Abstract
Williams syndrome (WS) is a rare genetic disorder caused by the deletion of ~25 genes on chromosome 7q11.23 and is characterized by both hypersociability and increases in specific phobia and anticipatory anxiety regarding non-social entities or circumstances. Alterations in amygdala reactivity and prefrontal regulation consistent with the observed behavioral pattern of social versus non-social abnormalities have been previously demonstrated in individuals with WS ( Meyer-Lindenberg et al., 2005). However, in that study, the social stimulus (faces) matching task was more difficult than the non-social scene (IAPS stimuli) matching task, making it impossible to disambiguate the relative contributions of task difficulty and stimulus type (social versus non-social). In the present study, we examined the performance of the same group of participants with WS and normal IQs during a more cognitively demanding task using the same scene stimuli as in the prior study. Confirming previous findings, the results indicated (a) a differential response of prefrontal regions as a function of task difficulty and (b) a persistently increased activation of the amygdala to non-social scenes by individuals with WS regardless of cognitive load. These data provide further evidence of disruption in amygdala-prefrontal circuitry in individuals with WS.
Williams syndrome (WS) is a rare genetic disorder (estimated at 1 in 7500 live births; Strømme et al., 2002) caused by the hemideletion of some 28 genes on chromosome 7q11.23 (Hillier et al., 2003; Meyer-Lindenberg et al., 2006). WS is associated with a variety of physical signs including cardiovascular abnormalities (Beuren et al., 1962; Pober and Morris, 2007; Williams et al., 1961), failure to thrive or growth deficiencies (Morris 2006), coordination problems (Chapman et al., 1996), and hypersensitivity to sound (Blomberg et al., 2006; Gothelf et al., 2006; Levitin et al., 2005). Phenotypically, WS is characterized by a dysmorphic facial appearance, a distinctive cognitive profile that is typically superimposed on mild to moderate intellectual disability (Mervis and Becerra, 2007), and a unique personality profile (Donnai and Karmiloff-Smith, 2000; Jarvinen-Pasley et al., 2008; Mervis and Klein-Tasman, 2000; Mervis et al., 2003). The cognitive profile of persons with WS has high sensitivity and diagnostic specificity (Mervis et al., 2000) and consists of pronounced deficits in visuospatial construction [associated with structural and functional impairments in the dorsal visual processing stream (Eckert et al., 2006; Meyer-Lindenberg et al., 2004; Reiss et al., 2004; Sarpal et al., 2008)] along with relative strengths in verbal short-term memory and (concrete) language (Mervis et al., 2000).
Individuals with WS also display high levels of hypersociability and empathy (Jarvinen-Pasley et al., 2008; Klein-Tasman and Mervis, 2003; Mervis and Klein-Tasman, 2000; Meyer-Lindenberg et al., 2006) associated with limitations in social judgment (Einfeld et al., 1997; Gosch and Pankau, 1997). Accordingly, individuals with WS often show little reserve in situations where individuals in the general population approach with considerable caution (e.g., encountering strangers). At the same time as their social anxiety is strikingly reduced, individuals with WS frequently evidence specific phobia and anticipatory anxiety regarding non-social entities or circumstances (Cherniske et al., 2004; Dykens, 2003; Gallo et al., 2008; Leyfer et al., 2006; Udwin and Yule, 1991), with ~50% meeting DSM-IV criteria for specific phobia (Cherniske et al., 2004; Leyfer et al., 2006, 2009). Because of these marked distinctions, WS is of particular interest as a model for the neural and genetic mechanisms involved in social cognition.
We previously studied the neural basis of emotional cognition in individuals with WS who have normal IQs by testing for differences in processing socially versus non-socially relevant threatening stimuli (Meyer-Lindenberg et al., 2005). We found that individuals with WS exhibited decreased amygdala reactivity in response to matching threatening facial expressions (social) when compared to IQ-matched controls from the general population, i.e., normal controls (NC), but increased amygdala reactivity when matching fearful scenes [non-social; taken from the International Affective Picture Series (IAPS)]. Further analyses indicated that this significant difference in amygdala reactivity was associated with altered prefrontal regulation. To study prefrontal cortex involvement, the tasks used in that experiment differed in cognitive load, with the face-matching task being more difficult (involving three different facial identities) than the scene-matching task (in which participants were asked to select which of two scenes was identical to a target scene). Consistent with previous studies (Hariri et al., 2003), the control group evidenced greater reactivity in the dorsolateral prefrontal (DLPFC), medial prefrontal (MPFC), and orbitofrontal (OFC) cortex in the face-matching task relative to the scene-matching task. The WS group, however, did not show a differential response in any of these brain areas. Since extensive preclinical data implicate these cortical regions in regulation of amygdala (Adolphs, 2003b), these results suggested an abnormal PFC–amygdala regulatory system.
