Abstract
Insight into copper-oxygen species proposed as intermediates in oxidation catalysis is provided by the identification of a Cu(II)-superoxide complex supported by a sterically hindered, pyridinedicarboxamide ligand. A tetragonal, end-on superoxide structure is proposed based on DFT calculations and UV-vis, NMR, EPR, and resonance Raman spectroscopy. The complex yields a trans-1,2-peroxodicopper(II) species upon reaction with [(tmpa)Cu(CH3CN)]OTf, and, unlike other known Cu(II)-superoxide complexes, acts as a base rather than an electrophilic (H-atom abstracting) reagent in reactions with phenols.
An important first step in copper-promoted aerobic oxidations in biology1 and catalysis2 is the formation of a 1:1 Cu/O2 adduct, in which the O2 molecule is activated for subsequent reactions, either with substrate or to form different copper-oxygen species. In one approach aimed at understanding such adducts, synthetic 1:1 Cu/O2 complexes have been targeted for detailed structural, spectroscopic, and reactivity studies.3 To date, three types have been identified: (a) end-on, triplet Cu(II)-superoxos supported by tetradentate tripodal4 or, in one case, tridentate5 N-donor ligands, (b) side-on, singlet Cu(II)-superoxos supported by facially coordinating tris(pyrazolyl)hydroborates,6 and (c) side-on, singlet Cu(III)-peroxos supported by strongly electron-donating, bidentate β-diketiminates or anilido-imines.3,7 A key finding from reactivity studies of type (a) compounds is that they are electrophilic, with the ability to perform biologically relevant H-atom abstractions from phenols and weak C-H bonds; 4b,5 investigations of the reactivity of type (b) compounds have not been reported, and type (c) compounds are relatively unreactive with external organic substrates. Herein we report that in seeking to expand the repertoire of available 1:1 Cu/O2 structures for comparative evaluations, we have discovered an end-on Cu(II)-superoxide complex that displays unique characteristics, including a tetragonal geometry and non-electrophilic reactivity.
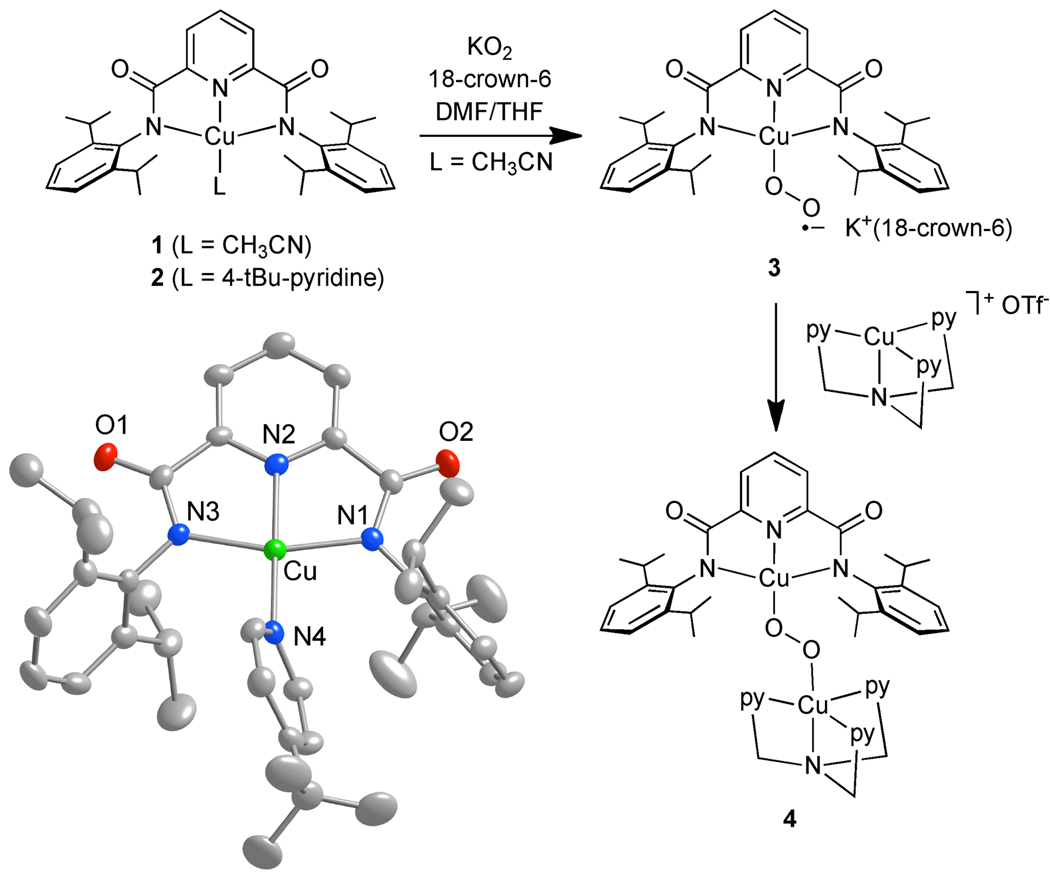
Inspired by a recent report,8 we prepared complex 1 (Scheme 1) by treating N,N′-bis(2,6-diisopropylphenyl)-2,6-pyridinedicarboxamide9 with NaOMe followed by CuCl2 in the presence of CH3CN.10 In methanol or THF solution, 1 is green, whereas it is red-brown in the presence of CH3CN or pyridine. Consistent with this solvatochromism (Figure S2), attempts to obtain crystals of 1 suitable for X-ray crystallographic analysis were complicated by apparent CH3CN lability. The addition of 4-tBu-pyridine, however, yielded X-ray quality dark red crystals of 2 (Scheme 1). The complex is square planar with a geometry similar to other known 2,6-pyridinedicarboxamide Cu(II) complexes.11
Scheme 1.
