Abstract
NF-κB is a set of multifunctional transcriptional factors that regulate expression of genes involved in numerous normal cellular activities. They also are activated in many inflammatory and neoplastic conditions in which their expression may be stimulated by pro-inflammatory cytokines. NF-κB in turn, regulates the expression of cytokines and so can mediate autocrine, self-amplifying cycles of cytokine release and NF-κB activation leading to maintenance of inflammatory reactions beyond the initial stimulus, as seen in rheumatoid arthritis and asthma. Since discovery of the requirement of NF-κB for basal and cytokine-induced osteoclast formation in the mid-1990s, much has been learned about the role of NF-κB in bone. NF-κB has roles in skeletal development, endochondral ossification, osteoclast and osteoblast functions, and common bone diseases. NF-κB inhibitors have been developed, but none has made it to clinical trials for the treatment of common bone diseases. Here we review the roles for NF-κB in bone and in common bone diseases.
INTRODUCTION
NF-κB (nuclear factor of activated B cells) is a family of transcription factors, which were first identified as regulators of B cell differentiation by their ability to bind to the κB site of the kappa light chain gene in B cells. They were shown subsequently to be involved in innate and adaptive immunity in response to pathogens and auto-immune stimuli as well as in many aspects of normal cellular functions (1, 2). Further studies showed that NF-κB activation is dysregulated in other common conditions, including cancer, diabetes and atherosclerosis, further emphasizing the wide spectrum of roles it plays in normal and disease states (3).
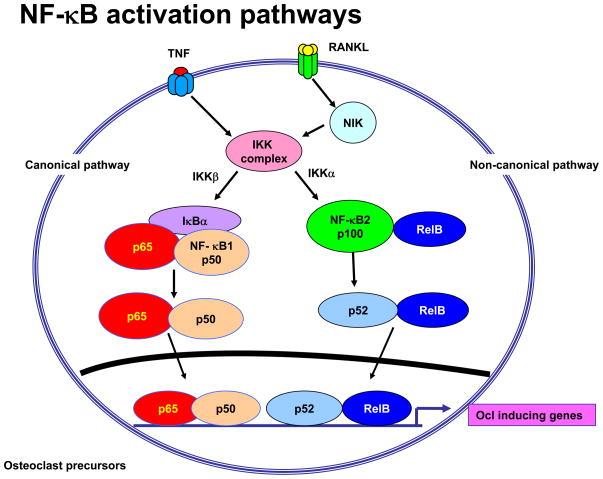
There are 5 original members of the NF-κB family: RelA (p65), p50, p52, RelB and c-Rel. They all have an N-terminal Rel homology domain for formation of homo- and heterodimers of family members and for sequence-specific DNA binding. RelA, RelB, and c-Rel also have a C-terminal transcription activation domain (TAD). p50 and p52 do not have TADs and must rely on interactions with other factors to positively regulate gene transcription (3). p50 and p52 are formed as larger proteins, NF-κB1 consisting of p50 and its precursor p105, and NF-κB2 consisting of p52 and its precursor p100 (1). RelA and c-Rel preferentially heterodimerize with p50 and signal in the classical pathway (Figure 1) in response to many influences, including RANKL, TNF and IL-1 (4). RelB preferentially heterodimerizes with p100 and its processed smaller form, p52, in the alternative pathway also in response to RANKL, but apparently not to TNF (5).
Figure 1. Canonical and Non-canonical NF-κB signaling induced by TNF and RANKL.
Both TNF and RANKL activate canonical signaling through IKKβ leading to phosphorylation of IκBα and release of p65/p50 heterodomers and their translocation to the nucleus. This results in activation of c-Fos and NFATc1, two other transcription factors necessary for osteoclast precursor differentiation. In addition, p65/p50 induce expression of NF-κB p100, which acts as an inhibitory κB protein by binding to RelB and preventing its translocation to the nucleus. RANKL induces activation of NIK and IKKα, which leads to proteasomal processing of p100 to p52, which then goes along with RelB to the nucleus to induce target gene expression. TNF increases NIK cytoplasmic levels in osteoclast precursors, but does not appear to activate it and thus p100 levels are increased in these cells and limits their differentiation (25).
