Abstract
Background
One of the principal theories regarding the biological basis of Major Depressive Disorder (MDD) implicates a dysregulation of emotion processing circuitry. Gender differences in how emotions are processed and relative experience with emotion processing might help to explain some of the disparities in the prevalence of MDD between women and men. The current study sought to explore how gender and depression status relate to emotion processing.
Methods
This study employed a 2 (MDD status) × 2 (gender) factorial design to explore differences in classifications of posed facial emotional expressions (N = 151).
Results
For errors, there was an interaction between gender and depression status. Women with MDD made more errors than did non-depressed women and men with MDD, particularly for fearful and sad stimuli (ps < .02), which they were likely to misinterpret as angry (ps < .04). There was also an interaction of diagnosis and gender for response cost for negative stimuli, with significantly greater interference from negative faces present in women with MDD compared with non-depressed women (p = .01). Men with MDD, conversely, performed similarly to control men (p = .61).
Conclusions
These results provide novel and intriguing evidence that depression in younger adults (< 35 years) differentially disrupts emotion processing in women as compared to men. This interaction could be driven by neurobiological and social learning mechanisms, or interactions between them, and may underlie differences in the prevalence of depression in women and men.
Keywords: psychiatric disorders, affect perception, sex differences
There is nearly a twofold prevalence of Major Depressive Disorder (MDD) in women as compared to men. Numerous biological, cognitive, and interpersonal hypotheses have been generated and tested to explain this difference(1–3). Among these hypotheses is the possibility that cognitive processes, such as emotion processing, underlie the differences between women and men in prevalence and pattern of depressive episodes(4).
Emotion processing and categorization are essential to successful communication and adaptive social behavior, as they involve both cognitive and interpersonal elements. These skills have emerged as key areas of inquiry in MDD. Inaccuracies in classification of emotional facial expressions are more frequent among depressed than non-depressed individuals(5;6). However, it is not clear whether categorization inaccuracies occur only with certain types of emotions. Also, despite known differences in the prevalence and sequelae of MDD between women and men(7), to our knowledge, no study has explored gender as a factor influencing emotion processing abnormalities in MDD.
Findings of emotion processing inaccuracies and biases in MDD have been demonstrated in studies of posed facial expressions, both during categorization and when self-simulated(8–13).The errors characteristic of MDD vary, however. For example, Gur and colleagues(10) found that depressed patients made more false negative categorizations for positive stimuli, more true positive categorizations for negative stimuli, and more frequently interpreted neutral faces as sad than did controls. In contrast, other studies have found that depressed patients are more likely than controls to classify sad faces incorrectly and are no different from controls in identifying neutrally posed expressions(12;13).
Methodological variability may partly explain the inconsistencies in the emotion processing and depression literature. For example, some studies have presented facial expressions for very brief periods of time (80–300 ms) using a computer(5;12), whereas others have presented stimuli manually with no time limit(6). From the perspective of ecological validity, it is critically important for these types of experiments to simulate the real-time demands of processing emotions, because it is more likely to reflect the real-life challenges of those who experience depression(5).
Gender and Emotion Processing
Although gender may be an important moderating variable in emotion processing and categorization during MDD, none of the aforementioned behavioral studies explored gender differences, and some samples were composed primarily or exclusively of women(5;6;10;13) or only of men(11;12). Gender differences in how emotions are processed and relative experience with emotion processing might help to explain some of the disparities in the prevalence of MDD between women and men. In non-depressed samples, gender differences in facial emotion processing have consistently favored women, both in terms of accuracy and speed of processing emotional information(14–17).
To address the limitations of the literature, the current study tested two primary hypotheses and one exploratory hypothesis. First, we hypothesized that individuals with MDD would demonstrate poorer emotion processing and greater negative response cost compared to non-depressed (control) participants. Second, we hypothesized accuracy and processing speed advantages for women in emotion processing. Finally, owing to a lack of previous literature, we speculated that there would be an interaction between MDD status and gender in emotion processing. Given the expectations that women would outperform men and controls would outperform individuals with MDD, it seemed likely that decrements in emotion processing accuracy for women and men during MDD would not be equal. Importantly, we only studied patients with MDD below the age of 35, to conservatively remove any effects of late onset MDD (e.g., common cerebrovascular causes).