Compounds prepared in this work, and a representation of the X-ray crystal structure of 2 shown as 50% thermal ellipsoids (H atoms omitted for clarity). Selected bond distances (Å) and angles (deg): Cu-N1, 2.004(2); Cu-N2, 1.920(2); Cu-N3, 1.994(2); Cu-N4, 1.973(2); N1-Cu-N2, 80.56(10); N1-Cu-N3, 161.19(9); N1-Cu-N4, 99.57(9); N2-Cu-N3, 80.84(10); N2-Cu-N4, 173.28(10); N3-Cu-N4, 99.23(10).
Addition of 1 to a slurry of KO2 and 18-crown-6 in DMF/THF (1:1 v/v) at −80 °C immediately yielded an azure solution comprising compound 3. This species is stable at −80 °C for hours, but decomposes upon warming above −60 °C as indicated by bleaching of its UV-vis spectral features. These features are compared to those of the starting material 1 in Figure 1. Particularly notable is an absorption at 627 nm (ε ~ 1700 M−1 cm−1) with sufficient intensity to suggest that it is a charge transfer transition. Laser excitation at 647 nm yields a resonance Raman (rR) spectrum with an O-isotope sensitive peak at 1104 cm−1 (Δ18O = 60 cm−1; Figure 2a). These data support formulation of 3 as a Cu(II)-superoxo complex with ν(O-O) = 1104 cm−1, a value slightly lower than reported for other end-on Cu(II)-superoxos (1120 cm−1).4,5 Attempts at characterizing 3 by negative-ion electrospray ionization mass spectrometry have not been successful due to its thermal instability. Monitoring by EPR spectroscopy (X-band, parallel and perpendicular modes, 2 K) of a titration of 3 by KO2/18-crown-6 showed only disappearance of the axial signal for 1 (Figure S1) and appearance of the g ~ 2 signal for superoxide; complex 3 is apparently EPR silent, which is consistent with a singlet or a triplet ground state.12 1H NMR spectra of 1 and 3 in 1:1 d8-THF/d7-DMF at −80 °C only showed residual solvent peaks, with the spectrum of 3 also showing peaks in the diamagnetic region for 18-crown-6 overlapping that of CH3CN. These data rule out an S = 0 ground state formulation and are consistent with both 1 and 3 being paramagnetic species, with the absence of paramagnetically shifted peaks for both suggesting very fast relaxation leading to extensive peak broadening.
Figure 1.
(top) UV-vis spectra of compounds 1, 3, and 4 (−80 °C, DMF/THF). (middle) Resonance Raman difference spectrum (16O-18O) for compound 3 prepared from K16O2 or K18O2 (−196 °C, λex = 647 nm). (bottom) Resonance Raman spectra for compound 4 prepared from 3(16O) (black line) or 3(18O) (blue line), overlaid with spectrum of [(tmpa)2Cu2(16O2)](OTf)2 (red dotted line) (−196 °C, λex = 647 nm).
Figure 2.
Calculated structure of end-on triplet Cu(II)-superoxo complex 3. Carbon (grey), nitrogen (blue), oxygen (red), and copper (green) are shown. Hydrogens omitted for clarity.
