivity to excitotoxicity. Since palmitoylation of PSD-95 is itself activity-dependent (El-Husseni et al., 2002), and this process targets PSD-95 to synapses and promotes its interaction with NMDARs, regulation of NMDAR desensitization by PSD-95 binding may be a highly dynamic mechanism for modulating neuronal signaling.
Figure 5.
PKC activation alters desensitization of recombinant NMDA receptors in HEK cells that express PSD-95. A. Representative traces of NMDAR currents in HEK cells expressing NR1 and NR2A subunits, with PSD-95-GFP or GFP, in control conditions or treated for 10 or 60 minutes with TPA. Current amplitudes were normalized for comparison of desensitization. B. Treatment with TPA for 60 min increased the extent of NMDA-evoked current desensitization in NR1/NR2A- and PSD-95- expressing cells. Control, n=8; 10-min TPA, n=8; 60-min TPA, n=8, **P<0.01, ***P<0.001; one-way ANOVA followed by Bonferroni post-test. Treatment with the phorbol ester, either for 10 or 60 minutes, did not induce a significant effect in GFP-expressing cells. Control, n=4; 10-min TPA, n=6; 60-min TPA, n=4.
Acknowledgments
We thank Esther Yu for assistance with neuronal culture preparation. Current address of B.L. is: Department of Neuroscience, University of California San Diego, 9500 Giman Drive, La Jolla, CA, 92093.
Grants
This work was supported by Canadian Institutes of Health Research (CIHR) operating grants to L.A.R. (MOP-129029) and A.E.H. L.A.R. is a CIHR Investigator and Michael Smith Foundation for Health Research (MSFHR) Senior Scholar. A.E.H. was a MSFHR Senior Scholar and University Distinguished Scholar.
Abbreviations
- BAPTA
1,2-bisethane-N,N,N',N'-tetraacetic acid
- DIV
days in vitro
- DMSO
dimethyl sulfoxide
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- EPSC
excitatory postsynaptic currents
- GFP
green fluorescent protein
- GAP43
growth associated protein-43
- GluR
glutamate receptor
- GK
guanylate kinase-like
- HEK293
human embryonic kidney 293
- Ip
peak current
- Iss
steady-state current
- LTP
long-term potentiation
- NBM
neurobasal media
- NMDA
N-methyl-D-aspartate
- NMDAR
NMDA receptor
- PBS
phosphate-buffered saline
- PDL
poly-D lysine
- PSD-95
post-synaptic density 95kDa
- PDZ
PSD-95/Discs large/zona occludens-1
- PKC
protein kinase C
- RO
RO-320432
- shRNA
short hairpin RNA
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SH3
Src homology 3
- TTX
tetrodotoxin
- TPA
12-O-tetradecanoylphorbol-13-acetate
- 2-BP
2-bromo-palmitate
- VH
holding potential
References
- Aoki C, Fujisawa S, Mahadomrongkul V, Shah PJ, Nader K, Erisir A. NMDA receptor blockade in intact adult cortex increases trafficking of NR2A subunits into spines, postsynaptic densities, and axon terminals. Brain Res. 2003;963:139–149. doi: 10.1016/s0006-8993(02)03962-8. [DOI] [PubMed] [Google Scholar]
- Auerbach A, Zhou Y. Gating reaction mechanisms for NMDA receptor channels. J Neurosci. 2005;25:7914–7923. doi: 10.1523/JNEUROSCI.1471-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci U S A. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchall AM, Bishop J, Bradshaw D, Cline A, Coffey J, Elliott LH, Gibson VM, Greenham A, Hallam TJ, Harris W, et al. Ro 32–0432, a selective and orally active inhibitor of protein kinase C prevents T-cell activation. J Pharmacol Exp Ther. 1994;268:922–929. [PubMed] [Google Scholar]
- Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Chen N, Moshaver A, Raymond LA. Differential sensitivity of recombinant N-methyl-D-aspartate receptor subtypes to zinc inhibition. Molecular pharmacology. 1997;51:1015–1023. doi: 10.1124/mol.51.6.1015. [DOI] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Sweeney NT, Craven SE, Kang R, El-Husseini Ael D, Bredt DS. Lipid- and protein-mediated multimerization of PSD-95: implications for receptor clustering and assembly of synaptic protein networks. J Cell Sci. 2003;116:3213–3219. doi: 10.1242/jcs.00617. [DOI] [PubMed] [Google Scholar]
