SUMMARY
Objective
Sphingomyelin deposition and metabolism occurs in the atherosclerotic plaque, leading to the formation of sphingosine 1-phosphate (S1P), which activates G protein-coupled receptors to regulate vascular and immune cells. The role of S1P receptors in atherosclerosis has not been examined.
Methods and Results
We tested the hypothesis that Sphingosine 1-phosphate receptor-2 (S1PR2) regulates atherosclerosis. Apoe−/−S1pr2−/− mice showed greatly attenuated atherosclerosis compared to the Apoe−/− mice. Bone marrow transplant experiments indicate that S1PR2 function in the hematopoietic compartment is critical. S1PR2 is expressed in bone marrow-derived macrophages and in macrophage-like foam cells in atherosclerotic plaques. Reduced macrophage-like foam cells were found in the atherosclerotic plaques of Apoe−/−S1pr2−/− mice, suggesting that S1PR2 retains macrophages in atherosclerotic plaques. Lipoprotein profiles, plasma lipids and oxidized LDL uptake by bone marrow-derived macrophages were not altered by the S1pr2 genotype. In contrast, endotoxin-induced inflammatory cytokine (IL-1β, IL-18) levels in the serum of S1PR2 knockout mice were significantly reduced. Further, treatment of wild-type mice with S1PR2 antagonist JTE-013 suppressed IL-1β and IL-18 levels in plasma.
Conclusion
These data suggest that S1PR2 signaling in the plaque macrophage regulates macrophage retention and inflammatory cytokine secretion, thereby promoting atherosclerosis.
Keywords: atherosclerosis, sphingolipids, sphingosine 1-phosphate, macrophages, inflammation
INTRODUCTION
Risk factors for atherosclerosis such as hyperlipidemia, smoking, and hypertension injure the vascular endothelium, leading to lipoprotein deposition in the arterial vessel wall 1, 2. Although cholesterol entry into the atherosclerotic plaque is well accepted to play a major role in atherogenesis, sphingomyelin (SM), which has an affinity for cholesterol in membranes is also deposited. Metabolism of SM by the sphingomyelinase pathway produces sphingolipid metabolites - ceramide, sphingosine and sphingosine 1-phosphate (S1P) 3–5, whose function in vascular disease is not understood. Indeed, inflammatory cytokines stimulate the metabolism of sphingomyelin by inducing the secretion of sphingomyelinase from endothelial cells and macrophage6. However, suppression of SM synthesis with the fungal metabolite myriocin attenuates atherosclerosis in animal models and SM and its metabolites are elevated in plasma of patients with coronary artery disease, suggesting that sphingolipid metabolites are important in atherosclerosis 7–9.
Among the sphingolipid metabolites, extracellular S1P signals via G protein-coupled receptors (S1PR1–5) and regulates vascular permeability, angiogenesis and immune cell trafficking10–13. S1P bound to HDL mediates the vascular protective functions of this lipoprotein via the S1PR114–16. On the other hand, clinical studies suggest that serum S1P could be a predictor of obstructive coronary artery disease 17. Interestingly, FTY720, a structural analog of myriocin and a S1P receptor modulator, inhibits atherosclerosis in mouse models even though the mechanisms involved remain unclear 18. However, the role of S1P in atherosclerosis is likely complex since S1P receptor subtypes exhibit redundant as well as antagonistic signaling properties 10. For example, S1PR1 and S1PR2 activate the small GTPases Rac and Rho, respectively, and induce cytoskeletal changes to regulate vascular endothelial adherens junctions and permeability in both positive and negative manner19–23. The role of the specific S1P receptors in atherosclerotic vascular disease is virtually unknown. In this report, we describe the pro-atherogenic role of S1PR2. In addition, we provide strong evidence that the atherogenic function of S1PR2 involves macrophage retention in the plaques and pro-inflammatory cytokine production.
Materials and Methods
A detailed expanded Materials and Methods section is available online at http://atvb.ahajournals.org).
Animals
Mice with targeted disruption of the S1pr2 gene 24 were maintained on a mixed C57BL/6×129Sv genetic background. Experiments on KO mice were performed with appropriate WT littermate controls. All procedures involving mice were approved by the University of Connecticut Health Center Animal Care Committee. Mice were fed with a high cholesterol diet (TD31857) and atherosclerosis was analyzed after 13 weeks.
Histology and Immunohistochemistry
Serial cross sections of the aortic leaflet were stained with the following antibodies: rat-anti-MOMA-2 (Serotec), polyclonal anti-S1pr2 25, 26 (1:200).
