Abstract
Objective:
The aim of this study is to characterize baseline regional oxygen saturations (rSO2) in stable preterm infants during the first weeks of life.
Study Design:
Cerebral, renal and abdominal rSO2 were continuously monitored from the time of birth to 21 days in twelve preterm infants of 29–34 weeks gestation. Regional saturations were evaluated for trends over time, variability and differences between gestational ages (GAs) and reported pediatric values.
Result:
Both cerebral (66–83%) and renal (64–87%) rSO2 baselines were within the range of reported neonatal values but consistently decreased over the first weeks of life (P<0.01). The baseline abdominal rSO2 was 32–66% and increased with GA (P=0.05). The rSO2 variability was lowest for cerebral measurements and highest at the abdomen. Abdominal rSO2 variability decreased over time (P⩽0.05).
Conclusion:
Daily baseline rSO2 in preterm infants changes over the first weeks of life, especially at the abdomen. Evolution in baseline rSO2 over time may indicate regional developmental maturation of physiological oxygen balance.
Keywords: regional, oximetry, mesenteric, splanchnic, near-infrared spectroscopy
Introduction
In the neonatal population, assessments of vital organ oxygen delivery and consumption are complicated by vascular shunts, vasomotor immaturity, patient size and complex cardiovascular changes during postnatal adaptation. Although traditional measures of lactic acidosis, prolonged capillary refill time, temperature instability, apnea, bradycardia, decreasing blood pressure and urine output are often readily available, they remain as indirect, nonspecific and insensitive markers of neonatal cardiovascular compromise.1
A promising solution to continuous end-organ perfusion assessment in the neonatal intensive care unit (NICU) is the current generation of spatially resolved near-infrared spectroscopy (NIRS) devices; however, peer-reviewed normative neonatal data are lacking. Although some information can be extracted from previous studies measuring regional oxygen saturation (rSO2) over short periods of time in premature infants during illness (Table 1), a comprehensive evaluation of rSO2 over time has not been carried out. Before widespread use in the NICU, characteristics of normal rSO2 in premature neonates should be established to appropriately interpret changes related to clinical pathology.5 The objective of this study was to evaluate the rSO2 baseline trends and normal physiological variability seen in healthy, stable, preterm infants over the first weeks of life. To examine this, rSO2 was continuously monitored at cerebral, renal and abdominal sites in sample of stable preterm infants from the time of birth up to 21 days.
Table 1. Neonatal rSO2 values with Somanetic NIRS systems and pediatric sensors.
| Site/source | rSO2 (%) | N | Population characteristics |
|---|---|---|---|
| Cerebral | |||
| Somanetics (Dr MD Wider, personal communication, 2008)2 | 60−80 | Adult and pediatric | |
| Petrova2 | 66±10 | 10 | >7 days old; 24–32 weeks GA; SaO2 >85% |
| Lemmers3 | 70±8 | 38 | 6–72 h old; <32 weeks GA |
| Renal | |||
| Somanetics (Dr MD Wider, personal communication, 2008) | 5−15% above cerebral | Adult and pediatric | |
| Petrova2 | 80±10 | 10 | >7 days old; 24–32 weeks GA; SaO2 >85% |
| Li4 | 40−83 | 11 | 4–92 days old; post Norwood |
| Abdominal | |||
| Somanetics (Dr MD Wider, personal communication, 2008) | 5−15% above cerebral | Adult and pediatric | |
| Vanderbilt pilot data (neonatal sensors) | |||
| Cerebral | 78±9 | 7 | 6–72 h old; >37 weeks GA |
| Renal | 88±6 | 7 | 6–72 h old; >37 weeks GA |
| Abdominal | 68±13 | 7 | 6–72 h old; >37 weeks GA |
Abbreviations: GA, gestational age; NIRS, near-infrared spectroscopy; rSO2, regional oxygen saturation; SaO2, arterial oxygen saturation.
Methods
In all, 14 stable premature infants between 24 and 36 weeks gestation admitted to the NICU at Vanderbilt University between April and September of 2008 were selected for monitoring. Patients at risk for potential problems with sensor interference and/or alterations in perfusion were excluded from analysis (Table 2).
