SUMMARY
Aging-associated muscle insulin resistance has been hypothesized to be due to decreased mitochondrial function, secondary to cumulative free radical damage, leading to increased intramyocellular lipid content. To directly test this hypothesis we examined both in vivo and in vitro mitochondrial function, intramyocellular lipid content and insulin action in lean healthy mice with targeted overexpression of the human catalase gene to mitochondria (MCAT mice). Here we show that MCAT mice are protected from age-induced decrease in muscle mitochondrial function (~30%), energy metabolism (~7%) and lipid-induced muscle insulin resistance. This protection from age-induced reduction in mitochondrial function was associated with reduced mitochondrial oxidative damage, preserved mitochondrial respiration and muscle ATP synthesis and AMP-activated protein kinase-induced mitochondrial biogenesis. Taken together these data suggest that the preserved mitochondrial function maintained by reducing mitochondrial oxidative damage may prevent age-associated whole body energy imbalance and muscle insulin resistance.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) and impaired glucose tolerance affect ~40% of the population over the age of 65 (Harris et al., 1998), and more than half of the 16 million Americans estimated to have T2DM are over age 60 (NDIC, 2002). However the underlying mechanism for the increased prevalence of T2DM associated with aging is unknown. Using multinuclear magnetic resonance spectroscopy to directly assess rates of muscle mitochondrial oxidative-phosphorylation activity and intramyocellular lipid content in vivo Petersen et al. found that healthy lean elderly individuals had an ~35% reduction in basal rates of muscle mitochondrial oxidative-phosphorylation activity which was associated with an ~30% increase in intramyocellular lipid content and severe muscle insulin resistance (Petersen et al., 2003). These results led to the hypothesis that age-associated reductions in muscle insulin sensitivity may be secondary to reduced mitochondrial activity resulting in increased intramyocellular lipid content leading to defective insulin signaling (Griffin et al., 1999; Shulman, 2000; Yu et al., 2002). However it remains to be determined whether the increased intramyocellular lipid content and muscle insulin resistance associated with aging is a cause or consequence of the mitochondrial dysfunction. Furthermore the nature of the mitochondrial dysfunction associated with aging remains unknown. Although cumulative oxidative stress has been proposed to cause age-associated reductions in mitochondrial function (Cadenas and Davies, 2000; Jang et al., 2009; Perez et al., 2008; Shigenaga et al., 1994; Stadtman, 2002; Wei et al., 1998) this remains a controversial topic (Bashan et al., 2009; Bonnard et al., 2008; Evans et al., 2005; Houstis et al., 2006; Loh et al., 2009; Ristow et al., 2009) and in vivo studies assessing the potential role of oxidative stress study in causing muscle mitochondrial function are lacking.
In order to directly examine whether age-associated reductions in mitochondrial function were due to cumulative oxidative damage and whether age-associated reductions in muscle mitochondrial function would lead to intramyocellular lipid accumulation and muscle insulin resistance, we examined both in vitro and in vivo mitochondrial function and intramuscular lipid content and insulin action in young and lean healthy old mice with targeted overexpression of human catalase to the mitochondria (MCAT). Whole body and tissue specific effects of insulin were assessed in awake young and old wild type (WT) and MCAT mice using a hyperinsulinemic-euglycemic clamp in combination with 3H/14C labeled glucose and in vivo rates of muscle mitochondrial ATP synthesis were assessed in vivo using 31P MRS.