Bone Marrow Transplantation
Male Apoe−/− mice were lethally irradiated with two 550rad doses, 4 hours apart and reconstituted with 5×106 bone marrow cells from Apoe−/−S1pr2−/− and Apoe−/−S1pr2+/+ donors. Recipients were maintained on normal chow for 4 weeks, then placed on high fat diet for 13 weeks and analyzed for lesion development.
RNA isolation and RT-PCR analysis
RNA was extracted from mouse aortas and quantitative RT-PCR assay was done using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems).
Cytokine measurements
Mouse serum levels of IL-1β, IL-18 (Invitrogen) and TNF-α (e-Bioscience) were measured by Enzyme Linked ImmunoSorbent assay (ELISA).
RESULTS
Requirement for S1PR2 in atherosclerosis
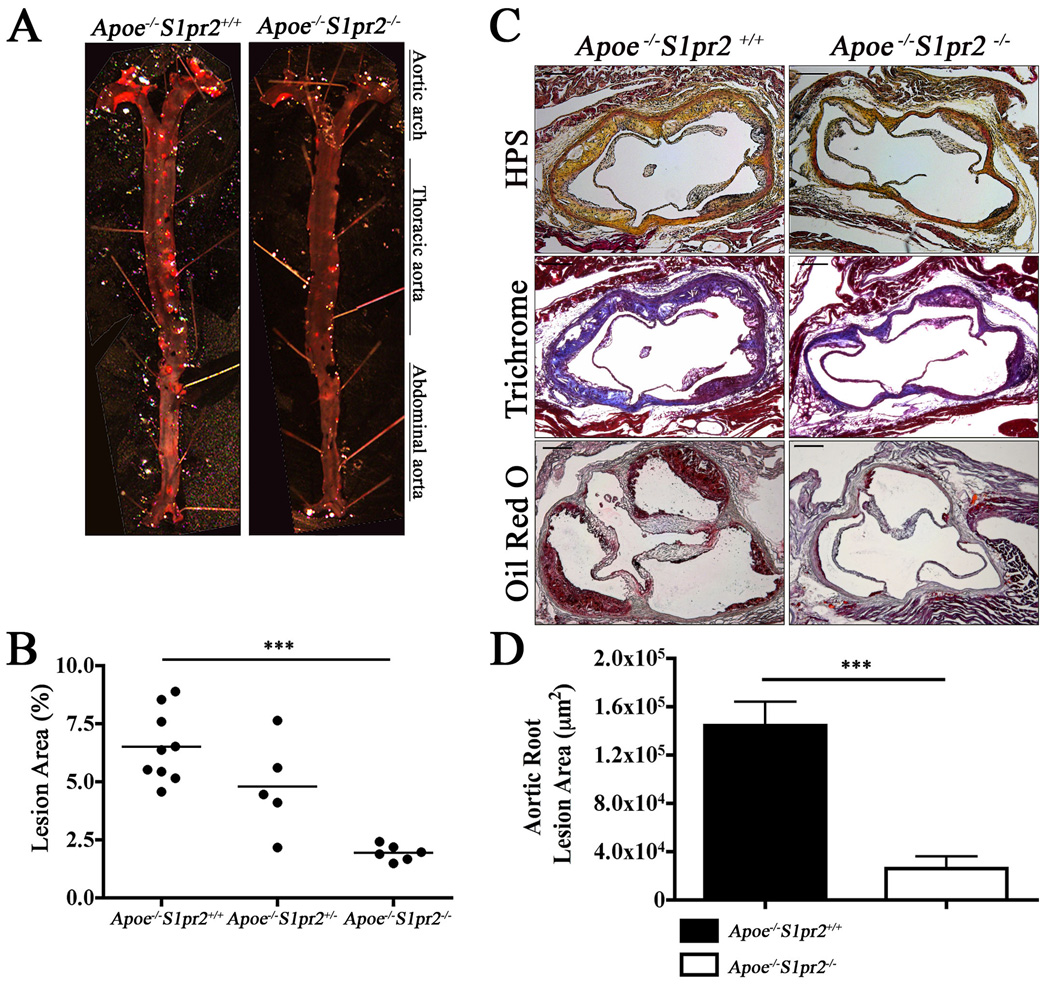
To investigate the role of the S1PR2 in atherosclerosis, we placed Apoe−/−S1pr2−/− mice and Apoe−/−S1pr2+/+ littermate controls on a high fat “Western” diet for 13 weeks 24, 27. Mouse aortae were dissected and stained with Oil Red O to highlight the atherosclerotic plaques of aortic arch, thoracic and abdominal aorta (Figure 1A). En face analysis of atheromatous lesions revealed significant and uniform reduction (~70%) in Apoe−/−S1pr2−/− mice compared to Apoe−/−S1pr2+/+ littermates (Figure 1B). We also performed Hematoxylin-Phloxin-Saffron (HPS), Trichrome, and Oil Red O staining of serial cross-sections from the aortic sinus to examine the presence of fibrous caps, connective tissue and lipid deposition, respectively (Figure 1C). As shown in Figure 1D, loss of S1PR2 led to a marked reduction (>80%) in atheromatous plaque area (fibrous tissue and collagen content) as well as lipid deposition. Necrotic core from the plaques of the Apoe−/−S1pr2−/− mice was decreased to a similar extent (Figure S1A) 28. In sharp contrast, nuclear-specific TUNEL staining did not reveal changes in apoptosis (Figure S1B) 29.