Table 2. Exclusion criteria.
| Potential sensor interference |
| Disorders of/nonkeratinized skin |
| Hydrops/anasarca |
| Severe jaundice |
| Congenital anomaly |
| Small for gestational age |
| Potentially altered perfusion |
| Pneumatosis/Necrotising Enterocolitis (NEC)/perforation |
| Encephalopathy/seizure |
| Congenital heart disease (including clinically significant PDA) |
| Indomethacin treatment |
| Renal failure |
| 5-min Apgar <5 |
| Hypotension requiring pressors |
| Other confounding factors |
| Chromosomal abnormality |
| Inborn errors of metabolism |
The study had a prospective observational design and the institutional review board approval was obtained. After parental consent, monitoring was initiated within the first 12 h of life. rSO2 was measured using neonatal sensors, which are approved by the Food and Drug Administration for use in preterm neonates (Somanetics 5100 INVOS System, Troy, MI, USA). A piece of gel-impregnated gauze (Mepitel, Mölnlycke, Sweden) was used as a barrier between the patient and sensor at all times. Somanetics laboratories confirmed INVOS signal integrity and validity when used in conjunction with the Mepitel (MD Wilder; personal communication) (Dr MD Wider, personal communication, 2008). As the neonatal sensors were recently developed, seven term newborns were studied to ensure they returned similar rSO2 values to those achieved with the Somanetic pediatric sensors (Table 1).
Sensors were placed over the forehead for cerebral (rSO2-C), right posterior-lateral flank for renal rSO2 and infraumbilical abdomen for abdominal (rSO2-A) regional saturations. Because of the contour of the neonatal abdomen and possible confounding contribution of the liver, infraumbilical probe placement was chosen to best sample mesenteric tissue.
rSO2 was measured every 30 s for 21 days or until discharge. Sensors were removed for 6 h of skin rest every 48 h and skin integrity was inspected daily. Sensors were reused as long as a value was returned. Individual sensors lasted from 1 to 10 days. Neither signal degradation nor augmentation was noted during new sensor placement. Clinicians were blinded to saturation values.
Data were filtered and aligned by hour of life using a MatLab program (The Mathworks, Natick, MA, USA). Isolated drop-out rSO2 values with continuous data for 5 min before and after were interpolated. Sensors with correct skin contact and placement always had some degree of variability. Data with no variability were determined to be inconsistent with biological readings and periods with <5 min of continuous data were removed.
Daily mean rSO2 at each site was calculated for each individual subject and also for the group. They were then plotted against postnatal age defining individual and group rSO2 baseline values. Daily intraindividual variability was defined as the percentage of time each day that a subject's rSO2 measurements were either 15 above or below their daily mean rSO2. For each individual, every 30 s rSO2 value was compared with the subject's mean for that site for that day and then averaged. The percentage of time outside the ±15 range was calculated daily at each site for each subject and the group.
Differences in daily mean rSO2 and variability between gestational age (GA) groups (29–30 and 32–33 weeks) were assessed with the Mann–Whitney U for independent samples. Weekly changes were evaluated using Wilcoxon signed-rank test and Friedman analysis of variance for repeated measures. Change over time was also assessed by comparing the slopes of rSO2 baselines to zero. Daily mean rSO2 were compared with a range of expected values based on previously published preterm measurements (except abdominal that has no published values) and industry reported pediatric norms with single sample t-tests (cerebral 60–80, renal 65–95, abdominal 65–95)(Table 1). Hemoglobin concentration and daily weights extracted from the chart were correlated to daily mean rSO2 values with linear regression at all the three sites.
Results
Population characteristics
In all, 14 preterm infants were enrolled with two exclusions; one for receiving therapeutic indomethacin and one for prolonged nasal continuous positive airway pressure (nCPAP) spatially interfering with the cerebral measurement. Altogether, 12 subjects were included in the analysis. Six were between 29 and 30 weeks gestation and six were between 32 and 33 weeks gestation. Each GA group included one set of twins. In the 29–30 week GA group, all subjects were monitored continuously for 21 days. In the 32–33 week GA group, subjects were monitored continuously from 5 to 15 days until discharge. Prenatal and nursery characteristics are summarized in Table 3. Although absence of a PDA was not routinely confirmed by echocardiogram (ECHO), four subjects included in the analysis received ECHOs for evaluation of a murmur (one small muscular ventricular septal defect (VSD), two patent foramen ovale (PFO) and one small restrictive patent ductus arteriosus (PDA). None of these subjects had changes in clinical management.