Methods
Participants
Participants were 72 non-depressed controls (34 women, 38 men) and 79 patients diagnosed with MDD (56 women, 23 men). Fifty participants had MDD alone, 15 also met criteria for an anxiety disorder, and 11 also met criteria for Dysthymic disorder. Healthy Control (HC) participants were recruited through four separate studies. Diagnosis of schizophrenia, bipolar disorder, brain injury, neurological conditions, or conditions that would affect cognitive functioning (e.g., cardiovascular disease) served as exclusionary criteria. Substance use greater than two alcoholic drinks per day or abuse of illicit substances also was grounds for exclusion. Thirty-four of the HC participants were screened formally with the SCID-IV(18) by licensed psychologists or nurses. Thirty-eight of the HC participants were screened with a semi-structured psychiatric/neurologic interview(5), with rule-out diagnoses taken from the DSM-IV/SCID-IV.
Participants with MDD were recruited through four separate mechanisms. Seven completed the SCID-IV, 69 completed semi-structured interviews by a licensed psychologist and/or licensed psychiatrist as part of larger clinical evaluations, and three completed the semi-structured interview that includes rule-out diagnoses from the DSM-IV/SCID-IV. Research subjects were compensated either $15 or $25 per hour, or received course credit (Marquette University participants), depending upon the research protocol used. Informed consent (n = 10 MDD patients and all control subjects) or waiver of informed consent (n = 69 MDD patients with retrospective data collection) was completed in compliance with approved IRB protocols at the University of Michigan Medical Center and Marquette University and the Declaration of Helsinki.
As shown in Table 1, the control group had significantly more years of formal education compared to the MDD group, F(1,144) = 7.35, p = < .01), with no interaction between diagnosis and gender, F(1, 144) = 1.85, p = .18. As greater education would likely benefit the performance of the MDD groups, whom were expected to be impaired relative to the control group, we did not use education as a covariate (emotion classification errors with education, r = −.13, p = .13).
Table 1.
Demographic Characteristics
| Measure | MDD | Control | ||
|---|---|---|---|---|
| Men (n = 23) | Women (n = 56) | Men (n = 38) | Women (n = 34) | |
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Age | 26.13 (4.81) | 25.95 (5.05) | 23.84 (9.17) | 26.71 (10.77) |
| Education | 15.52 (2.39) | 14.89 (2.42) | 14.00 (1.79) | 14.38 (2.02) |
| PHQ-8 | 13.69 (7.09) | 13.40 (6.59) | ||
| Age of onset | 17.82 (7.56) | 17.26 (6.15) | ||
| Chronicity in years | 9.29 (6.86) | 8.78 (6.40) | ||
| % taking psychotropic medication | 52.2 (n = 10 SSRI-like; n = 2 SSRI-like plus) | 50.0 (n = 16 SSRI-like; n = 11 SSRI-like plus) | ||
Note. PHQ-8: 0 – 5 = no depression; 6 – 9 = mild depression; 10 – 14 = moderate depression; 15 – 19 = moderately severe depression; 20 – 27 = severe depression. PHQ-8 = Patient Health Questionnaire (Depression Scale); n =16 men and 46 women.
There were no differences between groups in age, based upon gender, F(1, 147) = 1.02, p = .31, or diagnosis, F (1,147) = 0.33, p = .57, nor was the interaction significant, F(1, 147) = 1.32, p = .25. However, there were significantly more women with MDD than control women and more control men than men with MDD, X2 (1, N = 151) = 8.76, p <.01, which is not atypical of population parameters. There were no gender differences in MDD symptoms as measured by the PHQ-8(19), F(1, 59) = 0.04, p = .88, in the percentage of participants taking psychotropic medications, X2(1, N = 74) = .03, p = .86, age of depression onset, F(1, 64) = 0.12, p = .74, or chronicity of depression, F(1, 64) = 0.80, p = .78.
Measures and Procedure
Participants were seated in front of a desktop computer. Emotion processing was assessed using the Facial Emotion Perception Task(5;20), a computerized measure described fully in Langenecker and colleagues(21). Briefly, faces taken from the Ekman series(22) are presented for 300 ms, followed by a 100 ms mask, and a 2600 ms response window with four key-press choices (fearful, angry, happy, and sad). A practice face was used with unlimited time to respond before beginning the timed task. There were also animal categorization blocks to rule out any basic visual processing or praxic differences between groups. In these trials, participants were presented pictures of animals using response timing parameters identical to the Faces task and made one of four key-press choices (bird, cat, dog, and primate).
Statistical Analyses
Factorial analysis of variance (ANOVA) and repeated-measures ANOVA were the main statistical analyses, with post hoc analyses as appropriate. A statistical threshold of p < .05 was used for each analysis for a family-wise error of p < .10. Of note, the results for all main analyses of interest were equivalent with and without inclusion of subjects with MDD and a comorbid condition (e.g., anxiety disorder); therefore, participants with comorbid diagnoses were retained for the present paper.