Figure 1. Requirement for S1PR2 in murine atherosclerosis.
(A) Aortae from Apoe−/−S1pr2+/+ (n=9), Apoe−/−S1pr2+/− (n=5) and Apoe−/−S1pr2−/− (n=6) mice were stained with Oil Red O to visualize plaques. En face analysis is shown from a representative aorta. (B) Quantitative analysis of aortae indicates eduction of atherosclerotic plaques in the entire aortic tree of Apoe−/−S1pr2−/− (n=6) compared to Apoe−/−S1pr2+/+ (n=9, P < 0.0001). (C) Aortic root cross-sections of Apoe−/−S1pr2+/+ (n=5) and Apoe−/−S1pr2−/− (n=5) stained with Hematoxylin Phloxin and Saffron (HPS) to characterize fibrotic areas, Trichrome to visualize collagen and Oil Red O to stain for lipid deposition. (D) Lack of the receptor markedly reduced the plaque area (P < 0.001). Scale bar, 100µm.
Critical role of myeloid S1PR2 in atheroma development
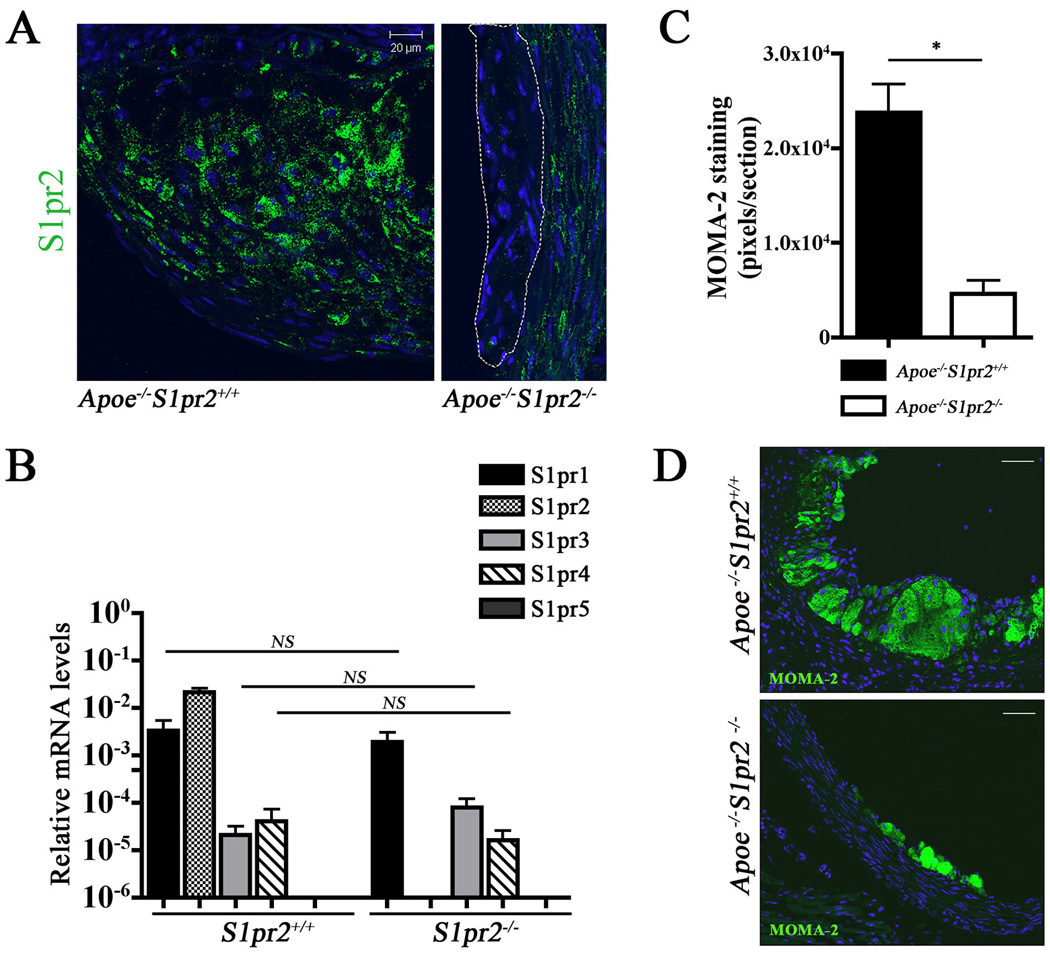
Given that macrophages play major roles in driving atherosclerotic plaque inflammation and progression 2, 30, 31, we examined the requirement for the S1PR2 in the hematopoietic compartment. Immunostaining for the S1PR2 in the aortic sinus showed that S1PR2 is expressed by macrophage-like foam cells in atherosclerotic plaques (Figure 2A). Indeed, bone marrow derived macrophages (BMDM) express high levels of S1PR2 and S1pr1 transcripts. Lack of S1PR2 in the knockout mice did not change the expression levels of S1pr1, S1pr3 and S1pr4 receptor transcripts (Figure 2B). Macrophage infiltration into the vessel wall was examined by immunostaining with a macrophage/monocyte specific antibody (MOMA-2). Apoe−/−S1pr2−/− mice showed a significant reduction in macrophage numbers (~80%) (Figure 2C and D). These data suggest that macrophage retention in the atherosclerotic plaques is regulated by the S1PR2.