Because this previous study employed a face-matching task of greater difficulty relative to the non-social scene-matching task, the relative contributions of task difficulty and stimulus type (social versus non-social) could not be disambiguated. The present study was conducted to achieve this goal. We studied the same groups of IQ-matched participants during a task that was more cognitively demanding but involved the same scene stimuli: selecting a label for a scene according to its emotional content. This experimental strategy applied to IAPS scenes has been shown to engage prefrontal target regions in a cohort of general-population controls (Hariri et al., 2003). In the present study, we compared brain reactivity during this more difficult task to that during the less cognitively demanding matching task reported previously (Meyer-Lindenberg et al., 2005), using the same set of aversive scenes for both.
We reasoned that if our previous findings of differential amygdala activation were a result of cognitive load and not dependent on the social content of the stimuli, the group with WS should show decreased amygdala activation relative to the general-population control group as previously observed in the more difficult (faces) condition. Conversely, if previous findings were a result of stimulus type (amygdala reactivity always greater in a condition involving non-socially relevant, non-facial stimuli in WS), one would predict the opposite result: increased amygdala reactivity in individuals with WS compared to general-population controls for scene-stimuli. Accordingly, we hypothesized that our results would demonstrate (a) a differential response of prefrontal regions as a function of task difficulty, as previously reported (Meyer-Lindenberg et al., 2005), and (b) a persistently increased activation of the amygdala to non-social scenes for the WS group regardless of task difficulty, implying an abnormality in the processing of danger stimuli consequent to stimulus type, rather than cognitive load. In addition to these between-group analyses, we also conducted exploratory within-group analyses.
Method
Participants
Demographics are summarized in Table 1. Thirteen normal-IQ individuals with WS and 13 general-population controls were matched for age, sex, and IQ. Three individuals in each group participated only in the matching experiment. All participants gave written informed consent and participated in the study according to the guidelines of the National Institute of Mental Health Institutional Review Board and were reimbursed for their time.
Table 1.
Demographics and behavioral data.
| WS label (n=10) | NC label (n=10) | WS match (n=13) | NC match (n=13) | P | |
|---|---|---|---|---|---|
| Gender | 6 M, 4 F | 6 M, 4 F | 6 M, 7 F | 6 M, 7 F | |
| Mean age | 26.7 (9.10) | 30.6 (7.83) | 28.3 (9.62) | 29.5 (7.08) | NS |
| Mean IQ | 93.7 (9.73) | 95.7 (7.01) | 92.1 (9.56) | 97.9 (7.58) | NS |
| Mean accuracy, labeling task (%) | 77.50 (18.86) | 87.50 (11.28) | NS | ||
| Mean reaction time, labeling task (ms) | 2613.62 (352.521) | 2316.98 (643.015) | NS | ||
| Mean accuracy, matching task (%) | 99.36 (2.310) | 96.15 (7.308) | NS | ||
| Mean reaction time, matching task (ms) | 1939.04 (284.369) | 1833.01 (454.631) | NS |
All participants were right-handed. One control participant was African-American; the other participants in both groups were Caucasian, Non-Hispanic. Details about IQ measurement are provided in Meyer-Lindenberg et al. (2004). Three individuals in each group did not participate in the labeling experiment. NS: Not significant.
To rule out psychiatric disorders, healthy controls were assessed with the Structured Clinical Interview for DSM-IV and/or screening interviews (Egan et al., 2000). Neither phobias nor any other conditions were present in any of the control population. Information relevant for diagnosis of phobia was available for eight participants with WS: the Anxiety Disorders Interview Schedule (ADIS-P; Silverman and Albano, 1996) was administered to two, and six were assessed with the Fear Survey Schedule (FSSC-R; Ollendick, 1983). Of the two assessed with the ADIS, one had a formal diagnosis of phobia. All six individuals assessed with FSSC-R endorsed specific phobia items at a level of “3” (=a lot of fear) and are considered likely to have phobias.
Stimuli
The stimuli used were 12 different threatening or fearful images (e.g., snakes, spiders, guns, car accidents, plane crashes, explosions) culled from the International Affective Picture Series (IAPS; Lang et al., 1997). None of the IAPS images used as part of this study depicted social interactions. The mean (±SEM) arousal and valence on a 9-point scale, where 1 represents minimum arousal or negative valence and 9 maximum arousal or positive valence, were 6.40±0.13 and 3.13±0.20, respectively, indicating that the pictures were rated as arousing and strongly negative. Simple geometric shapes (circles, vertical, and horizontal ellipses) were used as control stimuli.