RESULTS
MCAT protects mitochondria from cumulative oxidative damage and age-associated reductions in mitochondrial function
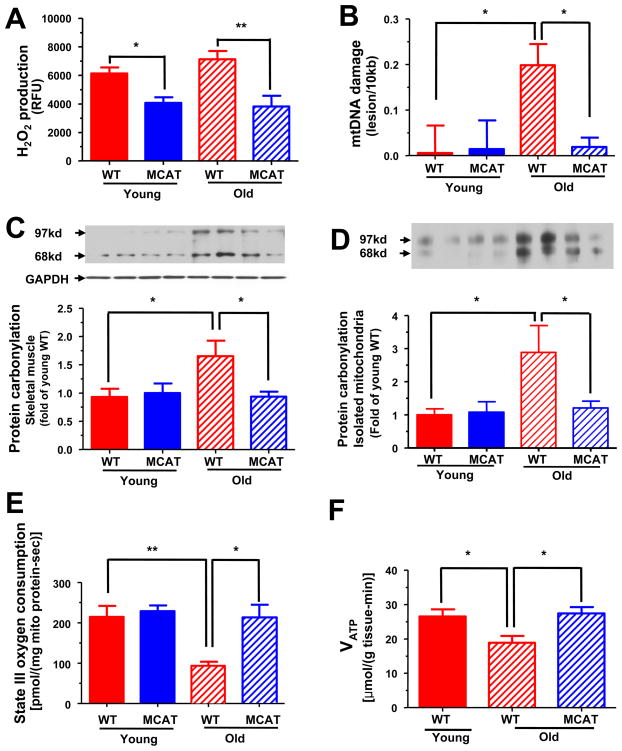
As an index of ROS induced mitochondrial damage and oxidative phosphorylation activity, we examined muscle mitochondrial hydrogen peroxide (H2O2) production, mitochondrial DNA (mtDNA) damage, oxidative protein carbonylation, mitochondrial oxygen consumption and in vitro and in vivo muscle mitochondrial ATP synthesis in age-weight matched young and old WT and MCAT mice. The mitochondrial targeted catalase is mainly overexpressed in both slow and fast twitch muscle tissues (Figure S1A, online available), and catalase overexpression attenuated muscle mitochondrial H2O2 production by ~45% in both young and old MCAT mice compared to WT mice (Figure 1A). There were no differences in superoxide dismutase 2 or glutathione peroxidase 1 protein expression (Figure S1B) or genomice DNA damage in gastrocnemius muscle (Figure S1C). The mtDNA damage (Figure 1B) and mitochondrial protein carbonylation (Figure 1C and 1D) were markedly increased in the old WT mice compared to the young WT and young MCAT mice. In contrast old MCAT mice were protected from these age-associated increases in mtDNA damage (Figure 1B) and protein carbonylation (Figure 1C and 1D). These age-associated increases in mtDNA damage and protein carbonylation were associated with reductions in both state III and state IV oxygen consumption (Figure 1E and S1D) and decreased rates of muscle mitochondrial ATP synthesis (Figure 1F) assessed by in vivo 31P magnetic resonance spectroscopy. In contrast old MCAT mice were protected from these age-associated reductions in both in vitro and in vivo mitochondrial function (Figure 1E and 1F).
Figure 1.
Hydrogen peroxide production, mitochondrial damage and mitochondrial function (both in vitro and in vivo) in skeletal muscle of young and old WT and MCAT mice. (A) Maximal H2O2 emission of mitochondria derived from skeletal muscle (n=10–15 per group). (B) Mitochondrial DNA damage in skeletal muscle (n=4–6 per group). (C) Oxidative protein carbonylation in skeletal muscle extracts (n=6 per group) and (D) in skeletal muscle mitochondria (n=6 per group). (E) State III oxygen consumption of mitochondria derived from skeletal muscle (n=6 per group). (F) Basal rates of ATP synthesis in skeletal muscle assessed by in vivo 31P MRS (n=6 per group). All data are mean ± SEM. Asterisk (*) indicates P<0.05 and **P<0.01 by ANOVA with post-hoc analysis.