Figure 2. S1PR2 is expressed and regulates macrophage/ foam cell retention in the plaque.
(A) Aortic root cross-sections were immunostained for S1PR2 with the affinity-purified antibody. Note positive signals in the atherosclerotic lesion of Apoe−/−S1pr2+/+ mice. Small plaques (encircled by dashed line) found in Apoe−/−S1pr2−/− animals are negative for S1PR2 expression. Scale bar, 20µm. (B) Expression of S1P receptor transcripts in bone marrow-derived macrophages was determined by Q RT-PCR (n=3). (C),(D) Aortic root sections from Apoe−/−S1pr2+/+ (n=3) and Apoe−/−S1pr2−/− (n=3) were immunostained with MOMA-2 (P < 0.05) to visualize macrophage/ foam cells. Scale bar, 50µm.
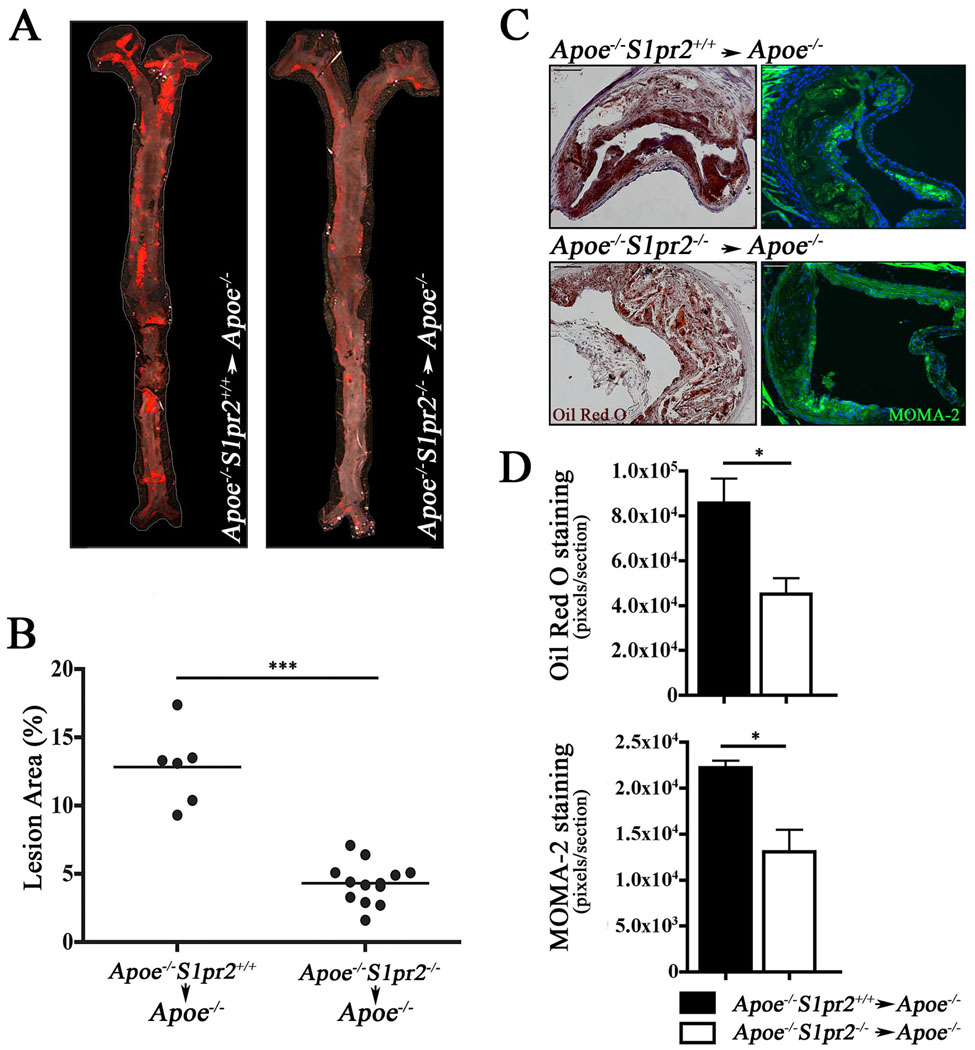
We generated bone marrow chimeras by transplanting lethally irradiated Apoe−/− mice with Apoe−/−S1pr2+/+ or Apoe−/−S1pr2−/− bone marrow. Following reconstitution of the hematopoietic system and after 13 weeks on a “western” diet, en face analysis demonstrated a significant reduction in atherosclerotic lesion area throughout the aorta (~ 65%) in mice receiving Apoe−/−S1pr2−/− bone marrow cells compared to Apoe−/−S1pr2+/+ counterparts (Figure 3A and 3B). Analysis of peripheral blood monocytes (CD11b+, CD115+), polymorphonuclear leukocytes (CD11b+, CD115−, Gr1+), and T- and B-lymphocytes (CD4+, CD8+, or B220+) revealed no differences between Apoe−/−S1pr2+/+ and Apoe−/−S1pr2−/− mice (Figure S2). Atherosclerotic lesions at the aortic root showed decreased lipid and macrophage accumulation (Figure 3C and 3D), suggesting that the S1PR2 signaling in macrophages in the Apoe−/− mice is sufficient to promote atherosclerosis.