Given the protean functions of NF-κB to positively regulate gene expression, it is not surprising that there is a family of NF-κB inhibitory proteins, called IκBs, which retain NF-κB dimers in the cytoplasm of un-stimulated cells. This family includes the classical IκBs: IκBα, IκBβ, and IκBε (6). These all have multiple ankyrin repeats, which bind to NF-κB dimers and thus interfere with the function of their nuclear localization signals (NLS). IκBα predominantly regulates RelA:p50 heterodimers in the classical pathway. The p65/p50 heterodimeric complex is the most common form in mammalian cells and this is what is commonly referred to as “NF-κB”. IκBε preferentially regulates RelA:RelA homodimers along with c-Rel:RelA heterodimers (3). IκBβ also binds to RelA:p50 heterodimers. In response to stimuli such as RANKL and TNFα, IκBα is degraded rapidly in the proteasome to allow NF-κB heterodimers to translocate to nuclei. There they induce expression not only of cytokines, enzymes and growth factors, but also of IκBα, which enters the nucleus and shuttles RelA/p50 dimers back to the cytoplasm for subsequent activation in a negative feedback loop (). There are other IκB-like proteins, including BCL3, IκBζ, and IκBNS, which are regulated differently from classical IκB family members and have different functions (3, 7), but further discussion of them is beyond the scope of this review.
The C-terminal portion of p105, called IκBγ, and of p100, called IκBδ, also contain multiple ankyrin repeats giving them IκB-like functions (8, 9). IκBγ selectively binds to RelA and c-Rel and retains them in the cytoplasm, but it can also bind to p50 molecules processed from p105 (3). p105 processing occurs constitutively in the proteasome of un-stimulated cells and removes the C-terminal portion to generate the p50 subunit (10). Upon stimulation by cytokines, such as RANKL and TNF, p105 is phosphorylated and rapidly degraded in the proteasome without release of p50 and this allows p50/Rel protein dimers to go to the nucleus. The IκBδ portion of p100 binds preferentially to RelB to retain it in the cytoplasm, but it also binds to and regulates RelA homodimers (11).
Classical NF-κB signaling is activated upon stimulation by a trimeric IκB kinase (IKK) complex consisting of the catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ (also called NEMO: NF-κB essential modulator) (3, 12). The IKK complex phosphorylates IκBα, leading to its polyubiquitination and degradation via the 26S proteasome and allowing nuclear translocation of RelA:p50 dimers (3). IKKβ mediates most of the IκB kinase activity in the classical pathway in most cell types, including RANKL-induced signaling in osteoclast precursors. In contrast, signaling in the alternative NF-κB pathway is activated by IKKα following its activation by NF-κB-inducing kinase (NIK) and leads to processing of p100 and production of RelB:p52 heterodimers (13–15) (Figure 1). An important additional effect of classical IKK signaling is that it also upregulates expression of NF-κB2 in the alternative pathway (16). As will be seen later, this has important inhibitory effects on TNF-induced osteoclastogenesis.
NF-κB is activated during embryogenesis to regulate certain aspects of endochondral ossification and in a number of pathologic conditions affecting the skeleton, including postmenopausal osteoporosis, inflammatory and osteoarthritis, infections, Paget’s disease, and metastatic bone disease (17). Studies to date indicate that NF-κB affects the skeleton predominantly by inducing enhanced osteoclast formation and activity, but it also has roles in osteoblasts and chondrocytes, which will be reviewed later in this review.
NF-κB SIGNALING IN OSTEOCLASTOGENESIS
A role for NF-κB in bone was first identified in the mid-1990s when 2 groups of investigators generated NF-κB1/2 double knockout (dKO) mice and discovered unexpectedly that they had failure of tooth eruption and osteopetrosis due to absence of osteoclasts (18, 19). They had generated these mice because both NF-κB1 and -2 single KO mice had modest immunodeficiencies and they wanted to determine the effect on immune cell functions of removing all or most NF-κB signaling by eliminating these two pivotal genes. These dKO mice also had severe B cell and T cell differentiation defects (18, 19) and in that respect are similar to RANKL and RANK KO mice (4), which were generated later after RANKL and RANK were discovered. The defect in osteoclastogenesis in NF-κB dKO mice is similar to that in RANKL KO mice in that there is a halt in osteoclast precursor (OCP) differentiation at the stage where OCPs express RANK, but fail to express other markers of osteoclast differentiation, including TRAP, cathepsin K and the calcitonin receptor (20). NF-κB is required for survival of many cell types, but surprisingly OCP numbers are increased in the dKO mice and do not have increased apoptosis (20). The defect in dKO OCP differentiation was not rescued by addition of cytokines, such as TNF, IL-1 or IL-6 and RANKL (21). The osteopetrosis was reversed in the mice by transplantation of hematopoietic cells from WT embryonic liver, indicating that the defect was osteoclast precursor cell autonomous (18).
