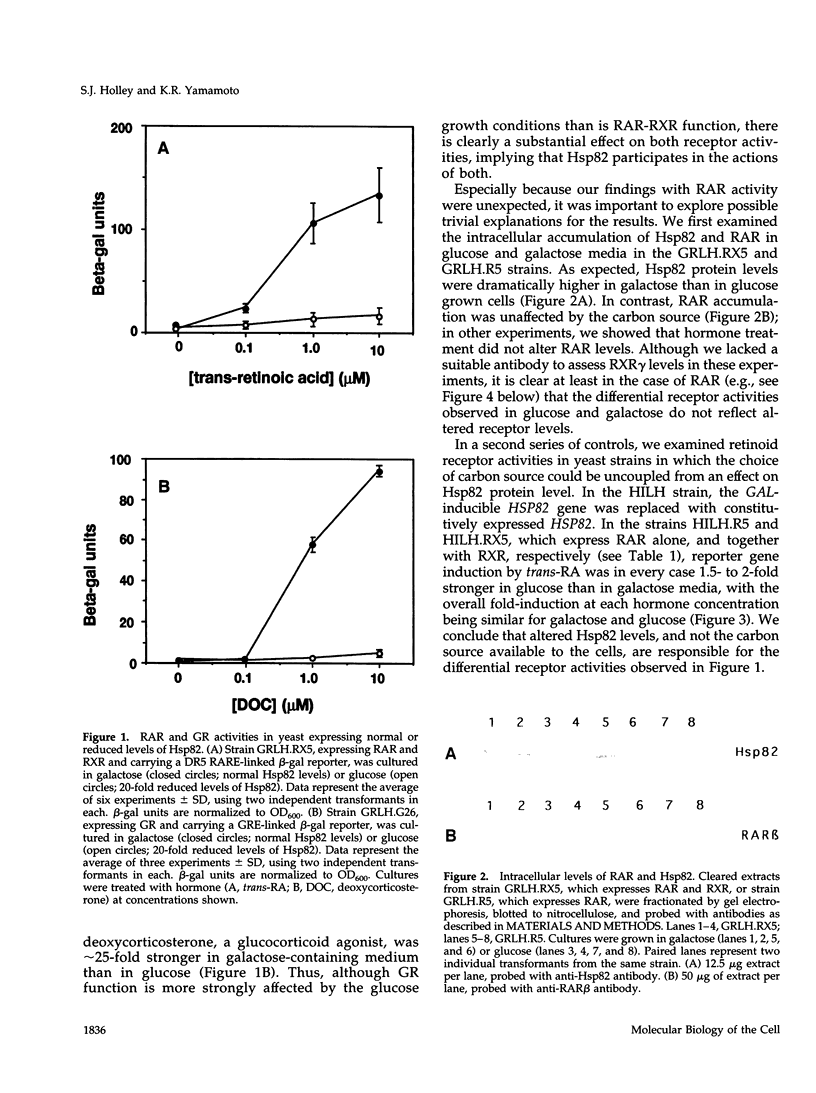
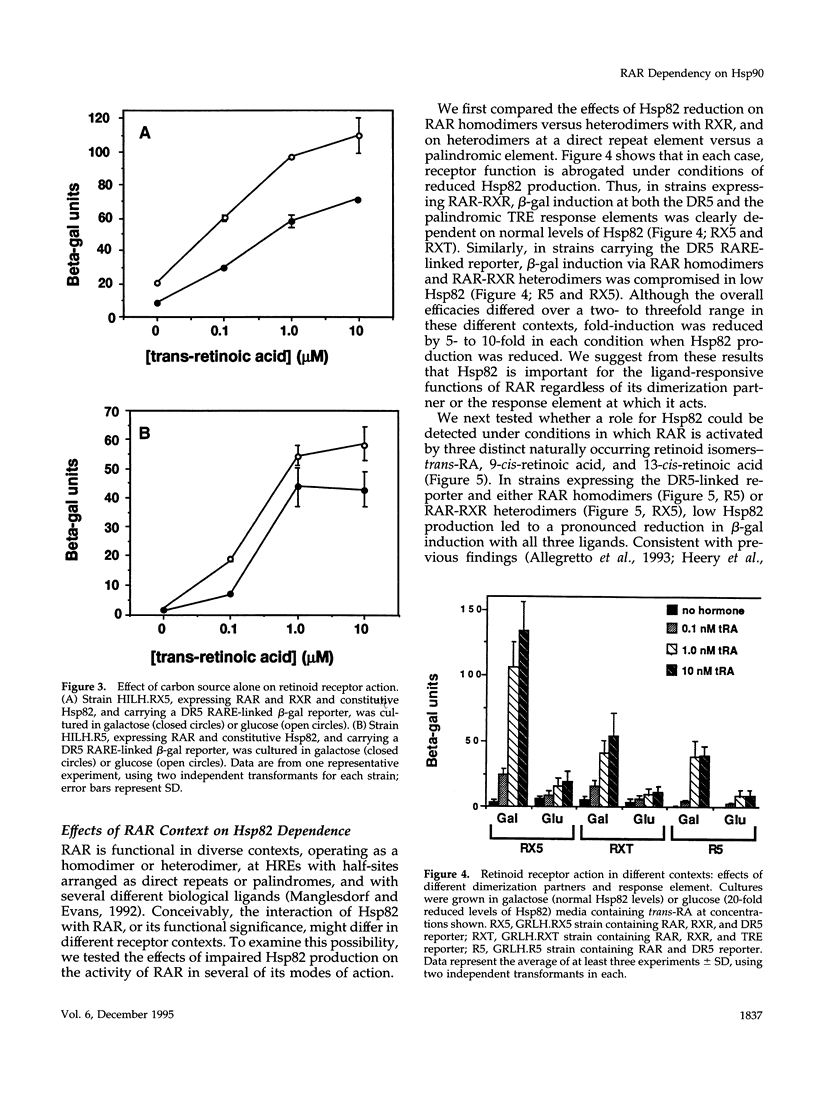
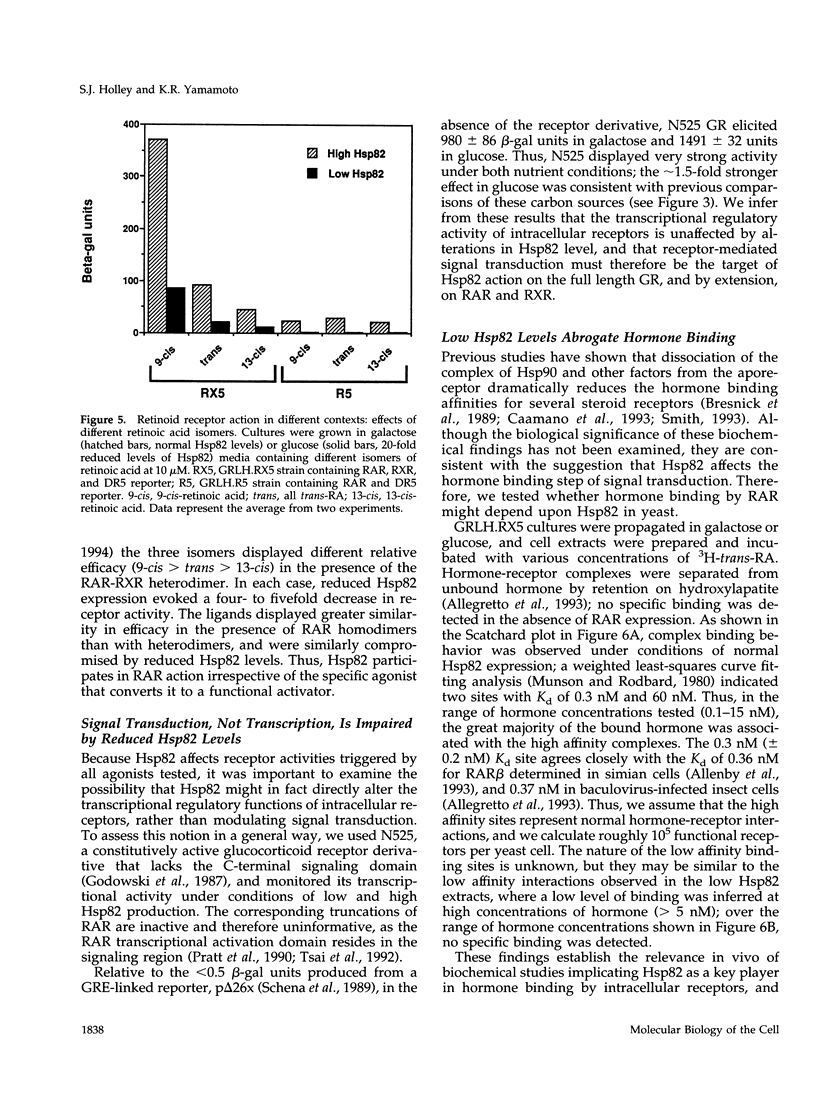
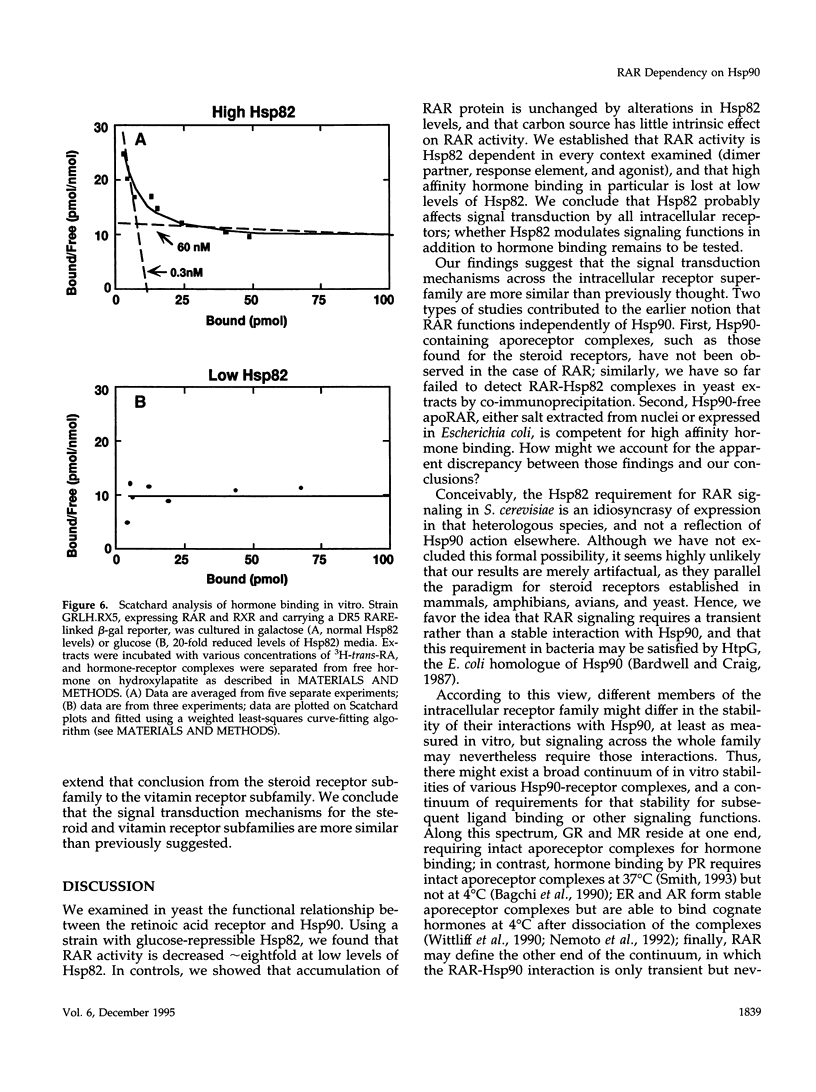
Abstract
The ubiquitous heat shock protein Hsp90 appears to participate directly in the function of a broad range of cellular signal transduction components, including steroid hormone receptors; however, an evolutionarily related subclass of intracellular receptors, exemplified by the retinoid receptors RAR and RXR, had been inferred from biochemical studies to function independently of Hsp90. To examine this issue genetically, we measured mammalian and avian retinoid receptor activity in a Saccharomyces cerevisiae strain in which the expression of the yeast Hsp90 homologue could be conditionally repressed approximately 20-fold relative to wild type. We tested transcriptional activation by RAR or RXR-RAR, from two types of retinoic acid response elements, triggered by three different agonist ligands. In every condition, we found that activation was severely compromised under conditions of low Hsp90 expression. We showed that the defect was in signal transduction rather than transcription activation per se, and that high affinity hormone binding was abolished in extracts of cells producing low levels of Hsp90. We suggest that Hsp90 may function in at least one step of signal transduction by all members of the intracellular receptor superfamily.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aligue R., Akhavan-Niak H., Russell P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994 Dec 15;13(24):6099–6106. doi: 10.1002/j.1460-2075.1994.tb06956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegretto E. A., McClurg M. R., Lazarchik S. B., Clemm D. L., Kerner S. A., Elgort M. G., Boehm M. F., White S. K., Pike J. W., Heyman R. A. Transactivation properties of retinoic acid and retinoid X receptors in mammalian cells and yeast. Correlation with hormone binding and effects of metabolism. J Biol Chem. 1993 Dec 15;268(35):26625–26633. [PubMed] [Google Scholar]
- Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Identification of a functional intermediate in receptor activation in progesterone-dependent cell-free transcription. Nature. 1990 Jun 7;345(6275):547–550. doi: 10.1038/345547a0. [DOI] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Eukaryotic Mr 83,000 heat shock protein has a homologue in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5177–5181. doi: 10.1073/pnas.84.15.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohen S. P., Kralli A., Yamamoto K. R. Hold 'em and fold 'em: chaperones and signal transduction. Science. 1995 Jun 2;268(5215):1303–1304. doi: 10.1126/science.7761850. [DOI] [PubMed] [Google Scholar]
- Bohen S. P., Yamamoto K. R. Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11424–11428. doi: 10.1073/pnas.90.23.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich K. A., Farrelly F. W., Finkelstein D. B., Taulien J., Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989 Sep;9(9):3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick E. H., Dalman F. C., Sanchez E. R., Pratt W. B. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J Biol Chem. 1989 Mar 25;264(9):4992–4997. [PubMed] [Google Scholar]
- Caamaño C. A., Morano M. I., Patel P. D., Watson S. J., Akil H. A bacterially expressed mineralocorticoid receptor is associated in vitro with the 90-kilodalton heat shock protein and shows typical hormone- and DNA-binding characteristics. Biochemistry. 1993 Aug 24;32(33):8589–8595. doi: 10.1021/bi00084a028. [DOI] [PubMed] [Google Scholar]
- Cutforth T., Rubin G. M. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994 Jul 1;77(7):1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Dalman F. C., Koenig R. J., Perdew G. H., Massa E., Pratt W. B. In contrast to the glucocorticoid receptor, the thyroid hormone receptor is translated in the DNA binding state and is not associated with hsp90. J Biol Chem. 1990 Mar 5;265(7):3615–3618. [PubMed] [Google Scholar]
- Dalman F. C., Sturzenbecker L. J., Levin A. A., Lucas D. A., Perdew G. H., Petkovitch M., Chambon P., Grippo J. F., Pratt W. B. Retinoic acid receptor belongs to a subclass of nuclear receptors that do not form "docking" complexes with hsp90. Biochemistry. 1991 Jun 4;30(22):5605–5608. doi: 10.1021/bi00236a038. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Eul J., Meyer M. E., Tora L., Bocquel M. T., Quirin-Stricker C., Chambon P., Gronemeyer H. Expression of active hormone and DNA-binding domains of the chicken progesterone receptor in E. coli. EMBO J. 1989 Jan;8(1):83–90. doi: 10.1002/j.1460-2075.1989.tb03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M. J., Yamamoto K. R. Genetic dissection of the signaling domain of a mammalian steroid receptor in yeast. Mol Biol Cell. 1992 Nov;3(11):1245–1257. doi: 10.1091/mbc.3.11.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz D., St Jean A., Woods R. A., Schiestl R. H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992 Mar 25;20(6):1425–1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Rusconi S., Miesfeld R., Yamamoto K. R. Glucocorticoid receptor mutants that are constitutive activators of transcriptional enhancement. Nature. 1987 Jan 22;325(6102):365–368. doi: 10.1038/325365a0. [DOI] [PubMed] [Google Scholar]
- Graupner G., Wills K. N., Tzukerman M., Zhang X. K., Pfahl M. Dual regulatory role for thyroid-hormone receptors allows control of retinoic-acid receptor activity. Nature. 1989 Aug 24;340(6235):653–656. doi: 10.1038/340653a0. [DOI] [PubMed] [Google Scholar]
- Hall B. L., Smit-McBride Z., Privalsky M. L. Reconstitution of retinoid X receptor function and combinatorial regulation of other nuclear hormone receptors in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6929–6933. doi: 10.1073/pnas.90.15.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heery D. M., Pierrat B., Gronemeyer H., Chambon P., Losson R. Homo- and heterodimers of the retinoid X receptor (RXR) activated transcription in yeast. Nucleic Acids Res. 1994 Mar 11;22(5):726–731. doi: 10.1093/nar/22.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard K. J., Distelhorst C. W. Evidence for intracellular association of the glucocorticoid receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Mar 5;263(7):3474–3481. [PubMed] [Google Scholar]
- Hutchison K. A., Dittmar K. D., Czar M. J., Pratt W. B. Proof that hsp70 is required for assembly of the glucocorticoid receptor into a heterocomplex with hsp90. J Biol Chem. 1994 Feb 18;269(7):5043–5049. [PubMed] [Google Scholar]