Analysis 1: Accuracy
A 2 (MDD status) × 2 (gender) ANOVA tested group differences in errors on the FEPT with accuracy (total errors) as the dependent variable [i.e., Hypothesis 1 (MDD effect), Hypothesis 2 (gender effect) and Hypothesis 3 (gender by MDD interaction)]. A similar ANOVA was conducted with animal categorization accuracy to rule out visual processing and praxis difficulties as alternative explanations for group differences or interaction effects.
Analysis 2: Response cost
A 2 (MDD status) × 2 (gender) × 3 (stimulus type) repeated-measures ANOVA addressed differences in response time (RT) for positively and negatively valenced stimuli. These RT analyses included only trials with correct responses. Positively valenced emotion was represented by the “happy” emotion category, whereas negatively valenced emotion was represented by an aggregate of all negative emotions (fear, anger, sadness). Similar to the previous analysis, MDD and gender were between-subjects factors and stimulus type (positive, negative, neutral valence) was the within-subject factor. This analysis also tested Hypotheses 1 through 3.
Results
Accuracy
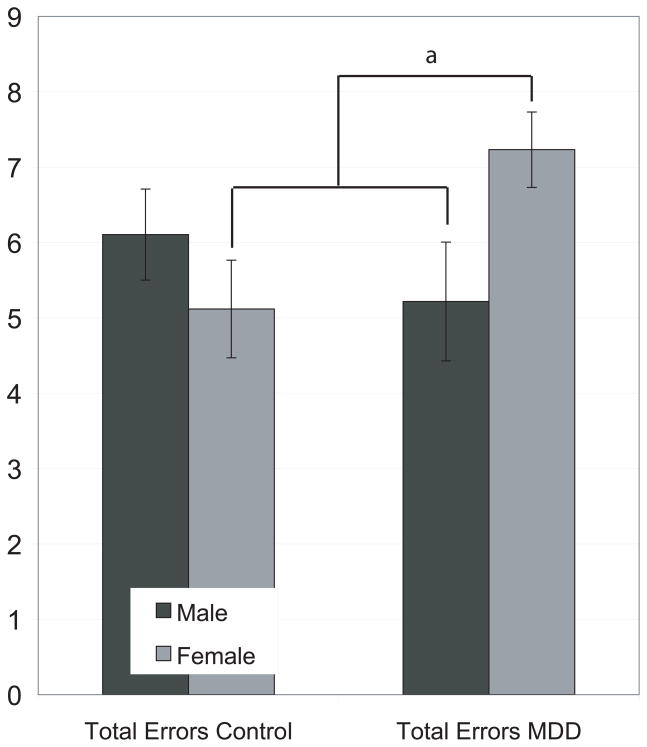
A 2 (gender) × 2 (MDD status) factorial ANOVA was computed, with the number of errors in classifying facial expressions of emotion as the dependent variable. The main effect of group was not significant, F(1,147) = .89, p = .35, nor was the main effect of gender, F(1,147) = 0.62, p = .43. The interaction between gender and MDD status was significant, F(1, 147) = 5.30, p = .02. Post hoc analyses indicated that women with MDD performed significantly worse than women controls, t(88) = −2.43, p = .02, but not compared to men controls, t(92) = −1.31, p = .20. Men with MDD made fewer errors than their control men counterparts, but this difference was not significant t(59) = 0.96, p = .34 (see Figure 1). Post hoc analyses also indicated that symptom severity (PHQ-8) was not significantly correlated with number of errors (r = .06, p = .63). Medication status was assessed by dividing the depressed group into those untreated (n = 39), those treated with a sole antidepressant such as an SSRI or buproprion (n = 24), and those treated with tranquilizers, anti-epileptics, and benzodiazepines (n = 24). There was no effect of accuracy based upon medication groups, F(2, 69) = 0.46, p = .64, nor was the interaction between gender and medication status significant, F(2, 69) = 0.16, p = .85.
Figure 1.
Errors by diagnosis and gender are shown. Women with MDD made more classification errors for emotions. They performed significantly worse than women controls (p = .02) and than men with MDD (p = .03), but not compared to men without MDD (p = .20).