Table 3. Maternal, neonatal and postnatal characteristics (n=12).
| Characteristic | Data |
|---|---|
| Maternal steroids, n (%) | 10 (83) |
| Pregnancy-induced hypertension, n (%) | 3 (25) |
| Preeclampsia, n (%) | 2 (17) |
| Placental abruption, n (%) | 2 (17) |
| Cesarean section, n (%) | 7 (58) |
| Chorioamnionitis, n (%) | 2 (17) |
| Gestational age, median (range) (in weeks) | 31 (29–33) |
| Birth weight, median (range) (in g) | 1640 (1278–2110) |
| Male gender, n (%) | 5 (42) |
| SNAPPE-II score, median (range) | 21 (0–34) |
| Change in hematocrit, median (range) | −8 (−22–+2) |
| Days on mechanical ventilation, median (range) | 1 (0–2) |
| Days on FiO2 >0.3, median (range) | 0 (0–2) |
| Clinical sepsis, n (%) | 3 (25) |
| DOL initial enteral feed, median (range) | 1 (1–4) |
| Days to full enteral feeds, median (range) | 10 (5–21+) |
| Number of blood transfusions n (%) | 0 (0) |
Abbreviations: DOL, day of life; SNAPPE-II, Score for Neonatal Acute Physiology Perinatal Extension, Version II.
Skin safety assessments
Skin integrity was evaluated daily as a measure of sensor safety. One infant developed a small linear bruise underneath a flank sensor. No skin concerns prompted removal of any sensors during the study period.
Regional saturation (rSO2): baselines
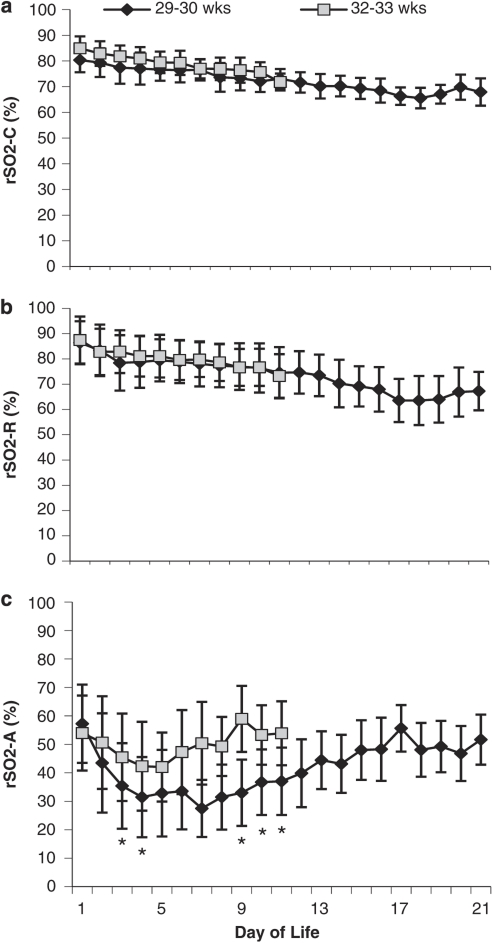
The daily mean rSO2 for preterm infants was determined at each site for each GA group plotted against time (Figure 1). The average slope of cerebral baselines was −1.01 and weekly mean rSO2-C significantly decreased over time (P<0.0001). There were no significant differences in cerebral measurements between GA groups.
Figure 1.
Daily mean rSO2±s.d. demonstrating interindividual daily variability at cerebral (a), renal (b) and abdominal (c) sites for two GA groups. n=6 for each GA group. *Significant difference from 32 to 33 weeks (P<0.05).
The average slope of renal baselines was –0.85 and weekly mean renal rSO2 significantly decreased over time (P<0.0001). There were no significant differences in renal measurements between GA groups. The relationship between cerebral and renal values within individuals was not consistent.
The abdominal rSO2 demonstrated a curvilinear baseline different from the renal and cerebral sites (Figure 1c). The median nadir was at day of life (DOL) 7 (range 3–9) for 29–30 weeks and DOL 4.5 (range 3–8) for 32–33 weeks. Individual daily mean abdominal rSO2 ranged widely from 18 to 80±11%. The older GA group had significantly higher daily means on DOL 3, 4, 9, 10, 11 and a significantly higher weekly mean in the second week of life (P⩽0.05). There were no abdominal baseline slope differences between the GA groups. The daily mean abdominal rSO2 was outside the range of industry reported normal values (P<0.001) (Dr MD Wider, personal communication, 2008).