The mechanisms whereby NF-κB induces osteoclastogenesis downstream of RANKL/RANK have been partly, but not completely explained. There is a rapid and transient (within a hour) increase in RelA and p50 (22). This is followed after ~2 hours by an increase in c-Fos and p52 expression, which is sustained during the subsequent 72–96 hours without any further change in RelA or p50 mRNA levels (23). NFATc1 expression increases around 48–72 after RANKL treatment (23) when OCP fusion takes place along with expression of TRAP and cathepsin K. These changes in mRNA levels do not necessarily reflect changes in NF-κB protein levels because, as stated above NF-κB dimers can be shuttled from the nucleus back to the cytoplasm for further rounds of cytokine-induced signaling. Further detailed time-course studies of levels of these proteins in nuclear and cytoplasmic extracts of OCPs will be required to more accurately define how the transcription factors regulate osteoclast formation and activity. Although NF-κB has been shown to activate c-Fos and NFATc1 to induce expression of cathepsin K, TRAP and other genes involved in osteoclast resorptive functions, the genes that are regulated by these transcription factors directly to mediate OCP differentiation remain to be identified.
These sequential changes in NF-κB, c-Fos and NFATc1 gene expression levels are also observed in response to TNF (23–25), which can induce OCP differentiation independently of RANKL/RANK signaling (26). However, TNF induces the formation of significantly fewer osteoclasts from WT OCPs than RANKL in vitro (23), which we have attributed recently to the inhibitory effects of TNF-induced p100 expression and inadequate p100 processing to p52 (25) and see below). RANKL and TNF, as members of the TNF superfamily, mediate signaling through their receptors by means of TRAFs (TNF receptor associated factors), adaptor molecules that recruit kinases for signaling because neither of their receptors has intrinsic kinase activity (27). Several TRAFs associate with RANK, but of these only TRAF6 appears to be essential for OCP differentiation (27). TNF/TNFR-induced osteoclastogenic signaling appears to be mediated predominantly by TRAF2 (28) and occurs in the absence of TRAF6 (our unpublished data).
RANKL activates both canonical and alternative NF-κB signaling in OCPs through TRAF6 and NIK, leading to activation of IKKβ and IKKα, respectively (4). Expression of IKKβ, but not IKKα, is required for basal osteoclastogenesis. Targeted deletion of IKKβ in myeloid cells in IKKβf/f;CD11b-Cre mice or IKKβf/f;Mx1-Cre mice eliminates osteoclastogenesis in vitro and in vivo (29, 30). Inhibition of IKKβ activation through a NEMO-binding domain peptide abolishes osteoclastogenesis, osteolysis, and inflammatory arthritis in various animal models of inflammation (31). These findings link IKKβ-mediated signaling to osteoclastogenesis, osteolysis, and inflammation. IKKβ also affects cell survival. Deletion of IKKβ renders immune cells, macrophages and OCPs susceptible to TNF-induced apoptosis through a gain of function in the JNK pathway. This is evident by the findings that inhibition of TNF or JNK restores the osteoclastogenic and inflammatory potential of these cells (29, 30).
In contrast to IKKβ−/− mice, IKKα−/− mice have normal bones and osteoclast numbers in vivo. OCPs from IKKα−/− mice do not differentiate into osteoclasts in response to RANKL in vitro, but they do form osteoclasts in response to TNF or IL-1 (30). Similar to IKKα, expression of NIK is not required for basal osteoclast formation in vivo, although NIK−/− OCPs have severely defective RANKL-induced osteoclast formation in vitro (32). NIK−/− mice also have decreased joint inflammation in response to TNF-induced arthritis (32). NF-κB1 and -2 single knockout mice have normal basal osteoclastogenesis in vivo and have increased osteoclastogenesis in response to IL-1, similar to WT mice (21). RelA−/− mice die early during embryogenesis due to TNF-mediated massive hepatocyte apoptosis, but mice generated with deficiency of RelA only in hematopoietic cells have defective response to RANKL in vivo (33). Furthermore, inhibition of RelA nuclear translocation in OCPs in vitro inhibits osteoclastogenesis (33). RelB−/− mice have mild osteopetrosis with near normal numbers of osteoclasts, but like NIK−/− mice their OCPs have an impaired response to RANKL in vitro (34). Also similar to NIK−/− mice, they have an impaired response to stimulated osteoclastogenesis in vivo in that unlike WT mice, they do not develop osteolysis around melanoma cells injected directly into their tibiae (34).