Experimental paradigm
Participants performed two experimental tasks and one control task while undergoing functional magnetic resonance imaging (fMRI) as part of the same study previously reported (Meyer-Lindenberg et al., 2005). Both experimental conditions involved presentation of IAPS stimuli, but they differed in how participants were instructed to evaluate the stimuli (Fig. 1). Because this work was aimed at assessing the effects of increased cognitive load, in one task participants were instructed to “label” the emotional content of the IAPS scene by selecting one of two simultaneously presented affective words (choices were between afraid – surprised – angry – happy). Performance was compared to that in a second task involving a simple perceptual processing of scenes, previously shown to engage amygdala circuitry, wherein participants were asked to match one of two simultaneously presented scenes with an identical target scene (Hariri et al., 2003; Hariri et al., 2002; Meyer-Lindenberg et al., 2005). As a sensorimotor control task, the participants were asked to match geometric shapes. The fMRI paradigm consisted of nine experimental blocks: two blocks each of labeling scenes and matching scenes interleaved with five control blocks, each lasting 32 seconds for a total scan length of 4:48 min. Each block began with a brief (2 seconds) instruction statement: “Label Pictures,”“Match Pictures,” or “Match Forms.” Each labeling or matching block consisted of six images. For each control block, six different geometric shapes were presented as targets. All images were presented sequentially, with no interstimulus interval, for a period of five seconds, randomized for all conditions. The order of the paradigms was counterbalanced across participants. During imaging, participants responded by pressing one of two buttons with their dominant (right) hands, allowing for the determination of accuracy and reaction time.
Fig. 1.
Each experimental condition presented threatening or fearful IAPS stimuli but differed in cognitive load. (A) In the first condition (“label”), participants were asked to judge which of two simultaneously presented affective labels best described the content of the target scene. (B) In the second condition (“match”), participants viewed the same target pictures but were asked to determine which of the two bottom pictures was identical to the target. (C) As a control task, the participants were asked to match geometric shapes.
Functional imaging
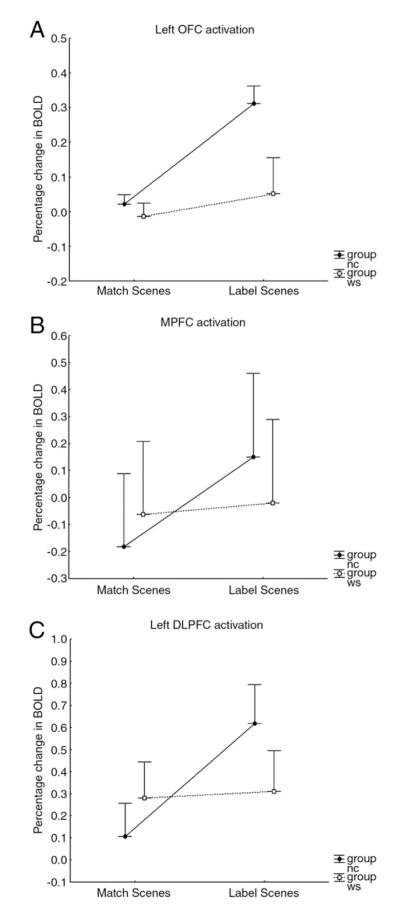
BOLD fMRI was performed on a GE Signa 3T system (Milwaukee, WI) using gradient echo EPI (24 axial slices, 4 mm thickness, 1 mm gap, TR/TE=2000/28 msec, FOV=24 cm, matrix=64×64). Images were processed as described previously (Meyer-Lindenberg et al., 2005) using SPM99 (http://www.fil.ion.ucl.ac.uk/spm). Briefly, images were realigned, spatially normalized into a standard stereotactic space (Montreal Neurologic Institute template) using a 12-parameter affine and nonlinear (4*5*4 basis–functions) transformation, and smoothed with an 8-mm FWHM Gaussian filter. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. A statistical image for each contrast—(1) match>control and (2) label>control—was obtained for each participant and these were analyzed in a second-level random effects model (ANOVA and one-tailed t-test), with appropriate contrasts to identify significant (p<0.05, corrected for multiple comparisons at a height threshold of T = 3) effects of group and task. Because of our strong a priori hypothesis regarding reactivity in the amygdala and previous results in this cohort (Meyer-Lindenberg et al., 2005), we chose to explore the differential response in this area using an anatomical mask created using the WFU Pick atlas (http://www.fmri.wfubmc.edu) software at a statistical threshold of p<0.05, corrected for multiple comparisons within the amygdala region of interest (ROI). For the ANOVAs exploring group by task effects (Fig. 3), mean activity was extracted from ROIs defined operator independently in normalized space delineated by spheres with an 8-mm radius centered at the coordinates identified by the main effect of scene-labeling>scene-matching (Table 5).
Fig. 3.
Estimated percentage change in BOLD response (mean±SEM) at maximum coordinates (Table 5): (A) left OFC, (B) MPFC, and (C) left DLPFC.
Table 5.
Within-group cortical activation in scene-labeling compared to scene-matching.
| Area | Talairach | T value | P |
|---|---|---|---|
| Control group | |||
| Left dorsolateral prefrontal cortex | −51 22 22 | 5.00 | 0.001 |
| Left orbitofrontal cortex | −49 25 −10 | 4.11 | 0.001 |
| Medial prefrontal cortex | −4 27 35 | 3.49 | 0.001 |
| Left middle temporal gyrus | −59 −47 2 | 4.71 | 0.001 |
| WS group | |||
| Right middle temporal gyrus | 49 −38 2 | 4.57 | 0.001 |
| Left middle temporal gyrus | −51 −44 2 | 4.94 | 0.001 |
Voxels were thresholded at p<0.001 (uncorrected) and reported if clusters were significant at p<0.05 whole-brain corrected for multiple comparisons.