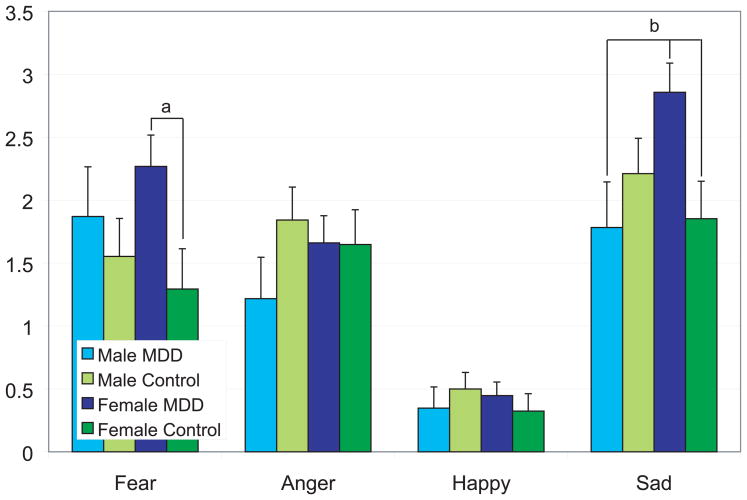
Additional post hoc t tests were conducted, examining the number of errors for the emotion categories (happy, sad, fearful, and angry) separately, each as a within-subject factor, and gender and MDD status as the between-subjects factors. This analysis assessed whether there was an equal distribution of errors based upon the stimulus properties (e.g., whether women with MDD made more errors for sad stimuli compared to non-depressed women). Each group was compared to the other three groups for each specific emotion, and differences are noted in Figure 2.
Figure 2.
Errors by emotion stimulus type, gender, and MDD status are shown. Women with MDD made significantly more errors in classifying fearful and sad stimuli compared to same-gender controls (ps < .02). Women with MDD performed worse than men with MDD (p = .01) and women without MDD (p = .01) but not as compared to men without MDD (p = .08) in classifying sad expressions.
Based upon the pattern of errors for different stimulus properties by gender and MDD status, follow-up analyses were conducted to examine incorrect choice response tendencies by women with MDD. Thus, whereas the preceding post hoc analysis addressed the stimulus characteristics when errors were made, these analyses addressed the emotion choices (i.e., actual choices made), irrespective of the stimulus properties. The goal was to determine whether there were tendencies to misinterpret emotional stimuli when choosing a specific type of emotion. Five 2 (MDD group) × 2 (gender) ANOVAs were conducted with each of five possible response choices: happy, sad, angry, fearful, and no response. For incorrect classifications of stimuli as angry, there was a significant interaction between gender and MDD status, F(1, 147) = 7.20, p < .01. Women with MDD were more likely to choose anger (incorrectly) compared to control women t(87.62) = −3.02, p < .01 and men with MDD, t(77) = −2.27, p = .03, but not compared to control men t(92) = −1.63, p = .11. No other effects for incorrect emotion classification choices were significant (ps > .20).
A second ANOVA, to rule out praxis and visual processing effects as contributing to any group, gender, or interaction effects, was conducted. Groups did not differ in animal classification accuracy by gender, F(1, 147) = 0.73, p = .40, or mood status, F(1, 147) = 0.79, p = .38, nor was the interaction significant, F(1, 147) = 1.57, p = .21.
Negative Response Cost
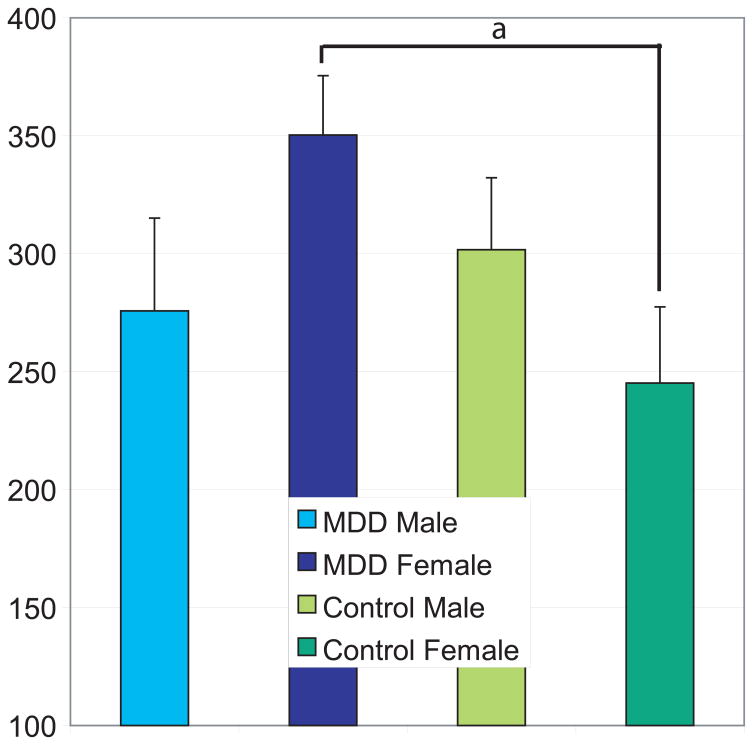
A difference score in RT was calculated between correct happy response and the average of correct responses for the three negative stimuli (fear, anger, sadness) to determine whether there was a differential response cost associated with processing negative stimuli in MDD patients compared to the control group: (Negative mean RT–Happy RT). The results of a 2 (MDD status) × 2 (gender) ANOVA demonstrated that the main effects of MDD status, F(1,147) = 1.51, p = .22 and gender, F(1,147) = 0.08, p = .78 were not significant. However, there was a significant interaction between gender and MDD status, F(1,147) = 4.13, p = .04. The expectations that both depressed persons and men would show generally inferior accuracy and speed of processing negative stimuli were not supported. Post hoc analyses indicated that only women with MDD showed greater negative processing cost relative to the control women, t(88) = −2.56, p = .01 (see Figure 3).