Combined daily mean rSO2 at each site with 95% confidence intervals (CI) for DOL 1–21 are available.
Regional saturation (rSO2): variability
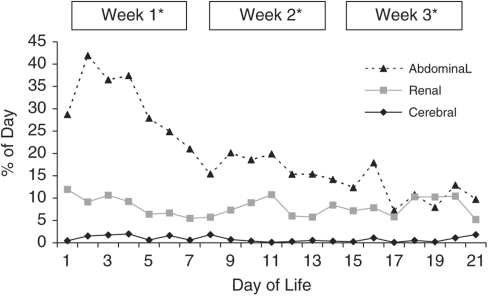
Although both cerebral and renal rSO2 showed almost 40% variability between individuals, the amount of variation within each individual throughout the day was much less. On average, cerebral rSO2 varied ±15 from the daily mean <1% of the day (95% CI 0–1.6%) and renal rSO2 8% (95% CI 4.5–10.7%). There were no differences in cerebral or renal variability between GA groups or over time (Figure 2). Abdominal daily variability was higher at 16% (95% CI 7.0–25.4), spending up to 40% of the day ±15 from the daily mean but decreased over time (P⩽0.05). There were no significant differences between renal and abdominal variability by the third week of life (P⩽0.05) (Figure 2).
Figure 2.
Mean percentage of time rSO2 measured 15 above or below an individual's daily mean at each site. Data are representative of daily intraindividual variability from baseline. *P⩽0.05; abdominal variability is significantly less each week with no significant difference between renal and abdominal means during week 3.
Influence of hemoglobin concentration and weight
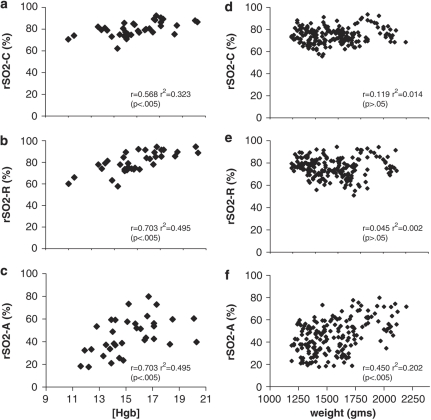
The effect of hemoglobin concentration on rSO2 in this population with evolving anemia was evaluated. All the three sites demonstrated a moderate-strength, positive correlation between rSO2 and [Hgb]. Coefficients of determination (r2) indicated that hemoglobin concentration contributed from 30 to 50% of the variation seen in rSO2 (Figures 3a–c).
Figure 3.
Correlation between hemoglobin concentration [Hgb] (g per 100 ml) and rSO2 at cerebral (a), renal (b) and abdominal (c) sites. Correlation between weight (g) and rSO2 at cerebral (d), renal (e) and abdominal (f) sites.
Large weight changes over the first weeks of life may affect rSO2 sensor depth reading. Weight had a weak positive correlation to rSO2 at the abdominal site only. The coefficient of determination that indicated 20% of variance in rSO2-A was attributable to changes in weight (Figures 3d–f).
Discussion
Despite increasing use in the NICU, little is known about neonatal rSO2 safety and normative values, including the potential effects of gestational and postnatal age.2, 6 This study confirms that continuous, long-term rSO2 monitoring of premature infants in the NICU is both safe and feasible. Our findings indicate that neonatal daily baseline rSO2 values not only change with postmenstrual age, but also decrease in variability as measured by the amount of time the rSO2 spends away from the daily mean value. These findings have important implications for using rSO2 as an indication of pathology in preterm infants during the first weeks of life. These data provide a basis from which to interpret changes in rSO2, stimulate speculation about normal transitional physiology, highlight the importance of longer rSO2 sampling periods, and introduce the use of abdominal rSO2 measurements in neonatal populations.
A valid concern for introduction of NIRS to the NICU is skin safety. To address this we used a Mepitel barrier, often used for wound care even in extremely low birth weight infants. Although this necessitated more frequent repositioning of the sensor, it enabled easy frequent removal for examination without skin disruption. Importantly, we found no adverse skin effects using this technique and believe that in conjunction with Mepitel, long-term NIRS monitoring can be safe in even the youngest GAs.