Results
Behavior
Behavioral results are summarized in Table 1. Reflecting task difficulty, there were significantly fewer correct responses during the scene-labeling task than during scene-matching (NC: F(1,21)=4.97, p<0.04; WS: F(1,21)=25.86, p<0.001) and reaction times were significantly longer in the labeling experiment (NC: F(1,21)=4.48, p<0.05; WS: F(1,21)=25.86, p<0.001), reflecting increased cognitive demand. Neither accuracy (Label: F(1,18)=2.07, p=0.17; Match: F(1,24)=2.27, p=0.15) nor reaction time (Label: F(1,18)=1.64, p=0.22; Match: F(1,24)=0.51, p=0.48) differed significantly between the WS and control group on any of the tasks used. There was no significant interaction between task accuracy and diagnosis (F(1,19)=2.54, p=0.13).
BOLD fMRI responses
Between-group analyses
Table 2 shows significant differential effects between groups on BOLD signal by experimental condition in the amygdala. Within the amygdala ROI, the WS group displayed significantly greater response than did the general-population control group in both the scene-labeling and scene-matching tasks.
Table 2.
Between-group amygdala activation in scene-labeling and scene-matching experiments.
| Left amygdala | T value | P | Right amygdala | T value | P | |
|---|---|---|---|---|---|---|
| Labeling task | ||||||
| NC>WS | NS | NS | ||||
| WS>NC | −30 7 −21 | 2.48 | 0.01 | 30 3 −14 | 2.25 | 0.02 |
| Matching task | ||||||
| NC>WS | NS | NS | ||||
| WS>NC | NS | 26 7 −21 | 3.26 | 0.002 | ||
All reported voxels are significant at p<0.05 corrected for multiple comparisons within the amygdala ROI.
In the cortex (Table 3), the WS group displayed hypoactivation in left DLPFC during the labeling task relative to the general-population control group. Additionally, during the matching task, the WS group exhibited significantly less activation in the right middle occipital gyrus compared to the control group. There were no regions in the cortex that were significantly greater in the WS group relative to the control group.
Table 3.
Between-group cortical activation in scene-labeling and scene-matching experiments.
| Talairach | T value | P | |
|---|---|---|---|
| Labeling task | |||
| NC>WS | |||
| Left dorsolateral prefrontal cortex | −41 16 27 | 5.60 | 0.001 |
| Matching task | |||
| NC>WS | |||
| Right middle occipital gyrus | 30 −72 17 | 5.35 | 0.001 |
Voxels were thresholded at p<0.001 (uncorrected) and reported if clusters were significant at p<0.05 whole-brain corrected for multiple comparisons.
Within-group analyses
Within the amygdala ROI, there was a significantly greater response in the scene-matching task relative to the scene-labeling task in both groups (Table 4). There were no voxels significantly more activated in scene-labeling compared to scene-matching within the amygdala ROI.
Table 4.
Within-group amygdala activation in scene-matching compared to scene-labeling.
| Area | Talairach | T value | P |
|---|---|---|---|
| Control group | |||
| Right amygdala | 30 −16 −20 | 2.11 | 0.02 |
| WS group | |||
| Right amygdala | 22 −1 −17 | 1.89 | 0.03 |
| Left amygdala | −26 −15 −16 | 2.30 | 0.01 |
All reported voxels are significant at p<0.05 corrected for multiple comparisons within the amygdala ROI.
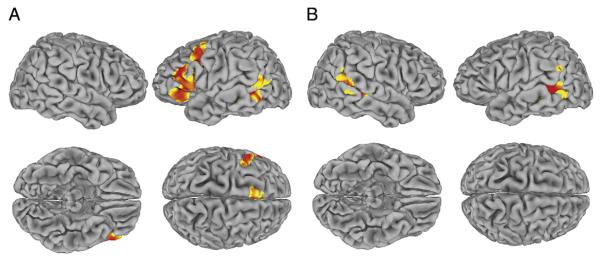
In the cortex, for the scene-labeling task compared to the scene-matching task, the control group showed greater prefrontal reactivity, specifically in left DLPFC, left OFC, and MPFC as well as in the left middle temporal gyrus. In contrast, the WS group showed no prefrontal differences between conditions, with only the posterior middle temporal gyrus more activated during scene-labeling than scene-matching (see Fig. 2, coordinates in Table 5).
Fig. 2.
Significant activation in the scene-labeling compared to the scene-matching task, p<0.05, corrected for multiple comparisons (height threshold, T=3) shown rendered on standard brain surface (more significant voxels in red). (A) General-population controls. (B) Participants with Williams syndrome. For coordinates, see Table 5.
These findings were confirmed by analyses of extracted mean BOLD signal, which demonstrated significant group-by-task interactions. As shown in Fig. 3, the control group, but not the WS group, showed differential reactivity as a function of task in left DLPFC [F(1,42)=8.21, p=0.006] and MPFC [F(1,42)=4.10, p=0.05], with higher activation in the more cognitively taxing labeling experiment. A trend in the same direction was observed in left OFC [F(1,42)=3.87, p=0.06].