In order to better characterize the electronic structure of 3, unrestricted DFT calculations were performed using the mPW functional and a broken-symmetry (BS) formalism for the singlet state.10 Various initial Cu(II)-superoxo complex geometries for either spin state all smoothly minimized to a structure showing end-on coordination of the superoxide to the copper center (Figure 2). The triplet state is computed to be lower in energy than the spin-purified singlet state by 6.2 kcal/mol. Previous analysis of various computational methods has suggested that the mPW functional may underestimate the energy of an end-on bound singlet state meaning that the triplet state may be even more strongly favored.13 The Cu-O distances were calculated to be 1.966 Å and 2.778 Å, with a Cu-O-O angle of 115.1°, and an O-O bond length of 1.292 Å, all consistent with superoxide character. The superoxide orients perpendicular to the plane of the N-donor ligand. A saddle point on the potential energy surface for superoxide rotation was identified with the O2 moiety in the plane of the ligand. After inclusion of zero-point energy, the minimum and saddle point structure are essentially degenerate in energy, suggesting that rotation about the Cu–O bond is effectively barrierless. A singlet structure with symmetrical η2 coordination of the superoxide was also located and identified as a transition-state structure corresponding to interconversion of one η1 superoxide species with another; the barrier on the singlet surface is 19.8 kcal/mol. Computed ν(O-O) frequencies for the end-on minima are 1182 cm−1 (Δ18O = 66 cm−1) for the triplet state and 1160 cm−1 (Δ18O = 64 cm−1) for the singlet state (for the side-on singlet TS structure, ν(O-O) is 1042 cm−1 (Δ18O = 58 cm−1)). The computed frequencies are somewhat high compared to the rR values, likely because the calculations do not include a counterion or solvation, thereby reducing the tendency for negative charge to concentrate on the O2 fragment and raising the stretching frequencies. Time dependent DFT calculations using the B98 functional10 identified a UV-vis transition at 625 nm. The orbitals involved correspond to the transition from the superoxide π* orbital to an antibonding combination of the Cu dx2-y2 and the four ligand sigma donors (Figure S8). The calculated data are collectively consistent with assigning 3 as an end-on, Cu(II)-superoxo species.
To further corroborate the formulation of 3, we sought to trap it with a Cu(I) complex to generate a (peroxo)dicopper species, a transformation known to occur in reactions of Cu(I) complexes with O2.14 Treatment of a solution of 3 with [(tmpa)Cu(CH3CN)]OTf15 at −80 °C yielded a purple solution with intense UV-vis absorptions at 624 nm (ε ~ 8300 M−1 cm−1) and a shoulder around 550 nm (4, Figure 1). The rR spectrum of this solution using 647 nm excitation showed an isotope sensitive peak at 832 cm−1 (Δ18O = 44 cm−1; Figure 1b) that we assign as the ν(O-O) for a (peroxo)dicopper complex. Taken together, the available evidence supports formulation of 4 as a (1,2-peroxo)dicopper species (Scheme 1). The similarities of the data to those reported for trans-1,2-peroxo complexes4d,16 suggest a similar geometry in 4, although the intensity pattern for the UV-vis absorptions is reversed from the typical pattern (higher energy peroxo πσ* → Cu(II) more intense than lower energy peroxo πv* → Cu(II) transition), perhaps due to distortions of the CuOOCu atoms from planarity. To confirm that the UV-vis and rR features attributed to 4 are not due to [(tmpa)2Cu2(O2)]2+, which might be envisioned to form from reaction of [(tmpa)Cu(CH3CN)]OTf with adventitious O2 or KO2, we collected UV-vis and rR data for [(tmpa)2Cu2(O2)](OTf)2 generated by purposeful addition of O2 to [(tmpa)Cu(CH3CN)]OTf in 1:1 DMF/THF at −80 °C. The pattern of UV-vis absorptions (Figure S2) and the ν(O-O) value (822 cm−1, Figure 2b) are similar to literature data16 but are different from the data obtained for 4.