No bone phenotypes have been reported in c-Rel−/− mice. C-Rel expression is not required for RANKL-induced osteoclast formation in vitro (34). However, most c-Rel−/− mice are resistant to collagen-induced arthritis and have significantly reduced joint inflammation and bone erosion. In contrast, c-Rel−/− mice develop acute arthritis in response to intra-articular injection of mBSA/IL-1. These findings led to the conclusion that c-Rel expression is required for systemic but not local joint disease (35). In the same study, NF-κB1−/− mice were reported to be resistant to both forms of arthritis. Furthermore, c-Rel binding activity is unchanged in RelA−/− OCPs (33), suggesting that c-Rel does not perform the same function as Rel A in osteoclasts
Since RANKL and TNF activate NF-κB, c-Fos and NFATc1 in a very similar manner (4, 23, 25), it has been surprising that, while TNF induces osteoclast formation from RANK−/− OCPs in vitro, it fails to induce osteoclastogenesis when given to RANK−/− mice in vivo (36). Like RANKL (5), TNF induces expression of p100, but unlike RANKL it induces processing of only a small amount of the protein to p52, resulting in high p100 cytoplasmic concentrations (16). We hypothesized that p100 induced by TNF, but inadequately processed to p52 in response to it in OCPs, acts as an NF-κB inhibitory protein limiting signaling in the alternative pathway in these cells and thus their differentiation. We found that treatment of NF-κB2−/− OCPs with TNF induced similar numbers of osteoclasts as RANKL (25), supporting our hypothesis. To determine if p100 inhibited TNF induction of osteoclastogenesis in RANK−/− mice in vivo, we generated NF-κB2/RANK dKO mice and gave them daily local injections of TNF into the subcutaneous tissues overlying their calvariae. In contrast to the effects in RANK−/− mice in which only occasional osteoclasts were observed in response to TNF, numerous bone resorbing osteoclasts formed in calvarial bones of the dKO mice (25). To determine if p100 also limited osteoclastogenesis in an animal model of rheumatoid arthritis, we crossed TNF-transgenic mice with NF-κB2−/− mice. We found that the TNF-Tg/NF-κB2−/− mice developed more severe bone erosions and increased inflammation in their joints one month earlier than TNF-Tg mice, further supporting a role for p100 to limit TNF-induced osteoclastogenesis (25).
To further explore the mechanism whereby p100 limits TNF-induced osteoclastogenesis, we examined its interactions with TRAFs and NIK in OCPs in response to TNF and RANKL. NIK activity is regulated negatively by its constitutive proteasomal degradation. This results from its association with TRAF3 (3, 13), which mediates NIK degradation and thus limits p100 processing and non-canonical NF-κB signaling. TRAF3 degradation is induced in B cells by CD40 or BAFF-R activation and its ablation in B cells prevents NIK degradation. This leads to progressive accumulation of NIK and processing of p100 to activate non-canonical NF-κB signaling (3, 13). TRAF3−/− mice have uncontrolled NIK activity resulting in early postnatal lethality, which is rescued by crossing the mice with nfkb2−/− mice (37). Thus, one potential mechanism to limit TNF-induced bone erosion in conditions, such as RA and postmenopausal osteoporosis, would be to induce stabilization of p100 or prevent its degradation by inhibiting the production or activity of NIK.
ROLES FOR NF-κB IN ENDOCHONDRAL OSSIFICATION AND ARTHRITIS
Most vertebrate bones are formed during fetal development from mesenchymal cell differentiation into chondrocytes, which form cartilage moulds of the bones followed by endochondral ossification (38, 39). These moulds are invaded by blood vessels typically in their mid-regions where chondrocytes become hypertrophic and the matrix around them calcifies. This calcified cartilage is removed and replaced by bone and a medullary cavity by poorly understood mechanisms that require Runx2 expression (39)) and appear to function most efficiently when osteoclasts are present. However, the whole process can proceed in the absence of osteoclasts, as seen in RANKL−/−, RANK−/−, and NF-κB dKO mice (4), all of which typically are dwarfed. In the absence of osteoclasts, hypertrophic chondrocytes could secrete matrix metalloproteinases to dissolve the matrix around them, undergo apoptosis and be replaced by new bone formation. Alternatively, the matrix could be removed by chondroclasts, which have not been characterized, but if chondroclasts exist, in rank−/− and rankl−/− mice they are TRAP- mononuclear cells (personal observation by BFB). Cartilage in the embryonic bones of these KO mice is thus removed by an as yet unexplained process and is replaced by bone matrix, which is not removed subsequently resulting in osteopetrosis. These mice have thickened zones of hypertrophic cartilage at their growth plates. The thickening of the hypertrophic zone is transient and disappears between 2–3 weeks postnatally in RANKL−/− and RANK−/− mice (our unpublished observations) similar to the phenotype in MMP9−/− mice (40). The thickened hypertrophic zone is corrected between 5 and 6 weeks in NF-κB dKO mice. The transient nature of this defect in all of these mice has not been adequately explained, but since MMP expression is regulated by NF-κB in many cell types, it presumably is related to defective NF-κB signaling in chondrocytes or other cell types, which is compensated for by some mechanism.