MCAT protects mice from age-associated reductions in whole body energy metabolism
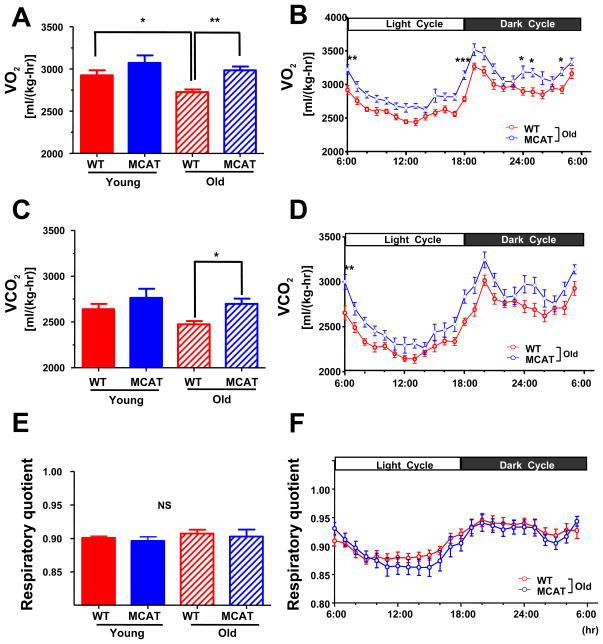
To assess the impact of preserved mitochondrial function on age-associated changes in whole body energy metabolism, body composition matched young and old MCAT and WT mice were studied by indirect calorimetry. Consistent with the decreased mitochondrial oxygen consumption and ATP synthesis observed in the old WT mice, whole body oxygen consumption (VO2) and energy expenditure were decreased by 6.8% and 6.9% respectively in old WT mice, compared to young WT mice (Figure 2A and S2A). In contrast old MCAT mice were protected from these age-associated reductions in oxygen consumption, CO2 production and energy expenditure (Figure 2B, 2D and S2B). Body weights and body fat composition were identical between young MCAT and WT mice, with similar increases in body weight occurring with aging in both groups (Table S1). There were no differences in respiratory exchange rate (Figure 2E and 2F) or locomotor activity (Figure S2E and S2F) between the young and old WT and MCAT mice although there was a tendency for the old MCAT mice to have a slight increase in food intake compared to the old WT mice (Figure S2C and S2D), which may explain the similar body weights and body composition in the old WT and MCAT mice despite an ~7% increase in whole body energy expenditure in the old MCAT mice. Taken together, these data demonstrate that aging is associated with ROS-induced mitochondrial damage, which are associated with reductions in muscle mitochondrial function and whole body energy metabolism, and that these changes are prevented by overexpression of catalase in mitochondria.
Figure 2.
Whole body energy metabolism. (A) Whole body oxygen consumption (VO2) consumption, (C) CO2 production (VCO2) and (E) respiratory quotient in young and old WT and MCAT mice during 72 hr analysis. Hour to hour average (B) VO2, (D) VCO2 and (F) respiratory quotient in old WT and MCAT mice during light/dark period. All data are mean ± SEM. N=8 in young groups; n=12–15 in old groups. *P<0.05; **P<0.01; ***P<0.001 by ANOVA with post-hoc analysis.