Figure 3. Myeloid S1PR2 mediates pro-atherosclerotic effects.
(A) En face Oil Red O staining analysis of aortae upon transplantation of Apoe−/−S1pr2+/+ (n=6) or Apoe−/−S1pr2−/−(n=13) bone marrow cells to Apoe−/−S1pr2+/+ recipients. Mice were placed on high fat diet for 13 weeks and analyzed as described. (B) Quantification of en face analysis of atherosclerotic plaques. (P < 0.0001). (C) MOMA-2 (monocytes and macrophages) and Oil Red O (lipid accumulation) staining in aortic root lesions from Apoe−/−S1pr2+/+ (n=3) or Apoe−/−S1pr2−/− (n=3) bone marrow transplanted to Apoe−/− S1pr2+/+ recipients and placed on high fat diet for 13 weeks. Scale bar, 100µm. (D) Quantitative analysis of aortic root lesions. Lipid accumulation (P < 0.04) and inflammatory component (P < 0.03) of the plaque were significantly reduced in Apoe−/−S1pr2+/+ animals transplanted with Apoe−/−S1pr2−/− bone marrow cells.
S1PR2 regulates inflammatory changes in macrophages
Body weight, plasma cholesterol, plasma triglyceride and lipoprotein profiles were not different between Apoe−/−S1pr2−/− mice and littermate controls, suggesting that alterations in sterol and triglyceride metabolism are unlikely to account for S1PR2-induced atherosclerosis (Figure S3). In addition, foam cell differentiation in vitro was not altered in S1pr2−/− cells compared to S1pr2+/+ control cells (Figure S4). These data suggest that S1PR2 signaling in macrophages rather than the changes in lipid metabolism or uptake is involved.