Discussion
The purpose of the present experiment was to disambiguate between the effects of cognitive load and stimulus type on differential amygdala and prefrontal activation in individuals with WS. Together with our previous results (Meyer-Lindenberg et al., 2005), the present experiment establishes (a) differential activation in prefrontal regions as a consequence of task difficulty and (b) hyperreactivity of amygdala to non-social fearful scenes in individuals with WS regardless of cognitive load.
Control participants, but not participants with WS, showed differential activation in prefrontal cortex regions during the scene-labeling task relative to the scene-matching task. This result is similar to that previously obtained comparing the more cognitively demanding face-matching task with scene-matching (Meyer-Lindenberg et al., 2005), indicating that the prefrontal response difference reflects increased cognitive demand (Table 5 and Fig. 3). Conversely, in the amygdala, participants with WS showed a significantly higher response than general-population controls to fearful scenes in both tasks (Table 2), demonstrating that this hyperreactive amygdala signaling cannot be attributed to cognitive load because the observed group difference in response to scenes was similar in conditions of both high and low cognitive demand (labeling and matching scenes, respectively). Rather, these results indicate that individuals with WS show increased amygdala reactivity (relative to general-population controls) when processing non-socially relevant aversive stimuli irrespective of cognitive demand. Interestingly, the within-group analyses showed that in both WS and NC, amygdala reactivity was relatively lower when stimuli were cognitively appraised (the label condition), confirming previous results in NC (Hariri et al., 2000). Even with this effect present, amygdala reactivity was still greater in participants with WS during the labeling condition. Further, our previous work using path analysis to investigate the functional relations between PFC regions and the amygdala during a face-matching task highlighted a significant difference between NC and WS participant groups (Meyer-Lindenberg et al., 2005). Whereas PFC regions were engaged in both WS and NC groups during this task, the OFC was functionally disconnected from the amygdala in the WS group only. These prior findings suggest an abnormality in PFC regulation of the amygdala in participants with WS. Taken together, the current results further support the conclusion that increased fear in non-social situations, such as the specific phobias of socially irrelevant stimuli commonly found in individuals with WS (Blomberg et al., 2006; Dykens, 2003; Klein-Tasman and Mervis, 2003), may result from abnormal amygdala–prefrontal regulation during the perceptual processing of non-social stimuli.
The amygdala is associated with fear processing, and bilateral amygdala damage has been implicated in errors assessing the trustworthiness of faces (Adolphs et al., 1998) as well as impairments in judging negative facial affect (Adolphs et al., 1994). Interestingly, prenatal amygdala lesions in non-human primates have a differential impact on social and non-social fear: These animals exhibit greater fear behaviors during social interactions than do control primates but display a blunted fear response to threatening non-social stimuli (Bauman et al., 2004; Prather et al., 2001). This profile, which is the opposite of that found in individuals with WS, reemphasizes the conclusion that the neural substrates of social and non-social fear are at least partly dissociable. Confirming our previous finding, the relative increase in amygdala response to non-socially relevant stimuli seen here in both tasks, excludes a primary deficiency of amygdala activation in WS (Bellugi et al., 1999). Further support of intact amygdala function is provided by the relative preservation of amygdala size in individuals with WS (Chiang et al., 2007). Interestingly, in a study comparing individuals with WS to both chronological and mental age-matched general-population controls, individuals with WS differed from both groups of controls in their judgments of a stranger’s approachability, based on facial photographs depicting either happiness or a negative emotion (e.g., fear, anger). In particular, individuals with WS rated the happy faces as significantly more approachable and the faces showing negative emotions as significantly less approachable than did both control groups. This result was obtained despite the fact that individuals with WS are considerably more likely to approach strangers indiscriminately, suggesting a generalized difficulty in inhibiting social approach, further supporting a prefrontal-amygdala dysregulation in WS (Frigerio et al., 2006).
In prefrontal regions that were more strongly activated in the labeling task compared to the matching task, the control group showed strong left lateralization. We hypothesize that this is due to obligate verbal processing in the labeling task; whereas, the matching task could be processed perceptually. This finding is in agreement with a large body of previous imaging studies in right-handed individuals that have indicated a lateralization based on material type, with verbal/language stimuli producing activation in the prefrontal and temporal regions of the left hemisphere (Binder et al., 1997; Frost et al., 1999; Ramsey et al., 2001) and visual pattern encoding resulting in an asymmetrical bias toward right hemisphere reactivity (Golby et al., 2001). Our results are also consistent with literature finding that the posterior left inferior parietal cortex (IPC) has been associated with viewing verbal stimuli, while the posterior right IPC has been differentially activated by visual processing (Wagner et al., 1998). In the WS group, no laterality difference in prefrontal activation was seen between tasks. The dependence of lateralization on processing mode is reemphasized by the observation that the regions differentially activated on the left by general-population controls corresponded to the regions found differentially activated on the right by the controls in our previous experiment (Meyer-Lindenberg et al., 2004), which was strictly in the visuospatial–perceptual domain. Importantly, in both the current and previous experiments, the involved regions participate in key regulatory interactions with the amygdala, implicating a brain circuit for emotional control and social cognition.