The differentiation of mesenchymal cells into chondrocytes is promoted by a number of genes, including Nkx3.2, which also maintains chondrocyte proliferation and negatively regulates their maturation (41). Nkx3.2 also inhibits chondrocyte apoptosis by direct or indirect modulation of NF-κB signaling (42). Nkx3.2 recruits RelA–IκBα heteromeric complexes into the nucleus through direct protein–protein interactions, and induces IκBα phosphorylation, ubiquitination and proteasome-dependent degradation, which ultimately leads to functional activation of RelA in chondrocytes (42). Sox9, a master regulator of chondrocyte differentiation, induces transcription of type II collagen and two other cartilage extracellular matrix molecules, aggrecan and link (43). TNF down-regulates Sox9 expression through NF-κB activation and in this way can inhibit chondrocyte differentiation in a number of pathologic settings in which its expression is increased (44). These include rheumatoid and osteoarthritis and fracture repair in which expression of TNF and IL-1 are increased (45). These cytokines are catabolic for cartilage. They reduce type II collagen production and stimulate the expression of metalloproteinases and aggrecanases, which induce cartilage degradation (46). They also inhibit mesenchymal stem cell differentiation to chondrocytes associated with marked activation of NF-κB (47). These anti-chondrogenic responses are rescued by a dominant-negative IκB super-repressor (47). IL-1 also activates NF-κB p65 in human chondrocytes to enhance the transcription of the inhibitory Smad7, which competes with Smad2 and Smad3 to bind activated TβRI to inhibit its anabolic role in chondrocytes (48). IL-1 receptor expression is increased in chondrocytes of OA patients, suggesting that they may be more sensitive to IL-1 than normal articular chondrocytes and more susceptible to injury (49). Since NF-κB regulates TNF and IL-1 expression (3), activation of NF-κB by these cytokines can in turn stimulate their production in RA and OA, forming a positive regulatory cycle that may amplify and maintain the disease process.
NF-κB can also be activated in articular cartilage during strenuous exercise that induces excessive compressive dynamic forces (50). Compressive force appears to be essential for maintenance of cartilage integrity during normal physical activities (50), but when excessive it could induce inflammation and promote damage to cartilage leading to osteoarthritis. NF-κB is a key mediator of mechanical signals applied to chondrocytes (50), and dynamic compressive strain (DCS) applied to chondrocytes grown in 3-D scaffolds regulates the expression of nitric oxide synthase (50), an NF-κB-inducible pro-inflammatory enzyme. Low DCS inhibits IL-1β-induced pro-inflammatory gene expression by attenuating NF-κB signaling through the regulation of IκBα and IκBβ degradation and subsequent NF-κB p65 nuclear translocation (50). In contrast, high DCS stimulates NF-κB signaling. These studies support a role for classical NF-κB signaling in inflammation-induced arthritis. Our observation that the inflamed joints of TNF-Tg/nfkb2−/− mice have more severe erosion and inflammation than those of TNF-Tg control mice (25) suggest that the alternative pathway can also dampen inflammatory cytokine-induced joint inflammation.