Age-associated reductions in mitochondrial function predispose aged mice to intramyocellular lipid accumulation and insulin resistance
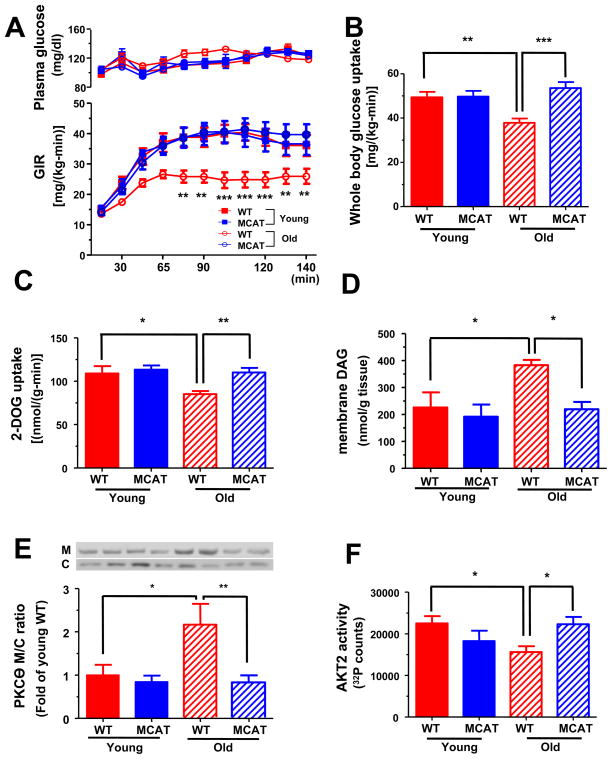
To determine whether protection from age-associated reductions in muscle mitochondrial function would result in protection from age-associated muscle insulin resistance, we performed hyperinsulinemic-euglycemic clamp studies in the body composition matched young and old WT and MCAT mice. Old WT mice were markedly insulin resistant as reflected by a 35% reduction in the glucose infusion rate (GIR) required to maintain euglycemia during the hyperinsulinemic-euglycemic clamp (Figure 3A). In contrast, old MCAT mice were totally protected from age-associated whole body insulin resistance as reflected by a GIR, which was undistinguishable from that of the young WT and MCAT mice (Figure 3A). Whole body insulin resistance in the old WT mice was due to decreased insulin-stimulated peripheral glucose uptake (Figure 3B) which could be attributed to decreased insulin-stimulated muscle glucose uptake, which were both normal in the old MCAT mice (Figure 3B and 3C). Although basal rates of hepatic glucose production were slightly increased in the old versus young WT mice, there were no differences in basal rates of hepatic glucose production (Table S1) or suppression of hepatic glucose production during the hyperinsulinemic-euglycemic clamp between the WT and MCAT mice (Table S2). Fasting plasma glucose and insulin concentrations were similar between WT and MCAT mice (Table S1).
Figure 3.
Insulin sensitivity, intramyocellular lipid accumulation and PKCθ activation in young and old WT and MCAT mice during hyperinsulinemic euglycemic clamp studies. (A) Glucose infusion rate during the hyperinsulinemic euglycemic clamp (n=8–10 per group) and (B) insulin stimulated whole body glucose uptake (n=8–10 per group). (C) Insulin stimulated 2-DOG uptakes in gastrocnemius muscle (n=8 per group). (D) Membrane DAG concentration (n=5–8 per group) and (E) membrane translocation of PKCθ (n=6–10 per group) in quadriceps muscle. M, membrane; C, cytosol. (F) AKT2 activity in gastrocnemius skeletal muscle (n=5–6 per group). All data are mean ± SEM. *P<0.05; **P<0.01; ***P<0.001 by ANOVA with post-hoc analysis.
Insulin resistance in skeletal muscle has been attributed to increases in diacylglycerol (DAG) content which in turn activates protein kinase C-θ (PKCθ) and inhibits insulin signaling at the level of IRS-1 tyrosine phosphorylation (Griffin et al., 1999; Shulman, 2000; Yu et al., 2002). Consistent with this hypothesis, skeletal muscle membrane DAG content was increased by ~70% in the old WT mice (Figure 3D) and was associated with increased PKCθ activation as reflected by increased PKCθ translocation to the plasma membrane (Figure 3E) and a ~30% reduction in insulin activation of AKT2 (Figure 3F). In contrast, MCAT mice exhibited no increases in membrane DAG content or PKCθ activation and similar activation of AKT2 compared to young WT and MCAT mice (Figure 3D to 3F). Thus catalase overexpression in the mitochondria prevented muscle mitochondrial dysfunction, which in turn prevented increases in intramuscular DAG content, PKCθ activation and muscle insulin resistance.