Consistent with our previous report, in individuals with WS, OFC was not activated in either the matching or labeling task, while DLPFC and MPFC were activated in both tasks but did not differ in magnitude between the high and low cognitive load conditions, as they did in the control group. Based on animal literature (Ghashghaei and Barbas, 2002) and previous studies in healthy humans (Adolphs, 2003a), it is, therefore, not surprising that abnormalities in the reactivity of these regions affect amygdala processing. Most importantly, the OFC, a region that we found unreactive in our previous experiment and where reduced grey matter volume and sulcal depth in WS have been found (Kippenhan et al., 2005; Meyer-Lindenberg et al., 2004; Van Essen et al., 2006), now showed the same lack of activation to cognitive load, reemphasizing a potential primary involvement of OFC in the genesis of the hypersociability and increased non-social fear response seen in individuals with WS. The OFC has long been identified as a key region in social processing (Adolphs, 2001), with a primary role in re-evaluating reward values in evolving social contexts (Kringelbach and Rolls, 2003; Rolls, 2004). OFC abnormalities may directly contribute to the presence of circumstance-inappropriate social behaviors (e.g., approaching and talking to strangers) or lead to dysfunctional regulatory adaptations of the prefrontal–amygdala network. It is of interest in this context that, at least in the macaque, some neurons in the OFC respond preferentially to faces, predicting an impairment in reversing face-reinforcement associations depending on the social context in WS (Rolls et al., 2005). In addition to this prefrontal regulatory network, recent data also show reduced functional connectivity between the amygdala and the fusiform face area in WS (Sarpal et al., 2008). This could indicate that in addition to the altered prefrontal regulatory network implicated by both the present study and Meyer-Lindenberg et al. (2005), a further reason for reduced amygdala responsivity to socially relevant cues could be found in abnormal interaction with posterior temporal regions that process and represent facial features. Very recently, the finding of reduced amygdala activation to fearful social visual stimuli was replicated in a group of individuals with WS who had intellectual disability (Haas et al., 2009). This study also showed increased activation to positive stimuli (happy faces), adding a new facet to the neural mechanisms underlying prosocial behaviour in WS and suggesting that dysregulation of amygdala by PFC may lead to changed activation profiles not only between socially relevant and less relevant stimuli but also across a range of stimulus valence.
The study of WS continues to contribute to our understanding of social cognition by offering a privileged access to genetic processes impacting upon relevant neural circuits. Nevertheless, there are several study limitations that should be noted. First, while results from this study may indicate that abnormalities found even in normal-IQ individuals with WS are likely to be core characteristics of the syndrome and to reflect its underlying genetic substrate, it would also be of interest to extend this work to individuals with WS who have intellectual disability in order to explore the generalizability of the present findings. In fact, our core finding of reduced amygdala activation to threatening fearful faces has been confirmed in such a participant group (Haas et al., 2009). Second, due to the small sample size of the current study, it was not possible to compare WS individuals with phobias to WS individuals without phobias; however, future studies should investigate potential differences between these two groups. Third, because we targeted WS individuals with IQs in the normal range, our sample size remained small. Additional studies in larger cohorts are thus desirable to replicate these studies. Finally, because this study utilized only emotionally negative stimuli, comparisons to positive and neutral stimuli could not be explored. More work is needed to investigate the potential differential responses to a broader range of valences. Additional work is also necessary to explore the underlying mechanisms mediating the findings observed in this study. In particular, imaging studies of populations with small deletions in the WS region would be beneficial in elucidating the contribution of specific genes to prefrontal–amygdala dysregulation (Meyer-Lindenberg et al., 2006).
Acknowledgments
We thank Neha Dixit, Aaron-Bonner Jackson, Rosanna Olsen, John Holt, and Yunxia Tong for research assistance and Daniel Weinberger for helpful discussion of this manuscript. This work was supported by DHHS/NIH/NIMH/IRP and NINDS grant no NS35102 (C.B.M., principal investigator). K.E.M. is supported by the Multimodal Neuroimaging Training Program Fellowship: 1T90DA022761. A.R.H is supported by the National Institute of Mental Health: MH072837 and the National Alliance for Research on Schizophrenia and Depression. A.M.L. acknowledges support during the preparation of the manuscript by Bundesministerium für Bildung und Forschung (Nationales Genom-forschungsnetz plus MooDs), Deutsche Forschungsgemeinschaft (SFB 636-B7) and NARSAD (2009 Distinguished Investigator Award).