Figure 3.
Negative processing cost by gender and MDD status are shown. Women with MDD exhibited greater negative processing cost (mean, sad, fearful, angry) RT – positive (happy) RT compared to women controls (p = .01), but not to either group of men (ps > .11).
Discussion
The present study was the first study to examine how gender relates to emotion processing accuracy and speed in adults with MDD. The expectations that MDD status and gender would individually influence accuracy and speed of processing negative stimuli were not supported. In contrast, only women without MDD showed the expected advantage in emotion processing. Consistent with our hypothesis, emotion processing depended on the combined characteristics of MDD status and gender. During depressed states, women were less accurate than non-depressed women, as well as men with and without MDD, in processing non-verbal emotional cues (i.e., facial expressions). In contrast, depressed men showed equivalent performance in emotion processing during depressed states as compared to non-depressed men. Women with MDD also were slower to respond to negative emotions (i.e., showed greater response cost) than non-depressed women, whereas men with MDD were equivalent to non-depressed men in speed of processing negatively posed expressions. Women with MDD demonstrated a tendency to misclassify facial expressions of fear and sadness as representing anger, showing clear and specific processing biases or skill deficits that were not present in men with and without MDD. Misclassifying negative facial expressions as angry suggests that women with MDD may engage in threat-related processing of emotions more than men with MDD. It is not entirely clear why gender appears to modulate the relationship between MDD and emotion processing, although social cognitive, socioemotional, and neurobiological processes may provide plausible hypotheses for future study.
Women and men process emotions differently during non-depressed and depressed states. Non-depressed women have been shown to be more emotionally aware than non-depressed men; a finding that is related to women’s superiority over men in recognizing, expressing, and interpreting emotional stimuli(17;23;24). During depressed states, however, the different cognitive strategies in which women and men engage may differentially affect their ability to process other’s emotions. For example, during depressed states, women are more likely to ruminate, whereas men are more likely to distract themselves(25). Given that rumination has been shown to influence appraisal of the past, present, and future(26;27), it is likely to lead to distorted perceptions of events, including those that arouse emotion. Rumination has also been shown to disrupt problem solving(28;29), and may similarly disrupt emotion processing during depressed states in women. Although this hypothesis has not been tested, it might explain increased processing time and reduced accuracy for sad and fearful stimuli, such that they are incorrectly appraised as anger.
Research suggests that from very early in life, women are taught to place greater value in interpersonal relations than are men, who are more likely to strive for individualism(30;31) and that interpersonal skill is strongly related to women’s self-esteem and well being(32;33). Consequently, difficulties in interpersonal skill degrade self-esteem in women(34;35), placing them at greater risk for MDD(36–38). Men, on the other hand, are more likely to become depressed in response to status or occupational loss(39;40).
Emotion processing is an essential component of interpersonal skill(41). Thus, the observation that women with such deficits are more likely to be depressed than women without such deficits may reflect the increased importance of interpersonal relationships in the etiology of MDD in women. Premorbid deficits in emotion processing may place some women at increased risk for interpersonal difficulties, and subsequently for MDD, whereas this may not be the case for men. Furthermore, the types of events that foment MDD in women may be related to these pre-existing social expectation biases and foundational interpersonal skills such as emotion processing.