MCAT reduce the aging associated declines in mitochondrial biogenesis
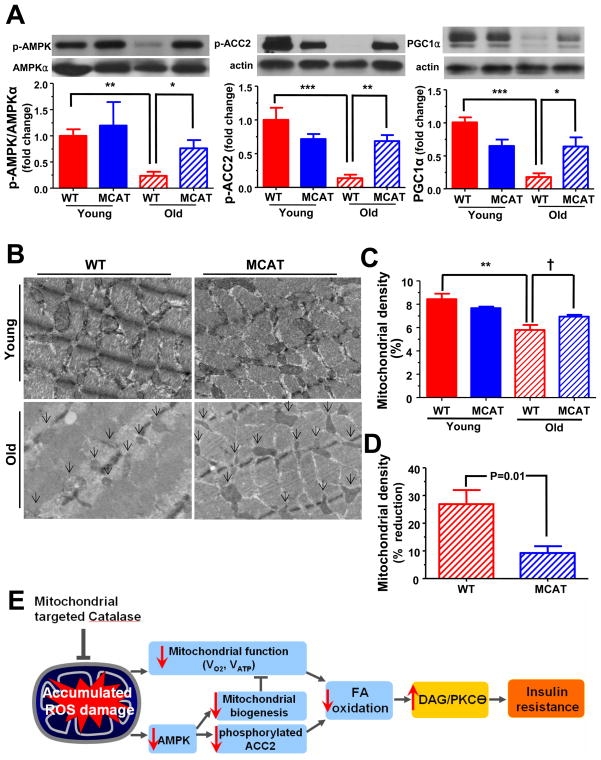
AMP-activated protein kinase (AMPK) is a critical regulator of mitochondrial biogenesis (Bergeron et al., 2001; Hardie, 2007; Zong et al., 2002) and aging is associated with a reduction in AMPK-induced mitochondrial biogenesis (Reznick et al., 2007). In the present study, age-associated decline in AMPK activation was also observed. Specifically, the ratio of phosphorylated AMPKT172 (p-AMPK) to AMPKα protein levels in skeletal muscle of WT mice was decreased and was accompanied by decreased ACC2 phosphorylation and decreased PGC1α expression (Figure 4A). Furthermore, there were morphological changes in mitochondrial structure during aging, mainly in size and density (Figure 4B). The intramyofibllilar mitochondrial density in soleus muscle was reduced by ~27% in old WT mice compared to young WT mice (Figure 4C and 4D). In contrast MCAT mice were protected from these age-associated declines in AMPK activity (Figure 4A) and mitochondrial density (Figure 4C and 4D). Consistent with this finding of protection from age-associated reductions in mitochondrial density we found a similar pattern of reduced VDAC mitochondrial membrane protein expression in the gastrocnemius and quadripcep muscles of the old WT mice which in contrast was normal in the old MCAT mice (Figure S3).
Figure 4.
AMPK activity and mitochondrial density in skeletal muscle in young and old WT and MCAT mice. (A) Representative immunoblots for each group and the ratio of p-AMPKT172 to AMPKα, the ratio of p-ACC2Ser212 and PGC1α to actin in quadriceps muscle (n=4–6 per group). (B) Representative figures for the intramyofibrillar mitochondria (arrows) in soleus skeletal muscle. (C) Mitochondrial density in soleus muscle and (D) the percent of declines in mitochondrial density between WT and MCAT during aging (n=3–5 per group). (E) Schematic fugure of the potential effect of mitochondrial oxidative damage on insulin sensitivity. All data are mean ± SEM. †P<0.05 by two-tailed unpaired Student’s t-tests. *P<0.05; **P<0.01; ***P<0.001 by ANOVA with post-hoc analysis.