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, Choudhary JS, Grant SG. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. Journal of neurochemistry. 2006;97 (Suppl 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- Cottrell JR, Borok E, Horvath TL, Nedivi E. CPG2: a brain- and synapse-specific protein that regulates the endocytosis of glutamate receptors. Neuron. 2004;44:677–690. doi: 10.1016/j.neuron.2004.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacological reviews. 1999;51:7–61. [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- El-Husseini Ael D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Traynelis SF. Zinc inhibition of rat NR1/NR2A N-methyl-D-aspartate receptors. J Physiol. 2008;586:763–778. doi: 10.1113/jphysiol.2007.143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong DK, Rao A, Crump FT, Craig AM. Rapid synaptic remodeling by protein kinase C: reciprocal translocation of NMDA receptors and calcium/calmodulin-dependent kinase II. J Neurosci. 2002;22:2153–2164. doi: 10.1523/JNEUROSCI.22-06-02153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hsueh YP, Sheng M. Requirement of N-terminal cysteines of PSD-95 for PSD-95 multimerization and ternary complex formation, but not for binding to potassium channel Kv1.4. J Biol Chem. 1999;274:532–536. doi: 10.1074/jbc.274.1.532. [DOI] [PubMed] [Google Scholar]
- Hu B, Zheng F. Molecular determinants of glycine-independent desensitization of NR1/NR2A receptors. The Journal of pharmacology and experimental therapeutics. 2005;313:563–569. doi: 10.1124/jpet.104.080168. [DOI] [PubMed] [Google Scholar]
- Jackson MF, Konarski JZ, Weerapura M, Czerwinski W, MacDonald JF. Protein kinase C enhances glycine-insensitive desensitization of NMDA receptors independently of previously identified protein kinase C sites. J Neurochem. 2006;96:1509–1518. doi: 10.1111/j.1471-4159.2006.03651.x. [DOI] [PubMed] [Google Scholar]
- Jain RK, Joyce PB, Molinete M, Halban PA, Gorr SU. Oligomerization of green fluorescent protein in the secretory pathway of endocrine cells. Biochem J. 2001;360:645–649. doi: 10.1042/0264-6021:3600645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. Differential K+ channel clustering activity of PSD-95 and SAP97, two related membrane-associated putative guanylate kinases. Neuropharmacology. 1996;35:993–1000. doi: 10.1016/0028-3908(96)00093-7. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. Calcium-dependent inactivation of recombinant N-methyl-D-aspartate receptors is NR2 subunit specific. Mol Pharmacol. 1996;50:1680–1688. [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Lester RA, Jahr CE. NMDA channel behavior depends on agonist affinity. J Neurosci. 1992;12:635–643. doi: 10.1523/JNEUROSCI.12-02-00635.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Chen N, Luo T, Otsu Y, Murphy TH, Raymond LA. Differential regulation of synaptic and extra-synaptic NMDA receptors. Nat Neurosci. 2002;5:833–834. doi: 10.1038/nn912. [DOI] [PubMed] [Google Scholar]
- Li B, Otsu Y, Murphy TH, Raymond LA. Developmental decrease in NMDA receptor desensitization associated with shift to synapse and interaction with postsynaptic density-95. J Neurosci. 2003;23:11244–11254. doi: 10.1523/JNEUROSCI.23-35-11244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao GY, Kreitzer MA, Sweetman BJ, Leonard JP. The postsynaptic density protein PSD-95 differentially regulates insulin- and Src-mediated current modulation of mouse NMDA receptors expressed in Xenopus oocytes. J Neurochem. 2000;75:282–287. doi: 10.1046/j.1471-4159.2000.0750282.x. [DOI] [PubMed] [Google Scholar]
- Lim IA, Hall DD, Hell JW. Selectivity and promiscuity of the first and second PDZ domains of PSD-95 and synapse-associated protein 102. J Biol Chem. 2002;277:21697–21711. doi: 10.1074/jbc.M112339200. [DOI] [PubMed] [Google Scholar]
- Lim IA, Merrill MA, Chen Y, Hell JW. Disruption of the NMDA receptor-PSD-95 interaction in hippocampal neurons with no obvious physiological short-term effect. Neuropharmacology. 2003;45:738–754. doi: 10.1016/s0028-3908(03)00276-4. [DOI] [PubMed] [Google Scholar]