Preliminary examination of the reactivity of 3 with phenols and acids revealed behavior distinct from that reported for other Cu(II)-superoxo complexes. For example, no reaction was observed upon addition of excess 4-t-butyl-, 2,4-di-t-butyl- or 2,4,6-tri-t-butyl-phenols to solutions of 3 at −80 °C, whereas other Cu(II)-superoxo complexes generate phenoxyl radicals or derived coupled products via H-atom abstraction from these substrates.4b,5 Even upon warming of the solution, no hydroxylated, coupled or other phenolic products could be identified from the reaction mixtures (GC/MS). Addition of PPh3 to a solution of 3 at −80 °C similarly yielded no change of the UV-vis spectral features and upon warming to room temperature and acidic workup, GC/MS analysis showed recovery of 78% of the PPh3 and no OPPh3. On the other hand, addition of HOAc or 4-nitrophenol to 3 resulted in immediate quenching of the features due to 3 and, for the latter case, the growth of an intense absorption at 407 nm attibutable to the 4-nitrophenoxide anion (likely complexed to Cu(II)). This was confirmed by observation of the same UV-vis spectrum upon addition of sodium 4-nitrophenoxide to a solution of 1 (Figure S3). Upon acidic workup of the product solution from the reaction of p-nitrophenol with 3, only 4-nitrophenol was identified in the reaction mixture (80% yield by GC/MS) suggesting that the Cu(II)-superoxo species was being protonated and not undergoing any radical processes. We surmise that the unique reactivity exhibited by 3 is due to the fact that it is anionic and thus more prone to act as a base or nucleophile, whereas other end-on Cu(II)-superoxo complexes are supported by neutral N-donor ligands and are therefore cationic and electrophilic.
In summary, a new type of tetragonal, anionic, end-on Cu(II)-superoxo species has been characterized by spectroscopy and theory. The reactivity of this species is significantly different from that of previously reported Cu(II)-superoxo complexes. Taken together, these results provide new insights into the chemistry of copper-oxygen species proposed to be key intermediates in oxidation catalysis.
Supplementary Material
Acknowledgment
We thank L. Que, Jr. and J. D. Lipscomb for access to the Raman and EPR spectroscopy facilities, respectively, L. Yang for collecting EPR data, and the NIH (GM47365 to W.B.T.) and NSF (CHE-0952054 to C. J. C.) for financial support of this work.
Footnotes
Supporting Information Available: Experimental procedures, spectra, computational details (PDF), and CIF. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Himes RA, Karlin KD. Curr. Opin. Chem. Biol. 2009;13:119–131. doi: 10.1016/j.cbpa.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rolff M, Tuczek F. Angew. Chem. Int. Ed. 2008;47:2344–2347. doi: 10.1002/anie.200705533. [DOI] [PubMed] [Google Scholar]; (c) Solomon E, Sarangi R, Woertink J, Augustine A, Yoon J, Ghosh S. Acc. Chem. Res. 2007;40:581–591. doi: 10.1021/ar600060t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Klinman JP. J. Biol. Chem. 2006;281:3013–3016. doi: 10.1074/jbc.R500011200. [DOI] [PubMed] [Google Scholar]; (e) Solomon EI, Sundaram UM, Machonkin TE. Chem. Rev. 1996;96:2563–2605. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 2.(a) Woertink JS, Smeets PJ, Groothaert MH, Vance MA, Sels BF, Schoonheydt RA, Solomon EI. Proc. Natl. Acad. Sci. USA. 2009;106:18908–18913. doi: 10.1073/pnas.0910461106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Que L, Jr, Tolman WB. Nature. 2008;455:333–340. doi: 10.1038/nature07371. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 3.(a) Cramer CJ, Tolman WB. Acc. Chem. Res. 2007;40:601–608. doi: 10.1021/ar700008c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Itoh S. Curr. Opin. Chem. Biol. 2006;10:115–122. doi: 10.1016/j.cbpa.