References
- Adolphs R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nat. Rev. Neurosci. 2003a;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Investigating the cognitive neuroscience of social behavior. Neuropsychologia. 2003b;41:119–126. doi: 10.1016/s0028-3932(02)00142-2. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J. Neurosci. 2004;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellugi U, Adolphs R, Cassady C, Chiles M. Towards the neural basis for hypersociability in a genetic syndrome. Neuroreport. 1999;10:1653–1657. doi: 10.1097/00001756-199906030-00006. [DOI] [PubMed] [Google Scholar]
- Beuren AJ, Apitz J, Harmjanz D. Supravalvular aortic stenosis in association with mental retardation and a certain facial appearance. Circulation. 1962;26:1235–1240. doi: 10.1161/01.cir.26.6.1235. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J. Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg S, Rosander M, Andersson G. Fears, hyperacusis and musicality in Williams syndrome. Res. Dev. Disabil. 2006;27:668–680. doi: 10.1016/j.ridd.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Chapman CA, du Plessis A, Pober BR. Neurologic findings in children and adults with Williams syndrome. J. Child. Neurol. 1996;11:63–65. doi: 10.1177/088307389601100116. [DOI] [PubMed] [Google Scholar]
- Cherniske EM, Carpenter TO, Klaiman C, Young E, Bregman J, Insogna K, Schultz RT, Pober BR. Multisystem study of 20 older adults with Williams syndrome. Am. J. Med. Genet. A. 2004;131:255–264. doi: 10.1002/ajmg.a.30400. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Reiss AL, Lee AD, Bellugi U, Galaburda AM, Korenberg JR, Mills DL, Toga AW, Thompson PM. 3D pattern of brain abnormalities in Williams syndrome visualized using tensor-based morphometry. Neuroimage. 2007;36:1096–1109. doi: 10.1016/j.neuroimage.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnai D, Karmiloff-Smith A. Williams syndrome: from genotype through to the cognitive phenotype. Am. J. Med. Genet. 2000;97:164–171. doi: 10.1002/1096-8628(200022)97:2<164::aid-ajmg8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Dykens EM. Anxiety, fears, and phobias in persons with Williams syndrome. Dev. Neuropsychol. 2003;23:291–316. doi: 10.1080/87565641.2003.9651896. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Tenforde A, Galaburda AM, Bellugi U, Korenberg JR, Mills D, Reiss AL. To modulate or not to modulate: differing results in uniquely shaped Williams syndrome brains. Neuroimage. 2006;32:1001–1007. doi: 10.1016/j.neuroimage.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. Am. J. Psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- Einfeld SL, Tonge BJ, Florio T. Behavioral and emotional disturbance in individuals with Williams syndrome. Am. J. Ment. Retard. 1997;102:45–53. doi: 10.1352/0895-8017(1997)102<0045:BAEDII>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Frigerio E, Burt DM, Gagliardi C, Cioffi G, Martelli S, Perrett DI, Borgatti R. Is everybody always my friend? Perception of approachability in Williams syndrome. Neuropsychologia. 2006;44:254–259. doi: 10.1016/j.neuropsychologia.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Language processing is strongly left lateralized in both sexes. Evidence from functional MRI. Brain. 1999;122:199–208. doi: 10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- Gallo FJ, Klein-Tasman BP, Gaffrey MS, Curran P. Expecting the worst: observations of reactivity to sound in young children with Williams syndrome. Res. Dev. Disabil. 2008;29:567–581. doi: 10.1016/j.ridd.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, Gabrieli JD. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 2001;124:1841–1854. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- Gosch A, Pankau R. Personality characteristics and behaviour problems in individuals of different ages with Williams syndrome. Dev. Med. Child. Neurol. 1997;39:527–533. doi: 10.1111/j.1469-8749.1997.tb07481.x. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Farber N, Raveh E, Apter A, Attias J. Hyperacusis in Williams syndrome: characteristics and associated neuroaudiologic abnormalities. Neurology. 2006;66:390–395. doi: 10.1212/01.wnl.0000196643.35395.5f. [DOI] [PubMed] [Google Scholar]
- Haas BW, Mills D, Yam A, Hoeft F, Bellugi U, Reiss A. Genetic influences on sociability: heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. J. Neurosci. 2009;29:1132–1139. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol. Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hillier LW, Fulton RS, Fulton LA, Graves TA, Pepin KH, Wagner-McPherson C, Layman D, Maas J, Jaeger S, Walker R, et al. The DNA sequence of human chromosome 7. Nature. 2003;424:157–164. doi: 10.1038/nature01782. [DOI] [PubMed] [Google Scholar]
- Jarvinen-Pasley A, Bellugi U, Reilly J, Mills DL, Galaburda A, Reiss AL, Korenberg JR. Defining the social phenotype in Williams syndrome: a model for linking gene, the brain, and behavior. Dev. Psychopathol. 2008;20:1–35. doi: 10.1017/S0954579408000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippenhan JS, Olsen RK, Mervis CB, Morris CA, Kohn P, Meyer-Lindenberg A, Berman KF. Genetic contributions to human gyrification: sulcal morphometry in Williams syndrome. J. Neurosci. 2005;25:7840–7846. doi: 10.1523/JNEUROSCI.1722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Tasman BP, Mervis CB. Distinctive personality characteristics of 8-, 9-, and 10-year-olds with Williams syndrome. Dev. Neuropsychol. 2003;23:269–290. doi: 10.1080/87565641.2003.9651895. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371–1383. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- Lang RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE. International Affective Pictures System (IAPS): Technical Manual and Affective Ratings. University of Florida; Gainesville: 1997. [Google Scholar]