- Ichikawa K., Hashizume K., Nishii Y., Takeda T., Kobayashi M., Suzuki S., Yamada T. Purification of human c-erb A beta protein. J Mol Endocrinol. 1991 Oct;7(2):123–129. doi: 10.1677/jme.0.0070123. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Yahara I., Lindquist S. Role of the protein chaperone YDJ1 in establishing Hsp90-mediated signal transduction pathways. Science. 1995 Jun 2;268(5215):1362–1365. doi: 10.1126/science.7761857. [DOI] [PubMed] [Google Scholar]
- Lombardo A., Costa E., Chao W. R., Toll L., Hobbs P. D., Jong L., Lee M. O., Pfahl M., Ely K. R., Dawson M. I. Recombinant human retinoic acid receptor beta. Binding of synthetic retinoids and transcriptional activation. J Biol Chem. 1994 Mar 11;269(10):7297–7303. [PubMed] [Google Scholar]
- Metzger D., White J. H., Chambon P. The human oestrogen receptor functions in yeast. Nature. 1988 Jul 7;334(6177):31–36. doi: 10.1038/334031a0. [DOI] [PubMed] [Google Scholar]
- Miyata Y., Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992 Apr 5;267(10):7042–7047. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nemoto T., Ohara-Nemoto Y., Ota M. Association of the 90-kDa heat shock protein does not affect the ligand-binding ability of androgen receptor. J Steroid Biochem Mol Biol. 1992 Sep;42(8):803–812. doi: 10.1016/0960-0760(92)90088-z. [DOI] [PubMed] [Google Scholar]
- Nervi C., Grippo J. F., Sherman M. I., George M. D., Jetten A. M. Identification and characterization of nuclear retinoic acid-binding activity in human myeloblastic leukemia HL-60 cells. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5854–5858. doi: 10.1073/pnas.86.15.5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Levinson W., Bishop J. M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Pratt M. A., Kralova J., McBurney M. W. A dominant negative mutation of the alpha retinoic acid receptor gene in a retinoic acid-nonresponsive embryonal carcinoma cell. Mol Cell Biol. 1990 Dec;10(12):6445–6453. doi: 10.1128/mcb.10.12.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalsky M. L. A subpopulation of the v-erb A oncogene protein, a derivative of a thyroid hormone receptor, associates with heat shock protein 90. J Biol Chem. 1991 Jan 25;266(3):1456–1462. [PubMed] [Google Scholar]