NF-κB SIGNALING IN OSTEOBLASTS
The role of NF-κB signaling has been studied less in osteoblasts than in osteoclasts. As stated earlier, nfkb1/2 dKO mice have a severe form of osteopetrosis resulting from a lack of osteoclasts. Their bone marrow cavities are largely filled with unremodeled bone trabeculae, suggesting that osteoblastic function during endochondral ossification is not impaired. To test this, we examined calvarial cells from neonatal dKO mice and found that bone nodule formation and alkaline phosphatase activity was similar in cells derived from their WT littermates (our unpublished observations). However, a recent study has identified an anti-anabolic function for p65 in the classical signaling pathway in mice. Inhibition of NF-κB p65 by a dominant-negative mutant IKKγ (IKK-DN) and a super IκBα repressor (SR-IκBα) enhanced osteoblastic differentiation of mesenchymal C2C12 cells in vitro (51). Furthermore, young transgenic mice with specific inhibition of NF-κB by a dominant-negative IKK driven by Bglap2, the osteocalcin promoter, in mature osteoblasts had significantly increased trabecular bone mass compared with WT littermates, with no effects on osteoclast activities (51). In addition, adult transgenic mice were protected from ovariectomy-induced bone loss. Further evidence of a role for NF-κB in osteoblasts is a report that 17 β-estradiol significantly antagonized TNF-induced NF-κB activity in MC3T3 osteoblastic cells associated with reduced osteoblastic function, although 17 β-estradiol had no effect on basal NF-κB activity (52). Suppression of NF-κB prevents TNF-induced inhibition of TGFβ-mediated Smad signaling and therefore potently stimulates differentiation and mineralization of MC3T3 pre-osteoblastic cells and primary murine bone marrow stromal cells (53). In addition, NF-κB has also been reported to repress osteoblast differentiation in Saos2 osteosarcoma cells by a mechanism involving Smad7 (54). In contrast to these catabolic effects in osteoblasts, NF-κB has been reported recently to have a pro-survival role in osteoblastic cells (55).
Hematopoietic stem cells (HSCs) and primitive hematopoietic progenitor cells (HPCs) reside in bone marrow adjacent to endosteal bone surfaces lined by osteoblasts. The specialized microenvironment or niche created by osteoblasts and other cells plays a critical role in hematopoiesis by providing survival, proliferation and differentiation signals (55, 57). HSCs and HPCs are very sensitive to injury, such as ionizing radiation (IR), which induces them to undergo apoptosis, while osteoblasts are more radiation-resistant (58). In response to IR, NF-κB p65 was activated through its phosphorylation at ser536 in osteoblasts to release many cytokines to support the survival of HSCs and HPCs (55). Inhibition of NF-κB p65 by siRNA not only induced the apoptotic death of osteoblastic cells, but also suppressed cytokine release from these osteoblasts in response to IR and thereby disrupted the supporting role of osteoblasts for HSC survival (55). In addition, previous studies have shown that NF-κB inhibitors had no adverse effects on osteoblast functions in vitro (59). Thus, further studies will be required to better define the role of NF-κB in osteoblastic cell functions.
SUMMARY
Studies to date have indicated that classical and/or alternative NF-κB signaling plays essential roles in certain aspects of osteoclast, osteoblast and chondroblast activities. These include the proliferation of precursors and the functions of differentiated cells. Some of these functions appear to be essential during embryonic development, such as the role of NF-κB 1 and 2 in osteoclastogenesis, while others, such as RelB are restricted to activation of NF-κB signaling in osteoclasts in disease states. NF-κB activation appears to play important roles in endochondral ossification to prevent dwarfism in mice and in cartilage destruction in animal models of RA and OA. There clearly are opportunities for development of drugs to promote or inhibit NF-κB activation to treat or prevent common bone diseases, but to date none is in clinical trials. The only drug that has been developed to target NF-κB-induced signaling for the treatment or prevention of bone disease is Denosumab, a human monoclonal antibody which binds to RANKL and prevents RANKL interaction with RANK and thus activation of NF-κB signaling (60).