It is also possible that women’s and men’s abilities to process emotions develop differently, with greater dependence upon limbic functioning in women as compared to men(42). Moreover, men do not rely as heavily as do women on inhibitory emotional repair strategies(43) that are associated with activation of the prefrontal cortex, which modulates activity in subcortical limbic circuits(44;45). Research on non-depressed samples indicates greater limbic activation among women than men during emotion perception tasks(42) and sad states(46). Thus, during depressed states, increased limbic activation may abrogate inhibitory emotional repair strategies in women, leading to greater emotion perception inaccuracy.
Study Limitations
To our knowledge, this is the first study to demonstrate a disproportionately greater adverse outcome of MDD on emotion processing in women as compared to men. Important limitations are present for this study. First, the sample is composed of women and men who chose to seek treatment for MDD, which may have disproportionately sub-sampled distinct groups of men and women. Substance abuse was an exclusion criteria for this study, which may have biased the sample in unknown ways, particularly with higher comorbidity of substance abuse in men. There is also an increased potential for Type I error and inaccurate estimates of effect sizes with unequal cell sizes across sex and diagnostic groups. To address this potential limitation, we ran post hoc analyses with equal numbers of women and men controls (n = 35) and population gender-representative MDD samples (n = 36 women, n = 23 men), with the same main findings. Third, it is not clear from this cross-sectional design whether MDD adversely affects emotion processing or whether these women with MDD were premorbidly impaired in emotion processing, which then served as a risk factor for subsequently developing MDD. Specifically, we can not comment on whether these emotion processing difficulties are a state or trait phenomena. Longitudinal research tracking changes in emotion processing over the course of depressive episodes would best address these two alternative interpretations, including impact of developmental experiences such as trauma on emotion processing acuity.
Conclusions
During depressed states, women were less accurate in processing sad and fearful facial stimuli, whereas men tended to show preserved accuracy in processing these stimuli as compared to same-gender controls. These findings suggest the need for further research into the mechanisms and functional correlates behind emotional processing differences in women and men with MDD. Future research might address the aforementioned limitations through employing community-based samples, perhaps using a longitudinal design. Future research might also measure MDD subtypes, severity and chronicity (e.g., hospitalizations, and number of depressive episodes). Moreover, to test the hypotheses generated by our findings, it would be necessary to measure rumination and correlate this with performance on a non-verbal emotion recognition task in depressed and non-depressed women and men. Finally, functional activation studies might enhance understanding of the interaction between gender and MDD status in emotion processing, specifically the comparison between depressed and non-depressed women. It would be very valuable toward understanding biological bases for early onset MDD to demonstrate that emotion processing circuits are affected in younger adult women with MDD in one way, with perhaps no effect in younger adult men with MDD.
Acknowledgments
This research was supported in part by Rachel Upjohn Clinical Scholars Awards (to SAL and SLW), K-12 Mentored Career Development Award (to SAL), NIH grant P01 MH 42251 (to HA, EAY, JKZ), internal support from the Depression and Neuropsychology Sections of the Department of Psychiatry, University of Michigan Medical Center, and the Department of Psychology, Marquette University. The present study is a further exploration of findings reported previously with a larger sample and there is some overlap (n = 124) in participants across both studies (Langenecker et al., 2007). A subset of these data was presented at the annual meeting of the Cognitive Neuroscience Society, 2007. The aid of a number of students and assistants was invaluable in completing this project: Ami S. Antonucci, Ph.D., Andrew Benway, Emily M. Briceno, Rachel Burns, Korey Cantrell, Luis Casenas, Stephen Crocker, Karla Felske, Caroline Freitag, Kristen Grabar, Leslie M. Guidotti, Najat M. Hamid, Thomas A. Hooven, Nicole Huby, Psy.D., Jessica Layne, Hadia Leon, Benjamin D. Long, Justin B. Miller, Rebecca Reiten, Michael-Paul Schallmo, Maureen Schrock, Megan Shaheen, Simrat Singh, Clare Tyson, Naalti Vats, Lesley Weitekamp, Yahong Yang, and Naomi Yodkovik.