- Lin Y, Jover-Mengual T, Wong J, Bennett MV, Zukin RS. PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proc Natl Acad Sci U S A. 2006;103:19902–19907. doi: 10.1073/pnas.0609924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi G, Prybylowski K, Fu Z, Luo J, Wenthold RJ, Vicini S. PSD-95 regulates NMDA receptors in developing cerebellar granule neurons of the rat. J Physiol. 2003;548:21–29. doi: 10.1113/jphysiol.2002.034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Wang Y, Yasuda RP, Dunah AW, Wolfe BB. The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B) Mol Pharmacol. 1997;51:79–86. doi: 10.1124/mol.51.1.79. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Vyklicky L, Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989;338:425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Futai K, Lashuel HA, Lo I, Okamoto K, Walz T, Hayashi Y, Sheng M. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–467. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Nuki G, Bresnihan B, Bear MB, McCabe D. Long-term safety and maintenance of clinical improvement following treatment with anakinra (recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis: extension phase of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:2838–2846. doi: 10.1002/art.10578. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Sala C, Niethammer M, Sheng M. Microtubule binding by CRIPT and its potential role in the synaptic clustering of PSD-95. Nat Neurosci. 1999;2:1063–1069. doi: 10.1038/15990. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP–2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski KL, Grossman SD, Wrathall JR, Wolfe BB. Expression of splice variants of the NR1 subunit of the N-methyl-D-aspartate receptor in the normal and injured rat spinal cord. Journal of neurochemistry. 2001;76:797–805. doi: 10.1046/j.1471-4159.2001.00069.x. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessoms-Sikes S, Honse Y, Lovinger DM, Colbran RJ. CaMKIIalpha enhances the desensitization of NR2B-containing NMDA receptors by an autophosphorylation-dependent mechanism. Mol Cell Neurosci. 2005;29:139–147. doi: 10.1016/j.mcn.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Sheng M, Pak DT. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu Rev Physiol. 2000;62:755–778. doi: 10.1146/annurev.physiol.62.1.755. [DOI] [PubMed] [Google Scholar]
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby O, Jensen V, Paulsen O, Andersen P, Kim JJ, Thompson RF, Sun W, Webster LC, Grant SG, Eilers J, Konnerth A, Li J, McNamara JO, Seeburg PH. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998;92:279–289. doi: 10.1016/s0092-8674(00)80921-6. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Regulation of glycine-insensitive desensitization of the NMDA receptor in outside-out patches. J Neurophysiol. 1994;72:754–761. doi: 10.1152/jn.1994.72.2.754. [DOI] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–1512. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagey R, Hu J, Pelech SL, Raymond LA, Krieger C. Modulation of NMDA-mediated excitotoxicity by protein kinase C. J Neurochem. 2001;78:715–726. doi: 10.1046/j.1471-4159.2001.00459.x. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Raouf R, Lu WY, Wang LY, Orser BA, Dudek EM, Browning MD, MacDonald JF. Regulation of N-methyl-D-aspartate receptor function by constitutively active protein kinase C. Mol Pharmacol. 1998;54:1055–1063. [PubMed] [Google Scholar]
- Yamada Y, Iwamoto T, Watanabe Y, Sobue K, Inui M. PSD-95 eliminates Src-induced potentiation of NR1/NR2A-subtype NMDA receptor channels and reduces high-affinity zinc inhibition. J Neurochem. 2002;81:758–764. doi: 10.1046/j.1471-4159.2002.00886.x. [DOI] [PubMed] [Google Scholar]
- Zheng F, Erreger K, Low CM, Banke T, Lee CJ, Conn PJ, Traynelis SF. Allosteric interaction between the amino terminal domain and the ligand binding domain of NR2A. Nat Neurosci. 2001;4:894–901. doi: 10.1038/nn0901-894. [DOI] [PubMed] [Google Scholar]
- Zheng F, Gingrich MB, Traynelis SF, Conn PJ. Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nature neuroscience. 1998;1:185–191. doi: 10.1038/634. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Thio LL, Clark GD, Clifford DB. Blockade of desensitization augments quisqualate excitotoxicity in hippocampal neurons. Neuron. 1990;5:61–66. doi: 10.1016/0896-6273(90)90033-c. [DOI] [PubMed] [Google Scholar]