References
- 1.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 2.Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–6843. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- 3.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 4.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, Teitelbaum SL. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198:771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Karin M. The IkappaB kinase - a bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 8.Dobrzanski P, Ryseck RP, Bravo R. Specific inhibition of RelB/p52 transcriptional activity by the C-terminal domain of p100. Oncogene. 1995;10:1003–1007. [PubMed] [Google Scholar]
- 9.Liou HC, Nolan GP, Ghosh S, Fujita T, Baltimore D. The NF-kappa B p50 precursor, p105, contains an internal I kappa B-like inhibitor that preferentially inhibits p50. Embo J. 1992;11:3003–3009. doi: 10.1002/j.1460-2075.1992.tb05370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 11.Fusco AJ, Savinova OV, Talwar R, Kearns JD, Hoffmann A, Ghosh G. Stabilization of RelB requires multidomain interactions with p100/p52. J Biol Chem. 2008;283:12324–12332. doi: 10.1074/jbc.M707898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata W, Maeda S, Hikiba Y, Yanai A, Ohmae T, Sakamoto K, Nakagawa H, Ogura K, Omata M. Cutting edge: The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J Immunol. 2007;179:2681–2685. doi: 10.4049/jimmunol.179.5.2681. [DOI] [PubMed] [Google Scholar]
- 13.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz ZB, Weih DS, Sivakumar V, Weih F. RelB is required for Peyer’s patch development: differential regulation of p52-RelB by lymphotoxin and TNF. Embo J. 2003;22:121–130. doi: 10.1093/emboj/cdg004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaisson ML, Branstetter DG, Derry JM, Armstrong AP, Tometsko ME, Takeda K, Akira S, Dougall WC. Osteoclast differentiation is impaired in the absence of inhibitor of kappa B kinase alpha. J Biol Chem. 2004;279:54841–54848. doi: 10.1074/jbc.M406392200. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Wu HF, Ang ES, Yip K, Woloszyn M, Zheng MH, Tan RX. NF-kappaB modulators in osteolytic bone diseases. Cytokine Growth Factor Rev. 2009;20:7–17. doi: 10.1016/j.cytogfr.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iotsova V, Caamano J, Loy J, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 20.Xing L, Bushnell TP, Carlson L, Tai Z, Tondravi M, Siebenlist U, Young F, Boyce BF. NF-kappaB p50 and p52 expression is not required for RANK-expressing osteoclast progenitor formation but is essential for RANK- and cytokine-mediated osteoclastogenesis. J Bone Miner Res. 2002;17:1200–1210. doi: 10.1359/jbmr.2002.17.7.1200. [DOI] [PubMed] [Google Scholar]
- 21.Xing L, Carlson L, Story B, Tai Z, Keng P, Siebenlist U, Boyce BF. Expression of either NF-kappaB p50 or p52 in osteoclast precursors is required for IL-1-induced bone resorption. J Bone Miner Res. 2003;18:260–269. doi: 10.1359/jbmr.2003.18.2.260. [DOI] [PubMed] [Google Scholar]
- 22.Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita T, Yao Z, Li F, Zhang Q, Badell IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282:18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- 24.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao Z, Xing L, Boyce BF. NF-κB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J Clin Invest. 2009 doi: 10.1172/JCI38716. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology. 2002;143:1108–1118. doi: 10.1210/endo.143.3.8701. [DOI] [PubMed] [Google Scholar]
- 27.Darnay BG, Besse A, Poblenz AT, Lamothe B, Jacoby JJ. TRAFs in RANK signaling. Adv Exp Med Biol. 2007;597:152–159. doi: 10.1007/978-0-387-70630-6_12. [DOI] [PubMed] [Google Scholar]
- 28.Kanazawa K, Kudo A. TRAF2 is essential for TNF-alpha-induced osteoclastogenesis. J Bone Miner Res. 2005;20:840–847. doi: 10.1359/JBMR.041225. [DOI] [PubMed] [Google Scholar]
- 29.Otero JE, Dai S, Foglia D, Alhawagri M, Vacher J, Pasparakis M, Abu-Amer Y. Defective osteoclastogenesis by IKKbeta-null precursors is a result of receptor activator of NF-kappaB ligand (RANKL)-induced JNK-dependent apoptosis and impaired differentiation. J Biol Chem. 2008;283:24546–24553. doi: 10.1074/jbc.M800434200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruocco MG, Maeda S, Park JM, Lawrence T, Hsu L-C, Cao Y, Schett G, Wagner EF, Karin M. IκB kinase (IKK)β, but not IKKα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med. 2005;201:1677–1687. doi: 10.1084/jem.20042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jimi E, Aoki K, Saito H, D’Acquisto F, May MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H, et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 32.Aya K, Alhawagri M, Hagen-Stapleton A, Kitaura H, Kanagawa O, Veis Novack D. NF-{kappa}B-inducing kinase controls lymphocyte and osteoclast activities in inflammatory arthritis. J Clin Invest. 2005;115:1848–1854. doi: 10.1172/JCI23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaira S, Alhawagri M, Anwisye I, Kitaura H, Faccio R, Novack DV. RelA/p65 promotes osteoclast differentiation by blocking a RANKL-induced apoptotic JNK pathway in mice. J Clin Invest. 