DISCUSSION
The role of ROS in causing age-associated reduction in mitochondrial function is controversial (Bashan et al., 2009; Bonnard et al., 2008; Evans et al., 2005; Houstis et al., 2006; Loh et al., 2009; Ristow et al., 2009). Furthermore it is unclear whether age-associated reductions in muscle mitochondrial function are responsible for the age-associated increases in intramyocellular lipid content and muscle insulin resistance or whether it is secondary in nature. In order to examine these fundamental questions we assessed muscle mitochondrial function and liver and muscle insulin responsiveness in young and old WT and MCAT mice. Using this approach we found that overexpression of catalase in mitochondria prevented age-associated mitochondrial damage and age-associated reductions in muscle mitochondrial function. Furthermore we found that prevention of age-associated reduction in muscle mitochondrial function prevented age-associated increases in muscle DAG content, PKCθ activation and muscle insulin resistance. These studies support the hypothesis that age-associated reductions in mitochondrial function predispose to intramyocellular lipid accumulation, PKCθ activation and muscle insulin resistance (Lowell and Shulman, 2005; Morino et al., 2006; Petersen et al., 2003). We also found that the overexpression of catalase in mitochondria protected mice from age-associated reductions in muscle AMPK activity and mitochondrial biogenesis (Reznick et al., 2007). To our knowledge this is the first demonstration that overexpression of catalase in mitochondria prevents age-associated: 1) reduction in muscle mitochondrial function in vivo, 2) decreases in whole body oxygen consumption, 3) increases in muscle DAG content, PKCθ activity and muscle insulin resistance, 4) decreases in AMPK activity and reductions in mitochondrial biogenesis.
Taken together these findings support the hypothesis that age-associated reductions in mitochondrial function are due to mitochondrial generated ROS production, which contribute to the pathogenesis of age-associated muscle insulin resistance and T2DM. Furthermore these data suggest that potential novel therapies targeted to reduce mitochondrial oxidative damage may prevent these age-associated changes.
EXPERIMENTAL PROCEDURES
Animals
Details of the generation of the mice overexpressing mitochondrial catalase (MCAT) used in this study have been described previously (Schriner et al., 2005). The ~3 to 6-month-old (young) and ~15 to 18-month-old (old) male MCAT mice and their age matched littermate wild-type control male mice (WT) were used. To minimize environmental differences, mice were individually housed for at least 2 weeks before each experiment. Fat and lean body composition were assessed by 1H nuclear magnetic resonance spectroscopy (Bruker BioSpin) on WT and MCAT mice, and expressed as percentages of total body weight. A comprehensive animal metabolic monitoring system (CLAMS: Columbus instruments, Columbus, OH, USA) was used to evaluate VO2, VCO2, respiratory quotient, energy expenditure, food consumption and activity for body weight and fat matched animals as previously described (Zhang et al., 2010). The study was conducted at the NIH-Yale Mouse Metabolic Phenotyping Center. All procedures were approved by the Yale University Animal Care and Use Committee.
Mitochondrial ROS production
Fresh skeletal muscle mitochondria were isolated from mixed hind limb skeletal muscle (quadriceps, gastrocnemius) from overnight fasted WT and MCAT mice using MITO-ISO kit (Sigma) according to the manufacturer’s instructions. Maximal mitochondrial H2O2 production was measured using dichlorodihydrofluorescein diacetate in the presence of horseradish peroxidase as described (Andrews et al., 2008). Data are expressed as arbitrary fluorescence units (RFU). Protein concentration was determined by the Bradford method.
Mitochondrial DNA damage and oxidative protein carbonylation
Total genomic DNA was isolated from snap frozen gastrocnemius skeletal muscle, and mtDNA copy number and integrity were determined using the QPCR method established by Van Houten and colleagues (Santos et al., 2006). Specific primers were used to amplify a fragment of the β-globin gene (13.5 kb) to determine nuclear DNA integrity; a large fragment of mtDNA (8.9 kb) to determine mtDNA integrity; and a small fragment (221 bp) of the mitochondrial genome to monitor changes in mtDNA copy number and to normalize the data obtained when amplifying the 8.9 kb fragment. Relative amplifications were calculated comparing each group with average of young WT, and used to estimate the lesion frequency, expressed as number of lesions per 10 kb, assuming a Poisson distribution of lesions on the template. Sensitivity limit of the technique is 1 lesion/105 bases. Oxidative protein carbonylation in both skeletal muscle (quadriceps) lysate and the isolated mitochondrial protein were measured by a western blot method using Oxyblot™ protein oxidation detection kit (Millipore).