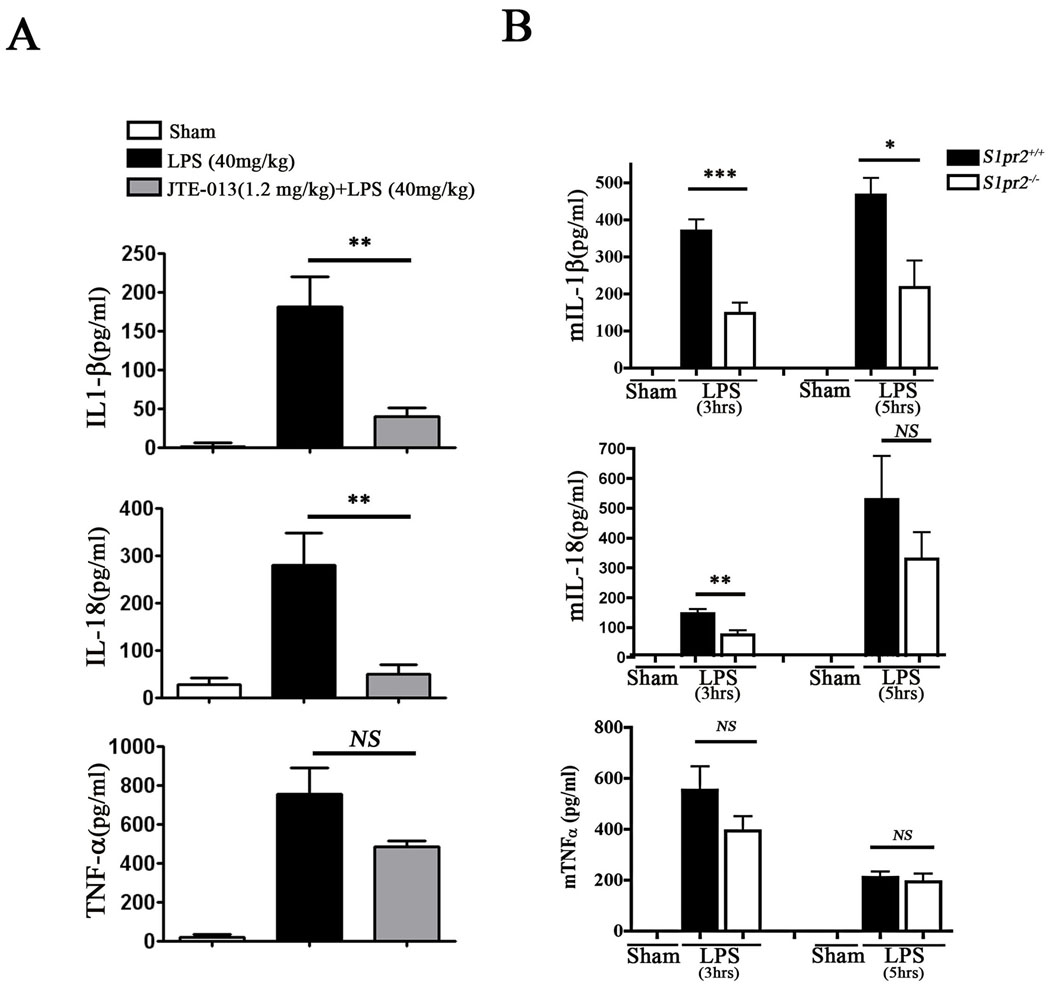
Although S1PR1 was proposed to regulate anti-inflammatory events, the role of S1PR2 in macrophage-dependent inflammation is not known 32. In order to test if S1PR2 regulates inflammatory pathways, we measured serum cytokine levels in WT or S1pr2−/− mice after treatment with LPS, which activates the TLR4 pathway. JTE-013, the S1PR2 antagonist inhibited LPS-induced IL-1β and IL-18 levels but not TNF-α (Figure 4A). In addition, S1pr2−/− mice had significantly reduced serum IL-1β and IL-18 levels compared to control S1pr2+/+ mice (Figure 4B). However, we did not observe differences in serum TNF-α. These data suggest that S1PR2 function is important in secretion of some inflammatory cytokines (such as IL-1β and IL-18) in vivo and thereby promotes atherosclerosis.
Figure 4. S1PR2 regulates pro-atherosclerotic cytokine release in vivo.
(A) Mice were treated with LPS (40mg/kg) for 3hrs and plasma cytokine levels were quantified (n=6–7). Mice pretreated with the S1PR2 antagonist (JTE-013, 1.2mg/kg, 30min pretreatment, n=7–9) had reduced serum IL-1β (P < 0.003) and IL-18 (P < 0.007) levels. TNF-α levels were not changed significantly. (B) Mice were treated with LPS (40mg/kg) for 3hrs and plasma cytokine levels were quantified. S1pr2−/− mice had reduced serum IL-1β and IL-18 compared to control group S1pr2+/+ mice (n=6, P < 0.001 and P < 0.01, respectively). At 5hrs of LPS treatment, the difference in serum IL-1β levels between WT and KO animals is still significant (n=6, P < 0.02).