By eliminating significant pathology and monitoring over time, we attempted to isolate the normal characteristics of rSO2 unique to prematurity and postnatal age. In this circumstance, changes in the rSO2 baseline over time may represent developmental maturation of the physiological control of oxygen delivery and utilization at regional organ vascular beds. This in turn could provide new insight into physiological patterns during neonatal transition in premature infants in addition to potential early identification and management of perfusion altering disease states.
The group mean cerebral rSO2 fell within the expected range of 60–80% however, several subjects demonstrated cerebral values consistently above 80% during the first week of life before declining. The etiology of an initially higher and gradually declining rSO2-C in the early days of life is unclear. A pattern of decreasing cerebral rSO2 contrasts the increase in cerebral artery blood flow described for this time period.7, 8 These novel data suggest a gradual increase in cerebral oxygen demand even during a physiological rise in oxygen delivery through increased blood flow. This contradicts previously published findings in which Naulaers et al.9 found that increased cerebral oxygenation values over the first 3 days of life presumably attributed to increased blood flow. This contradiction may be explained as the Naulaers study sampled infants over 30 min in contrast to our more robust 24 h measurement. In addition, Naulaers used tissue oxygenation index (a different NIRS technology), which has not been equivalent to rSO2.10 In contrast, Lemmers et al.3 did not see any change in cerebral oxygenation over DOL 1–3. The important difference here includes a shorter sampling period of 60 min twice daily. Such conflicting data highlights the importance of comparing similar technologies in this field and potential downfalls of shorter sampling periods, which may underestimate the degree of inherent variability.
The group mean renal rSO2 also fell within the expected range (65–95), but did not always maintain the same relationship to the cerebral measurement. Renal rSO2 was higher than cerebral for a majority of the days monitored in 10 out of 12 subjects, but did not reach significance. We speculate that these differences in cerebro-renal rSO2 relationship may be related to intermittent ductal influences on cerebro-renal blood flow. Similar to the cerebral site, decreasing rSO2 contrasts the increase in renal artery blood flow typical of this time period and may reflect increased renal oxygen consumption outweighing delivery.7, 11, 12
The unique abdominal rSO2 pattern suggests regional changes in oxygen balance not mirrored at the cerebral or renal sites. As Doppler studies indicate a progressive increase in superior mesenteric artery flow over the first days to weeks of life,7, 13 the rSO2-A pattern suggests a change in intestinal oxygen consumption around DOL 3–9. A net increase in intestinal oxygen utilization over the first days of life may switch to a net increase in oxygen delivery. This could reflect physiological compensations for the increasing metabolic demand of advancing enteral feeds.
Abdominal rSO2 was also the only site with differences between GA groups. Although not reaching significance, the younger GA group reached an rSO2-A nadir later and deeper than the 32–33 week group. Although these differences may be related to feeding schedules, the pattern suggests a possible GA-dependent maturation in the ability of the mesenteric vasculature to provide for the increasing metabolic activity of the intestinal tract. Although cyclic changes in rSO2-A were noted in association with feeds, in-depth analysis of such responses were beyond the scope of this paper.
Potential concerns with abdominal rSO2 measurement involve the increased variability in values and the related uncertainty of what tissue is being sampled. As the intestine is a hollow, peristalsing organ, the specific tissue sampled may change frequently despite static sensor placement. Measurement fluctuations are therefore expected to be greater and may represent saturation differences between enteral mucosa, smooth muscle and/or enteric contents. In addition, with infraumbilical placement, there is potential for intestinal displacement from bladder distension; however, in our experience, infants with urinary catheters demonstrate similar variability at the abdominal site. Although infraumbilical placement may inadvertently sample posterior pelvic or abdominal wall muscles in the smallest infants, supraumbilical sensor placement may sample the liver, stomach and spleen in the neonate. In our experience, supraumbilical placement returns rSO2 values very similar to the renal site, and different from infraumbilical. Lateral abdominal placement presents difficulty in maintaining adequate skin contact due to the contour of the abdomen.
In our experience, because of the large variability of the abdominal signal, we recommend caution in interpreting low absolute abdominal rSO2 values in young preterm infants. Many of our subjects registered rSO2-A at 15% for long periods (hours) while tolerating feeds with good sensor pick-up and appropriate sensor positioning.