Mitochondrial oxygen consumption
Skeletal muscle mitochondria were isolated from mixed hind limb skeletal muscle (quadriceps and gastrocnemius) from overnight fasted animals, and oxygen consumption was assessed as previously described (Andrews et al., 2008) using a Clark-type oxygen electrode (Hansatech Instruments, Norfolk, UK) at 37°C with succinate (10 mM). State III respiration was obtained by adding ADP and state IV respiration was obtained by adding oligomycin.
Unidirectional rate of muscle ATP synthesis by in vivo 31P-MRS
After an overnight fast, the mouse was sedated by using ~1% isoflurane, and the left hindlimb was positioned under a 15-mm-diameter 31P surface coil. The unidirectional rate of muscle ATP synthesis (VATP) was assessed by 31P saturation-transfer MRS using a 9.4 T superconducting magnet (Magnex Scientific) interfaced to a Bruker Biospec console as described previously (Choi et al., 2008).
Hyperinsulinemic-euglycemic clamp study
Seven days prior to the hyperinsulinemic-euglycemic clamp studies, indwelling catheters were placed into the right internal jugular vein extending to the right atrium. After an overnight fast, [3-3H]-glucose (HPLC purified; PerkinElmer) was infused at a rate of 0.05 μCi/min for 2 hours to assess the basal glucose turnover, and a hyperinsulinemic-euglycemic clamp in awake mice was conducted for 140 min with a primed/continuous infusion of human insulin (152.8 pmol/kg prime, 21.5 pmol/kg/min infusion; Novo Nordisk) as described previously (Samuel et al., 2006). During the clamp, plasma glucose was maintained at basal concentrations (~120 mg/dl). Rates of basal and insulin-stimulated whole-body glucose fluxes and tissue glucose uptake were determined by bolus (10μci) injection of 2-deoxy-D-[1-14C] glucose (2-DOG, PerkinElmer) as described (Samuel et al., 2006; Zhang et al., 2010).
Tissue lipid measurements
DAG extraction and analysis in both cytosolic and membrane samples from quadriceps muscle was performed as previously described (Neschen et al., 2005; Yu et al., 2002). Total DAG content was expressed as the sum of individual DAG species. Tissue triglyceride was extracted using the method of Bligh and Dyer (Bligh and Dyer, 1959) and measured using a DCL Triglyceride Reagent (Diagnostic Chemicals Ltd).
Insulin and AMPK signaling assays
AKT2 activity and PKCθ membrane translocation were assessed in protein extracts from gastrocnemius and quadriceps muscle, respectively, harvested after hyperinsulinemic-euglycemic clamp study using the methods previously described (Alessi et al., 1996; Choi et al., 2007; Samuel et al., 2004).
Phosphorylated AMP-activated protein kinase (T172) (p-AMPKT172), Peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC1α), phosphorylated acetyl-CoA carboxylase (p-ACC2) were assessed in protein extracts from quadriceps muscle harvested after overnight fasting. Immunoblots were quantified from multiple exposures using ImageJ (NIH). Relative values from band intensities normalized to actin were calculated comparing each sample with average of young WT. The primary antibodies used in the current study were as follows: PKCθ (Santa Cruz), p-AMPKT172 (Cell Signaling), AMPKα (Cell Signaling), pan-actin (Cell signaling), GAPDH (Cell signaling), PGC1α (Santacruz), phospho-ACC2S212 (Santacruz) and Catalase (Abcam).
Mitochondrial density assessed by transmission electron microscopy
Mitochondrial density was assessed by Transmission Electron Microscopy for soleus muscle as described (Morino et al., 2005). For each sample, 5 images of 3 random sections of individual muscle were taken. The average volume density of these 15 images was used to estimate the mitochondrial volume density for each muscle.
The study was conducted and analyzed at the Electron Microscopy Core Facility, Yale School of Medicine. Images were acquired using a Tecnai Biotwin TEM at 80kV, using a Morada CCD and iTEM software.