DISCUSSION
Despite the transformative nature of HMG CoA reductase inhibitors in the control of hypercholesterolemia and atherosclerosis, efforts continue to identify novel therapeutic agents. Sphingolipid signaling, which appears to be highly significant in atherogenesis 7, 9, is poorly understood at the mechanistic level. In particular, enzymes in the sphingolipid metabolic cascade and the receptors for sphingolipid metabolites may regulate the complex process of atherosclerosis. As such they represent novel opportunities in therapeutic development. Indeed, S1P receptor modulators were shown to be efficacious in clinical trials for the control of autoimmune inflammation in multiple sclerosis 33, 34. S1P signaling is complex and may have both pro- and anti-atherosclerotic properties 16, 32. The effect of S1P in atherosclerotic vascular disease might be different depending on the expression of different S1P receptors in the cells of the vessel wall or infiltrating hematopoietic cells. In addition, local production of S1P may also be important.
In this report, we demonstrate that the S1PR2 promotes atherosclerosis in the Apoe−/− mouse model. It is known that this receptor subtype, which potently activates the Rho GTPase, induces vascular permeability in endothelial cells 21. It is also associated with pathologic angiogenesis and is required for abnormal vascular tuft formation in the retinopathy of prematurity in the mouse 22. Bone marrow transplant experiments in this report clearly indicate that the function of S1PR2 in the macrophage compartment is critical in the promotion of atherosclerosis. Although the function of S1PR2 in endothelial cells may also contribute to atherosclerosis, it appears that macrophage may be the important cell type in which S1PR2 signaling is critical.
The function of S1PR2 in hematopoietic cells is less understood. Recent studies showed that S1PR2 inhibits chemoattractant-induced motility in murine primary macrophages in vitro and may limit macrophage infiltration into the inflamed peritoneum35. In these studies, we showed that S1PR2 induced the cAMP/ protein kinase A pathway to inhibit macrophage motility in vitro. Given that macrophage accumulation in the atherosclerotic plaque is significantly reduced in S1pr2−/− mice, it is likely that S1PR2 in the macrophage regulate trafficking in and out of the plaque. Myeloid adhesion proteins (VLA-4 and CD18) which are important in monocyte-endothelial cell adhesion, were not altered in bone marrow-derived macrophages isolated from S1pr2−/− mice (Figure S5). In vitro studies do not support the hypothesis that S1P is a direct chemoattractant for myeloid cells. In fact, it is known that chemokine signals, i.e., those induced by CCL2 (MCP-1) and CXCL11 (SDF-1) regulate the recruitment of monocytes into atherosclerotic plaques 36. In contrast, S1P action on S1PR2 inhibit motility of macrophages, suggesting that S1PR2 may retain these macrophage / foam cells in the atherosclerotic plaques. Interestingly, the related S1PR1 receptor is known to promote egress of immune cells (especially T and B cells) from lymphoid organs into lymph or blood 37. Thus, S1PR2 may be a retention promoting receptor in myeloid cells.
In addition, we show that the S1PR2 is needed for TLR4-induced expression and secretion of proinflammatory cytokines IL-1β and IL-18. It is known that these two cytokines are important for atherosclerosis 38. Thus, our findings suggest that specific inhibition of macrophage S1PR2 by pharmacological antagonists may be useful as adjuncts in the control of atherosclerotic vascular disease. Due to the pharmacokinetic and solubility properties of JTE-013, a pharmacologic antagonist for S1PR2 that is not optimal for chronic administration, its effect on atherosclerosis cannot be assessed at present. However, acute administration of JTE-013 was able to inhibit TLR4-stimulated IL-1β and IL-18 release in vivo. Development of a specific S1PR2 antagonist with better in vivo properties will address pharmacologic utility of this novel pro-atherogenic signaling pathway.
Interestingly, S1PR2 did not regulate TNF-α secretion. In contrast to IL-1β and IL-18, which are secreted by the caspase-dependent inflammasome pathway 39, TNF-α is produced by the classical ER/ Golgi secretory pathway. Thus, S1PR2 signaling may selectively regulate the non-classical cytokine secretion pathway.
In summary, the results of this study provide the first molecular genetic and pharmacologic evidence to support the hypothesis that S1PR2 pathway is a crucial step in the development of atherosclerotic disease. We speculate that similar mechanisms may be involved in human cardiovascular disease. Thus, the specific inhibition of S1PR2 function could lessen lesion formation and facilitates plaque stability.