2008;118:2088–2097. doi: 10.1172/JCI33392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaira S, Johnson T, Hirbe AC, Alhawagri M, Anwisye I, Sammut B, O’Neal J, Zou W, Weilbaecher KN, Faccio R, et al. RelB is the NF-kappaB subunit downstream of NIK responsible for osteoclast differentiation. Proc Natl Acad Sci U S A. 2008;105:3897–3902. doi: 10.1073/pnas.0708576105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell IK, Gerondakis S, O’Donnell K, Wicks IP. Distinct roles for the NF-kappaB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J Clin Invest. 2000;105:1799–1806. doi: 10.1172/JCI8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He JQ, Zarnegar B, Oganesyan G, Saha SK, Yamazaki S, Doyle SE, Dempsey PW, Cheng G. Rescue of TRAF3-null mice by p100 NF-kappa B deficiency. J Exp Med. 2006;203:2413–2418. doi: 10.1084/jem.20061166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 39.Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Genet Dev. 2001;11:527–532. doi: 10.1016/s0959-437x(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 40.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Provot S, Kempf H, Murtaugh LC, Chung UI, Kim DW, Chyung J, Kronenberg HM, Lassar AB. Nkx3.2/Bapx1 acts as a negative regulator of chondrocyte maturation. Development. 2006;133:651–662. doi: 10.1242/dev.02258. [DOI] [PubMed] [Google Scholar]
- 42.Park M, Yong Y, Choi SW, Kim JH, Lee JE, Kim DW. Constitutive RelA activation mediated by Nkx3.2 controls chondrocyte viability. Nat Cell Biol. 2007;9:287–298. doi: 10.1038/ncb1538. [DOI] [PubMed] [Google Scholar]
- 43.Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008;18:213–219. doi: 10.1007/s10165-008-0048-x. [DOI] [PubMed] [Google Scholar]
- 44.Seguin CA, Bernier SM. TNFalpha suppresses link protein and type II collagen expression in chondrocytes: Role of MEK1/2 and NF-kappaB signaling pathways. J Cell Physiol. 2003;197:356–369. doi: 10.1002/jcp.10371. [DOI] [PubMed] [Google Scholar]
- 45.Herman S, Kronke G, Schett G. Molecular mechanisms of inflammatory bone damage: emerging targets for therapy. Trends Mol Med. 2008;14:245–253. doi: 10.1016/j.molmed.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, Loeser RF. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J Immunol. 2005;174:5781–5788. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Muller PE, Evans CH, Porter RM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60:801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauge C, Attia J, Leclercq S, Pujol JP, Galera P, Boumediene K. Interleukin-1beta up-regulation of Smad7 via NF-kappaB activation in human chondrocytes. Arthritis Rheum. 2008;58:221–226. doi: 10.1002/art.23154. [DOI] [PubMed] [Google Scholar]
- 49.Martel-Pelletier J, McCollum R, DiBattista J, Faure MP, Chin JA, Fournier S, Sarfati M, Pelletier JP. The interleukin-1 receptor in normal and osteoarthritic human articular chondrocytes. Identification as the type I receptor and analysis of binding kinetics and biologic function. Arthritis Rheum. 1992;35:530–540. doi: 10.1002/art.1780350507. [DOI] [PubMed] [Google Scholar]
- 50.Nam J, Aguda BD, Rath B, Agarwal S. Biomechanical thresholds regulate inflammation through the NF-kappaB pathway: experiments and modeling. PLoS One. 2009;4:e5262. doi: 10.1371/journal.pone.0005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamaguchi M, Weitzmann MN. The estrogen 17beta-estradiol and phytoestrogen genistein mediate differential effects on osteoblastic NF-kappaB activity. Int J Mol Med. 2009;23:297–301. [PubMed] [Google Scholar]
- 53.Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN. Endogenous TNF alpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-kappaB. J Bone Miner Res. 2007;22:646–655. doi: 10.1359/jbmr.070121. [DOI] [PubMed] [Google Scholar]
- 54.Eliseev RA, Schwarz EM, Zuscik MJ, O’Keefe RJ, Drissi H, Rosier RN. Smad7 mediates inhibition of Saos2 osteosarcoma cell differentiation by NF-kappaB. Exp Cell Res. 2006;312:40–50. doi: 10.1016/j.yexcr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 55.Xiao M, Inal CE, Parekh VI, Li XH, Whitnall MH. Role of NF-kappaB in hematopoietic niche function of osteoblasts after radiation injury. Exp Hematol. 2009;37:52–64. doi: 10.1016/j.exphem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 57.Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, Williams B, Havens A, Schneider A, Ge C, Franceschi RT, et al. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008;26:2042–2051. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmed KM, Li JJ. NF-kappa B-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med. 2008;44:1–13. doi: 10.1016/j.freeradbiomed.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, Harris SE, Gallwitz W, Kim KB, Hu S, et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burkiewicz JS, Scarpace SL, Bruce SP. Denosumab in osteoporosis and oncology. Ann Pharmacother. 2009;43:1445–1455. doi: 10.1345/aph.1M102. [DOI] [PubMed] [Google Scholar]