Statistics
Values are expressed as mean ± SEM. The significance of the differences in mean values among two groups was evaluated by two-tailed unpaired Student’s t-tests. More than three groups were evaluated by ANOVA followed by post hoc analysis using the Bonferroni’s Multiple Comparison Test. P values less than 0.05 were considered significant.
HIGHLIGHTS.
Overexpression of catalase in mitochondria prevents age-associated reductions in muscle mitochondrial function in vivo.
Overexpression of catalase in mitochondria prevents age-associated increases in muscle DAG content, PKCθ activity and muscle insulin resistance.
Overexpression of catalase in mitochondria prevents age-associated decreases in AMPK activity and reductions in mitochondrial biogenesis.
Supplementary Material
Acknowledgments
We thank David Frederick, Xiaoxian Ma, Mario Kahn, Blas Guigni, Christopher Carmean, Irena Todorova, Yanna Kosover for their excellent technical support in these studies. These studies were support by grants from the United States Public Health Service (R01 DK40936, R01 AG23686, U24 DK076169, P01 ES01116, P01 AG001751), German Research Foundation Bi1292/2-1 and a VA Merit grant (VTS).
Footnotes
Supplemental information includes 2 supplemental tables and 4 figures and can be found with this article online.
AUTHOR CONTRIBUTIONS
H.-Y.L., C.S.C, V.T.S. and G.I.S. designed research; H.-Y.L., A.L.B., T.C.A, F.R.J., M.J.J., D.Z., D.K.W and J.H.S performed research; H.-Y.L., C.S.C., G.S.S, W.L, P.S.R, J.H.S, K.F.P., V.T.S. and G.I.S analyzed data; and H.-Y.L., C.S.C., G.S.S, W.L, P.S.R, J.H.S, K.F.P., V.T.S. and G.I.S edited manuscript; and H.-Y.L., V.T.S. and G.I.S wrote manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89:27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, et al. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci U S A. 2008;105:19926–19931. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CS, Fillmore JJ, Kim JK, Liu ZX, Kim S, Collier EF, Kulkarni A, Distefano A, Hwang YJ, Kahn M, et al. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1995–2003. doi: 10.1172/JCI13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7:1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Jang YC, Perez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, et al. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci. 2009;64:1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NDIC. Diabetes and Aging. National Diabetes Information Clearinghouse (NDIC); 2002. http://diabetes.niddk.nih.gov/about/dateline/spri02/8.htm. [Google Scholar]
- Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, et al. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Perez VI, Lew CM, Cortez LA, Webb CR, Rodriguez M, Liu Y, Qi W, Li Y, Chaudhuri A, Van Remmen H, et al. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic Biol Med. 2008;44:882–892. doi: 10.1016/j.freeradbiomed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Choi CS, Phillips TG, Romanelli AJ, Geisler JG, Bhanot S, McKay R, Monia B, Shutter JR, Lindberg RA, et al. Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes. 2006;55:2042–2050. doi: 10.2337/db05-0705. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- Santos JH, Meyer JN, Mandavilli BS, Van Houten B. Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol Biol. 2006;314:183–199. doi: 10.1385/1-59259-973-7:183. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Importance of individuality in oxidative stress and aging. Free Radic Biol Med. 2002;33:597–604. doi: 10.1016/s0891-5849(02)00904-8. [DOI] [PubMed] [Google Scholar]
- Wei YH, Lu CY, Lee HC, Pang CY, Ma YS. Oxidative damage and mutation to mitochondrial DNA and age-dependent decline of mitochondrial respiratory function. Ann N Y Acad Sci. 1998;854:155–170. doi: 10.1111/j.1749-6632.1998.tb09899.x. [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- Zhang D, Christianson J, Liu ZX, Tian L, Choi CS, Neschen S, Dong J, Wood PA, Shulman GI. Resistance to high-fat diet-induced obesity and insulin resistance in mice with very long-chain acyl-CoA dehydrogenase deficiency. Cell Metab. 2010;11:402–411. doi: 10.1016/j.cmet.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.