Supplementary Material
ACKNOWLEDGEMENTS
This work is supported by NIH grants HL67330, HL70694 and HL89934 to TH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Rader DJ, Puré E. Lipoproteins, macrophage function, and atherosclerosis: Beyond the foam cell? Cell Metabolism. 2005;1:223. doi: 10.1016/j.cmet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Tabas I, Williams KJ, Boren J. Subendothelial Lipoprotein Retention as the Initiating Process in Atherosclerosis: Update and Therapeutic Implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee S. Sphingolipids in Atherosclerosis and Vascular Biology. Arterioscler Thromb Vasc Biol. 1998;18:1523–1533. doi: 10.1161/01.atv.18.10.1523. [DOI] [PubMed] [Google Scholar]
- 5.Auge N, Nikolova-Karakashian M, Carpentier S, Parthasarathy S, Negre-Salvayre A, Salvayre R, Merrill AH, Jr, Levade T. Role of sphingosine 1-phosphate in the mitogenesis induced by oxidized low density lipoprotein in smooth muscle cells via activation of sphingomyelinase, ceramidase, and sphingosine kinase. J Biol Chem. 1999;274:21533–21538. doi: 10.1074/jbc.274.31.21533. [DOI] [PubMed] [Google Scholar]
- 6.Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A, Gold PW, Hirsch E, Williams KJ, Licinio J, Tabas I. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci U S A. 2000;97:8681–8686. doi: 10.1073/pnas.150098097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabas I. Sphingolipids and Atherosclerosis: A Mechanistic Connection? A Therapeutic Opportunity? Circulation. 2004;110:3400–3401. doi: 10.1161/01.CIR.0000150861.98087.56. [DOI] [PubMed] [Google Scholar]
- 8.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang X-C. Effect of Myriocin on Plasma Sphingolipid Metabolism and Atherosclerosis in apoE-deficient Mice. J. Biol. Chem. 2005;280:10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X-c, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR. Plasma Sphingomyelin Level as a Risk Factor for Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 10.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Seminars in Cell & Developmental Biology. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 12.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 14.Argraves KM, Argraves WS. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J. Lipid Res. 2007;48:2325–2333. doi: 10.1194/jlr.R700011-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Nofer J-R, van der Giet M, Tölle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Gödecke A, Ishii I, Kleuser B, Schäfers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. Journal of Clinical Investigation. 2004;113:569. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daum G, Grabski A, Reidy MA. Sphingosine 1-phosphate: a regulator of arterial lesions. Arterioscler Thromb Vasc Biol. 2009;29:1439–1443. doi: 10.1161/ATVBAHA.108.175240. [DOI] [PubMed] [Google Scholar]
- 17.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, Gleason LA, Nakajima N, Sabbadini RA. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 18.Nofer J-R, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen EAL. FTY720, a Synthetic Sphingosine 1 Phosphate Analogue, Inhibits Development of Atherosclerosis in Low-Density Lipoprotein Receptor Deficient Mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 19.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of Vascular Permeability by the Sphingosine-1-Phosphate Receptor-2 (S1P2R) and its Downstream Effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 22.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117:2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. Journal of Biological Chemistry. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 25.Singer II, Tian M, Wickham LA, Lin J, Matheravidathu SS, Forrest MJ, Mandala S, Quackenbush EJ. Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J Immunol. 2005;175:7151–7161. doi: 10.4049/jimmunol.175.11.7151. [DOI] [PubMed] [Google Scholar]
- 26.Forrest M, Sun SY, Hajdu R, Bergstrom J, Card D, Doherty G, Hale J, Keohane C, Meyers C, Milligan J, Mills S, Nomura N, Rosen H, Rosenbach M, Shei GJ, Singer II, Tian M, West S, White V, Xie J, Proia RL, Mandala S. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther. 2004;309:758–768. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- 27.Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 28.Thorp E, Kuriakose G, Shah YM, Gonzalez FJ, Tabas I. Pioglitazone Increases Macrophage Apoptosis and Plaque Necrosis in Advanced Atherosclerotic Lesions of Nondiabetic Low-Density Lipoprotein Receptor Null Mice. Circulation. 2007;116:2182–2190. doi: 10.1161/CIRCULATIONAHA.107.698852. [DOI] [PubMed] [Google Scholar]
- 29.Tabas I. Consequences and Therapeutic Implications of Macrophage Apoptosis in Atherosclerosis: The Importance of Lesion Stage and Phagocytic Efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 30.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 31.Tedgui A, Mallat Z. Cytokines in Atherosclerosis: Pathogenic and Regulatory Pathways. Physiol. Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 32.Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-Phosphate Induces an Antiinflammatory Phenotype in Macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, Pohlmann H, Kappos L. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology. 2009;72:73–79. doi: 10.1212/01.wnl.0000338569.32367.3d. [DOI] [PubMed] [Google Scholar]
- 34.Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 35.Michaud J, Im DS, Hla T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J Immunol. 2010;184:1475–1483. doi: 10.4049/jimmunol.0901586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 37.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 38.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 39.Martinon F, Mayor A, Tschopp The Inflammasomes: Guardians of the Body. Annual Review of Immunology. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.