Reference List
- 1.Geerts E, Bouhuys N. Multi-level prediction of short-term outcome of depression: non-verbal interpersonal processes, cognitions and personality traits. Psychiatry Res. 1998;79:59–72. doi: 10.1016/s0165-1781(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 2.Joiner TE. Depression’s vicious scree: Self-propagating and erosive processes in depression chronicity. Clinical Psychology-Science and Practice. 2000;7:203–218. [Google Scholar]
- 3.Lara ME, Klein DN. Psychosocial processes underlying the maintenance and persistence of depression: Implications for understanding chronic depression. Clinical Psychol Rev. 1999;19:553–570. doi: 10.1016/s0272-7358(98)00066-x. [DOI] [PubMed] [Google Scholar]
- 4.Nolen-Hoeksema S, Jackson B. Mediators of the gender difference in rumination. Psychology of Women Quarterly. 2001;25:37–47. [Google Scholar]
- 5.Langenecker SA, Bieliauskas LA, Rapport LJ, et al. Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol. 2005;27:320–333. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- 6.Persad SM, Polivy J. Differences between depressed and nondepressed individuals in the recognition of and response to facial emotional cues. J Abnorm Psychol. 1993;102:358–368. doi: 10.1037//0021-843x.102.3.358. [DOI] [PubMed] [Google Scholar]
- 7.McGrath E, Keita G, Strickland B, Russo NF. Women and depression: Risk factors and treatment issues: Final report of the American Psychological Association's National Task force on Women and Depression. Washington D.C: American Psychological Association; 1990. [Google Scholar]
- 8.Deldin PJ, Keller J, Gergen JA, Miller GA. Cognitive bias and emotion in neuropsychological models of depression. Cognition & Emotion. 2001;15:787–802. [Google Scholar]
- 9.Gotlib IH, Hammen CL. Psychological aspects of depression - Toward a cognitive-interpersonal integration. England: John Wiley & Sons Inc; 1992. [Google Scholar]
- 10.Gur RC, Erwin RJ, Gur RE, Zwil AS. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42:241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- 11.Jaeger J, Borod JC, Peselow E. Facial Expression of Positive and Negative Emotions in Patients with Unipolar Depression. J Affect Disord. 1986;11:43–50. doi: 10.1016/0165-0327(86)90058-3. [DOI] [PubMed] [Google Scholar]
- 12.Mikhailova ES, Vladimirova TV, Iznak AF, et al. Abnormal recognition of facial expression of emotions in depressed patients with major depression disorder and schizotypal personality disorder. Biol Psychiatry. 1996;40:697–705. doi: 10.1016/0006-3223(96)00032-7. [DOI] [PubMed] [Google Scholar]
- 13.Rubinow DR, Post RM. Impaired recognition of affect in facial expression in depressed patients. Biol Psychiatry. 1992;31:947–953. doi: 10.1016/0006-3223(92)90120-o. [DOI] [PubMed] [Google Scholar]
- 14.Hall JA, Matsumoto D. Gender differences in judgments of multiple emotions from facial expressions. Emotion. 2004;4:201–206. doi: 10.1037/1528-3542.4.2.201. [DOI] [PubMed] [Google Scholar]
- 15.Montagne B, Kessels RP, Frigerio E, et al. Sex differences in the perception of affective facial expressions: Do men really lack emotional sensitivity? Cognitive Processing. 2005;6:136–141. doi: 10.1007/s10339-005-0050-6. [DOI] [PubMed] [Google Scholar]
- 16.Mufson L, Nowicki S., Jr Factors affecting the accuracy of facial affect recognition. J Soc Psychol. 1991;131:815–822. doi: 10.1080/00224545.1991.9924668. [DOI] [PubMed] [Google Scholar]
- 17.Thayer JF, Johnsen BH. Sex differences in judgement of facial affect: a multivariate analysis of recognition errors. Scand J Psychol. 2000;41:243–246. doi: 10.1111/1467-9450.00193. [DOI] [PubMed] [Google Scholar]
- 18.Spitzer MB. Structured Clinical Interview for DSM-IV Axis I Disorders-Non-patient Edition. New York, New York: Biometrics Research Department; 1995. [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapport LJ, Friedman SR, Tzelepis A, Van VA. Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology. 2002;16:102–110. doi: 10.1037//0894-4105.16.1.102. [DOI] [PubMed] [Google Scholar]
- 21.Langenecker SA, Caveney AF, Giordani B, et al. The sensitivity and psychometric properties of a brief computer-based cognitive screening battery in a depression clinic. Psychiatry Res. 2007;152:143–154. doi: 10.1016/j.psychres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Ekman P, Friesen W. Pictures of facial affect. California: Consulting Psychologists Press; 1976. [Google Scholar]