- Privalsky M. L., Sharif M., Yamamoto K. R. The viral erbA oncogene protein, a constitutive repressor in animal cells, is a hormone-regulated activator in yeast. Cell. 1990 Dec 21;63(6):1277–1286. doi: 10.1016/0092-8674(90)90423-c. [DOI] [PubMed] [Google Scholar]
- Rose D. W., Wettenhall R. E., Kudlicki W., Kramer G., Hardesty B. The 90-kilodalton peptide of the heme-regulated eIF-2 alpha kinase has sequence similarity with the 90-kilodalton heat shock protein. Biochemistry. 1987 Oct 20;26(21):6583–6587. doi: 10.1021/bi00395a003. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Casanova J., Stanley F. Thyroid hormone action: in vitro characterization of solubilized nuclear receptors from rat liver and cultured GH1 cells. J Clin Invest. 1974 Oct;54(4):853–865. doi: 10.1172/JCI107825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E. R., Toft D. O., Schlesinger M. J., Pratt W. B. Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. J Biol Chem. 1985 Oct 15;260(23):12398–12401. [PubMed] [Google Scholar]
- Schena M., Freedman L. P., Yamamoto K. R. Mutations in the glucocorticoid receptor zinc finger region that distinguish interdigitated DNA binding and transcriptional enhancement activities. Genes Dev. 1989 Oct;3(10):1590–1601. doi: 10.1101/gad.3.10.1590. [DOI] [PubMed] [Google Scholar]
- Schena M., Yamamoto K. R. Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science. 1988 Aug 19;241(4868):965–967. doi: 10.1126/science.3043665. [DOI] [PubMed] [Google Scholar]
- Scherrer L. C., Dalman F. C., Massa E., Meshinchi S., Pratt W. B. Structural and functional reconstitution of the glucocorticoid receptor-hsp90 complex. J Biol Chem. 1990 Dec 15;265(35):21397–21400. [PubMed] [Google Scholar]
- Scherrer L. C., Hutchison K. A., Sanchez E. R., Randall S. K., Pratt W. B. A heat shock protein complex isolated from rabbit reticulocyte lysate can reconstitute a functional glucocorticoid receptor-Hsp90 complex. Biochemistry. 1992 Aug 18;31(32):7325–7329. doi: 10.1021/bi00147a017. [DOI] [PubMed] [Google Scholar]