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 4.(a) Würtele C, Gaoutchenova E, Harms K, Holthausen MC, Sundermeyer J, Schindler S. Angew. Chem. Int. Ed. 2006;45:3867–3869. doi: 10.1002/anie.200600351. [DOI] [PubMed] [Google Scholar]; (b) Maiti D, Lee D-H, Gaoutchenova K, Würtele C, Holthausen MC, Sarjeant AAN, Sundermeyer J, Schindler S, Karlin KD. Angew. Chem. Int. Ed. 2008;47:82–85. doi: 10.1002/anie.200704389. [DOI] [PubMed] [Google Scholar]; (c) Maiti D, Fry HC, Woertink JS, Vance MA, Solomon EI, Karlin KD. J. Am. Chem. Soc. 2007;129:264–265. doi: 10.1021/ja067411l. [DOI] [PubMed] [Google Scholar]; (d) Komiyama K, Furutachi H, Nagatomo S, Hashimoto A, Hayashi H, Fujinami S, Suzuki M, Kitagawa T. Bull. Chem. Soc. Jpn. 2004;77:59–72. [Google Scholar]; (e) Jazdzewski BA, Reynolds AM, Holland PL, Young VG, Jr, Kaderli S, Zuberbulhler AD, Tolman WB. J. Biol. Inorg. Chem. 2003;8:381–393. doi: 10.1007/s00775-002-0420-9. [DOI] [PubMed] [Google Scholar]
- 5.Kunishita A, Kubo M, Sugimoto H, Ogura T, Sato K, Takui T, Itoh S. J. Am. Chem. Soc. 2009;131:2788–2789. doi: 10.1021/ja809464e. [DOI] [PubMed] [Google Scholar]
- 6.(a) Chen P, Root DE, Campochiaro C, Fujisawa K, Solomon EI. J. Am. Chem. Soc. 2003;125:466–474. doi: 10.1021/ja020969i. [DOI] [PubMed] [Google Scholar]; (b) Fujisawa K, Tanaka M, Moro-oka Y, Kitajima N. J. Am. Chem. Soc. 1994;116:12079–12080. [Google Scholar]
- 7.(a) Aboelella NW, Lewis EA, Reynolds AM, Brennessel WW, Cramer CJ, Tolman WB. J. Am. Chem. Soc. 2002;124:10660–10661. doi: 10.1021/ja027164v. [DOI] [PubMed] [Google Scholar]; (b) Spencer DJE, Aboelella NW, Reynolds AM, Holland PL, Tolman WB. J. Am. Chem. Soc. 2002;124:2108–2809. doi: 10.1021/ja017820b. [DOI] [PubMed] [Google Scholar]; (c) Aboelella NW, Kryatov SV, Gherman BF, Brennessel WW, Victor G, Young J, Sarangi R, Rybak-Akimova EV, Hodgson KO, Hedman B, Solomon EI, Cramer CJ, Tolman WB. J. Am. Chem. Soc. 2004;126:16896–16911. doi: 10.1021/ja045678j. [DOI] [PubMed] [Google Scholar]; (d) Reynolds AM, Gherman BF, Cramer CJ, Tolman WB. Inorg. Chem. 2005;44:6989–6997. doi: 10.1021/ic050280p. [DOI] [PubMed] [Google Scholar]
- 8.Huang D, Holm RH. J. Am. Chem. Soc. 2010;132:4693–4701. doi: 10.1021/ja1003125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wasilke J-C, Wu G, Bu X, Kehr G, Erker G. Organometallics. 2005;24:4289. [Google Scholar]
- 10.For details, see Supporting Information.
- 11.Marlin DS, Olmstead MM, Mascharak PK. Inorg. Chem. 2001;40:7003–7008. doi: 10.1021/ic010523n. [DOI] [PubMed] [Google Scholar]
- 12.Lanci MP, Smirnov VV, Cramer CJ, Gauchenova EV, Sundermeyer J, Roth JP. J. Am. Chem. Soc. 2007;129:14697–14709. doi: 10.1021/ja074620c. [DOI] [PubMed] [Google Scholar]
- 13.Cramer CJ, Gour JR, Kinal A, Wtoch M, Piecuch P, Moughal Shahi AR, Gagliardi L. J. Phys. Chem. A. 2008;112:3754–3767. doi: 10.1021/jp800627e. [DOI] [PubMed] [Google Scholar]
- 14.Karlin KD, Tolman WB, Kaderli S, Zuberbühler AD. J. Mol. Catal. A. 1997;117:215–222. [Google Scholar]
- 15.Tyeklar Z, Jacobson RR, Wei N, Murthy NN, Zubieta J, Karlin KD. J. Am. Chem. Soc. 1993;115:2677–2689. [Google Scholar]
- 16.(a) Baldwin MJ, Ross PK, Pate JE, Tyeklar Z, Karlin KD, Solomon EI. J. Am. Chem. Soc. 1991;113:8671–8679. [Google Scholar]; (b) Henson MJ, Vance MA, Zhang CX, Liang H-C, Karlin KD, Solomon EI. J. Am. Chem. Soc. 2003;125:5186–5192. doi: 10.1021/ja0276366. [DOI] [PubMed] [Google Scholar]; (c) Garcia-Bosch I, Company A, Frisch JR, Torrent-Succarrat M, Cardellach M, Gamba I, Güell M, Casella L, Que L, Jr, Ribas X, Luis JM, Costas M. Angew. Chem. Int. Ed. 2010;49:2406–2049. doi: 10.1002/anie.200906749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.