- 23.Barrett LF, Lane RD, Sechrest L, Schwartz GE. Sex differences in emotional awareness. Personality and Social Psychology Bulletin. 2000;26:1027–1035. [Google Scholar]
- 24.Kring AM, Gordon AH. Sex differences in emotion: Expression, experience, and physiology. J Pers Soc Psychol. 1998;74:686–703. doi: 10.1037//0022-3514.74.3.686. [DOI] [PubMed] [Google Scholar]
- 25.Nolen-Hoeksema S, Larson J, Grayson C. Explaining the gender difference in depressive symptoms. J Pers Soc Psychol. 1999;77:1061–1072. doi: 10.1037//0022-3514.77.5.1061. [DOI] [PubMed] [Google Scholar]
- 26.Lyubomirsky S, Caldwell ND, Nolen-Hoeksema S. Effects of ruminative and distracting responses to depressed mood on retrieval of autobiographical memories. J Pers Soc Psychol. 1998;75:166–177. doi: 10.1037//0022-3514.75.1.166. [DOI] [PubMed] [Google Scholar]
- 27.Lyubomirsky S, Nolenhoeksema S. Effects of Self-Focused Rumination on Negative Thinking and Interpersonal Problem-Solving. J Pers Soc Psychol. 1995;69:176–190. doi: 10.1037//0022-3514.69.1.176. [DOI] [PubMed] [Google Scholar]
- 28.Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- 29.Nolen-Hoeksema S. Gender differences in depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. New York, NY, US: Guilford Press; 2002. pp. 492–509. [Google Scholar]
- 30.Jack DC. Silencing the self: Women and depression. Massachusetts: Harvard University Press; 1991. [Google Scholar]
- 31.Miller JB. Towards a new psychology of women. Massachusetts: Beacon Press; 1976. [Google Scholar]
- 32.Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- 33.Stein JA, Newcomb MD, Bentler PM. The effect of agency and communality on self-esteem: Gender differences in longitudinal data. Sex Roles. 1992;26:465–483. [Google Scholar]
- 34.Moran PB, Eckenrode J. Gender differences in the costs and benefits of peer relationships during adolescence. Journal of Adolescent Research. 1991;6:396–409. [Google Scholar]
- 35.Zuckerman DM. Stress, self-esteem, and mental health: How does gender make a difference? Sex Roles. 1989;20:429–444. [Google Scholar]
- 36.Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. Am J Psychiatry. 2002;159:1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- 37.Kernis MH, Whisenhunt CR, Waschull SB, et al. Multiple facets of self-esteem and their relations to depressive symptoms. Personality and Social Psychology Bulletin. 1998;24:657–668. [Google Scholar]
- 38.Roberts JE, Kassel JD. Labile self-esteem, life stress, and depressive symptoms: Prospective data testing a model of vulnerability. Cognitive Therapy and Research. 1997;21:569–589. [Google Scholar]
- 39.Emslie C, Ridge D, Ziebland S, Hunt K. Men’s accounts of depression: Reconstructing or resisting hegemonic masculinity? Soc Sci Med. 2006;62:2246–2257. doi: 10.1016/j.socscimed.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 40.Robbins A. Biopsychosocial aspects in understanding and treating depression in men: A clinical perspective. Journal of Men’s Health & Gender. 2006;3:10–18. [Google Scholar]
- 41.Kohler CG, Martin EA. Emotional processing in schizophrenia. Cognitive Neuropsychiatry. 2006;11:250–571. doi: 10.1080/13546800500188575. [DOI] [PubMed] [Google Scholar]
- 42.Hall GBC, Witelson SF, Szechtman H, Nahmias C. Sex differences in functional activation patterns revealed by increased emotion processing demands. Neuroreport. 2004;15:219–223. doi: 10.1097/00001756-200402090-00001. [DOI] [PubMed] [Google Scholar]
- 43.Bjorklund DF, Kipp K. Parental investment theory and gender differences in the evolution of inhibition mechanisms. Psychol Bull. 1996;120:163–188. doi: 10.1037/0033-2909.120.2.163. [DOI] [PubMed] [Google Scholar]
- 44.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Advancing from the Ventral Striatum to the Extended Amygdala. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 45.Thayer JF, Friedman BH. Stop that! Inhibition, sensitization, and their neurovisceral concomitants. Scand J Psychol. 2002;43:123–130. doi: 10.1111/1467-9450.00277. [DOI] [PubMed] [Google Scholar]
- 46.George MS, Ketter TA, Parekh PI, et al. Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biol Psychiatry. 1996;40:859–871. doi: 10.1016/0006-3223(95)00572-2. [DOI] [PubMed] [Google Scholar]