- Levitin DJ, Cole K, Lincoln A, Bellugi U. Aversion, awareness, and attraction: investigating claims of hyperacusis in the Williams syndrome phenotype. J. Child. Psychol. Psychiatry. 2005;46:514–523. doi: 10.1111/j.1469-7610.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Woodruff-Borden J, Klein-Tasman BP, Fricke JS, Mervis CB. Prevalence of psychiatric disorders in 4 to 16-year-olds with Williams syndrome. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2006;141B:615–622. doi: 10.1002/ajmg.b.30344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyfer OT, Woodruff-Borden J, Mervis CB. Anxiety disorders in children with Williams syndrome, their mothers, and their siblings: implications for the etiology of anxiety disorders. J. Neurodev. Disord. 2009;1:4–14. doi: 10.1007/s11689-009-9003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Becerra AM. Language and communicative development in Williams syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2007;13:3–15. doi: 10.1002/mrdd.20140. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Klein-Tasman BP. Williams syndrome: cognition, personality, and adaptive behavior. Ment. Retard. Dev. Disabil. Res. Rev. 2000;6:148–158. doi: 10.1002/1098-2779(2000)6:2<148::AID-MRDD10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF, Bertrand J, Morris CA, Klein-Tasman BP, Armstrong SC. The Williams syndrome cognitive profile. Brain. Cogn. 2000;44:604–628. doi: 10.1006/brcg.2000.1232. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF, Rowe ML, Becerra AM, Klein-Tasman BP. Language abilities of individuals with Williams syndrome. Int. Rev. Res. Ment. Retard. 2003;27:35–81. [Google Scholar]
- Meyer-Lindenberg A, Kohn P, Mervis CB, Kippenhan JS, Olsen RK, Morris CA, Berman KF. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43:623–631. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, Berman KF. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat. Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat. Rev. Neurosci. 2006;7:380–393. doi: 10.1038/nrn1906. [DOI] [PubMed] [Google Scholar]
- Morris CA. The dysmorphology, genetics, and natural history of Williams–Beuren syndrome. In: Morris CA, Lenhoff HM, Wang PP, editors. Williams–Beuren syndrome: Research, Evaluation, and Treatment. Johns Hopkins University Press; Baltimore: 2006. pp. 3–17. [Google Scholar]
- Ollendick TH. Reliability and validity of the Revised Fear Survey Schedule for Children (FSSC-R) Behav. Res. Ther. 1983;21:685–692. doi: 10.1016/0005-7967(83)90087-6. [DOI] [PubMed] [Google Scholar]
- Pober BR, Morris CA. Diagnosis and management of medical problems in adults with Williams–Beuren syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 2007;145:280–290. doi: 10.1002/ajmg.c.30139. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Sommer IE, Rutten GJ, Kahn RS. Combined analysis of language tasks in fMRI improves assessment of hemispheric dominance for language functions in individual subjects. Neuroimage. 2001;13:719–733. doi: 10.1006/nimg.2000.0722. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, Chang M, Reynolds MF, Kwon H, Galaburda A. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J. Neurosci. 2004;24:5009–5015. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Browning AS, Inoue K. Face-selective and auditory neurons in the primate orbitofrontal cortex. Exp. Brain Res. 2005:1–14. doi: 10.1007/s00221-005-0191-y. [DOI] [PubMed] [Google Scholar]
- Sarpal D, Buchsbaum BR, Kohn PD, Kippenhan JS, Mervis CB, Morris CA, Meyer-Lindenberg A, Berman KF. A genetic model for understanding higher order visual processing: functional interactions of the ventral visual stream in Williams syndrome. Cereb. Cortex. 2008;18:2402–2409. doi: 10.1093/cercor/bhn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WK, Albano AM. The Anxiety disorders interview schedule for DSM-IV: Parent interview schedule. Graywind Publications, a Division of the Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J. Child. Neurol. 2002;17:269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- Udwin O, Yule W. A cognitive and behavioural phenotype in Williams syndrome. J. Clin. Exp. Neuropsychol. 1991;13:232–244. doi: 10.1080/01688639108401040. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dierker D, Snyder AZ, Raichle ME, Reiss AL, Korenberg J. Symmetry of cortical folding abnormalities in Williams syndrome revealed by surface-based analyses. J. Neurosci. 2006;26:5470–5483. doi: 10.1523/JNEUROSCI.4154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Poldrack RA, Eldridge LL, Desmond JE, Glover GH, Gabrieli JD. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- Williams JC, Barratt-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation. 1961;24:1311–1318. doi: 10.1161/01.cir.24.6.1311. [DOI] [PubMed] [Google Scholar]