- Shaknovich R., Shue G., Kohtz D. S. Conformational activation of a basic helix-loop-helix protein (MyoD1) by the C-terminal region of murine HSP90 (HSP84). Mol Cell Biol. 1992 Nov;12(11):5059–5068. doi: 10.1128/mcb.12.11.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993 Nov;7(11):1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Schowalter D. B., Kost S. L., Toft D. O. Reconstitution of progesterone receptor with heat shock proteins. Mol Endocrinol. 1990 Nov;4(11):1704–1711. doi: 10.1210/mend-4-11-1704. [DOI] [PubMed] [Google Scholar]
- Spindler B. J., MacLeod K. M., Ring J., Baxter J. D. Thyroid hormone receptors. Binding characteristics and lack of hormonal dependency for nuclear localization. J Biol Chem. 1975 Jun 10;250(11):4113–4119. [PubMed] [Google Scholar]
- Stancato L. F., Chow Y. H., Hutchison K. A., Perdew G. H., Jove R., Pratt W. B. Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J Biol Chem. 1993 Oct 15;268(29):21711–21716. [PubMed] [Google Scholar]
- Tai P. K., Maeda Y., Nakao K., Wakim N. G., Duhring J. L., Faber L. E. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986 Sep 9;25(18):5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Tsai S., Bartelmez S., Heyman R., Damm K., Evans R., Collins S. J. A mutated retinoic acid receptor-alpha exhibiting dominant-negative activity alters the lineage development of a multipotent hematopoietic cell line. Genes Dev. 1992 Dec;6(12A):2258–2269. doi: 10.1101/gad.6.12a.2258. [DOI] [PubMed] [Google Scholar]
- Wiech H., Buchner J., Zimmermann R., Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992 Jul 9;358(6382):169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson A., Cuthill S., Denis M., Wikström A. C., Gustafsson J. A., Poellinger L. The specific DNA binding activity of the dioxin receptor is modulated by the 90 kd heat shock protein. EMBO J. 1990 Jan;9(1):69–76. doi: 10.1002/j.1460-2075.1990.tb08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittliff J. L., Wenz L. L., Dong J., Nawaz Z., Butt T. R. Expression and characterization of an active human estrogen receptor as a ubiquitin fusion protein from Escherichia coli. J Biol Chem. 1990 Dec 15;265(35):22016–22022. [PubMed] [Google Scholar]
- Yang N., Schüle R., Mangelsdorf D. J., Evans R. M. Characterization of DNA binding and retinoic acid binding properties of retinoic acid receptor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3559–3563. doi: 10.1073/pnas.88.9.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]