The promising benefit of rSO2 is the potential for real time assessments of change in regional tissue oxygen balance. The significance of such a change depends on the inherent variability of that measurement. Many pediatric intensive care units and operating rooms use an acute drop in cerebral rSO2 from baseline (10–20% change), or an absolute rSO2-C value below 50% as a trigger for intervention. These numbers have been extrapolated from both animal and human data correlating rSO2 with structural ischemia.14, 15, 16, 17 Without knowing the true ischemic rSO2 thresholds at each site in neonates, we opted to assign a change of 15 from the daily mean (baseline) as an indicator of possible clinical change and evaluated how often the rSO2 hit that mark over the course of a day in a stable patient. We defined this as rSO2 variability.
The cerebral rSO2 remained fairly constant, spending <1% of the day on an average outside the ±15 range, thus by making changes from baseline values potentially meaningful as indicators of clinical change. The renal and abdominal rSO2 were more variable, spending ∼10% of the day ±15 from the daily mean thereby making changes more difficult to interpret as pathologic, especially in the first 2 weeks of life. This variability highlights the importance of establishing an individual's baseline over an adequate amount of time and suggests a greater role in long-term monitoring at the renal and abdominal sites.
A potential confounding factor to interpret rSO2 in the premature infant is the presence of anemia. Preterms are prone to iatrogenic and/or physiological anemia, and hemoglobin concentration is an important contributor to oxygen delivery that weakly correlates with rSO2 in adults.18 Similarly, the natural decline in fetal hemoglobin may affect rSO2. We found only moderate correlations between rSO2 and hemoglobin concentration explaining 30–50% of the changes seen. The contribution of fetal hemoglobin is more difficult to evaluate, but studies suggest that the switch to hemoglobin A is likely delayed beyond our period of observation in this GA group.19 Although anemia appears to contribute to rSO2 variability, it does so only to a minor degree.
Weaknesses of this study include small numbers and subject attrition in the third week for the older GA group. In addition, slight differences in sensor repositioning could have introduced some variability.
Reflecting changes in the balance between oxygen consumption and delivery, these neonatal rSO2 patterns suggest the presence of a longer transitional period to ex-utero steady state than perhaps traditionally recognized. Future studies should identify time to steady state baseline for all sites and further define abdominal characteristics as the field moves toward detecting and managing pathological physiology using regional oximetry technology in the NICU.
Conclusion
This paper characterizes for the first time normal rSO2 patterns in a sample of stable premature infants using the Somanetics NIRS system. We have shown that baseline rSO2 in preterm infants change during the first weeks of life, change with postnatal age and may be GA dependent at the abdomen. Such characteristics are important to recognize and establish in moving toward interpretation of rSO2 in the NICU.
Acknowledgments
We thank the NICU staff at Vanderbilt University and the Monroe Carell Jr Children's Hospital at Vanderbilt for their support.
Dr McNeill used NIRS equipment donated by Somanetics Corp. to complete this research. Dr Engelhardt is a member of an unsalaried neonatal research panel working with Somanetics Corporation. Dr Gatenby and Dr McElroy declare no conflict of interest.
References
- Noori S, Seri I.Etiology, pathophysiology, and phases of neonatal shockIn: Kleinman CS, Seri I, Polin RA (eds).Hemodynamics and Cardiology: Neonatology Questions and Controversies Saunders: Philadelphia; 20083–18. [Google Scholar]
- Petrova A, Mehta R. Near-infrared spectroscopy in the detection of regional tissue oxygenation during hypoxic events in preterm infants undergoing critical care. Pediatr Crit Care Med. 2006;7:449–454. doi: 10.1097/01.PCC.0000235248.70482.14. [DOI] [PubMed] [Google Scholar]
- Lemmers PMA, Toet M, van Schelven LJ, van Bel F. Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: the impact of respiratory distress syndrome. Exp Brain Res. 2006;173:458–467. doi: 10.1007/s00221-006-0388-8. [DOI] [PubMed] [Google Scholar]
- Li J, Van Arsdell GS, Zhang G, Cai S, Humpl T, Caldarone CA, et al. Assessment of the relationship between cerebral and splanchnic oxygen saturations measured by near-infrared spectroscopy and direct measurements of systemic haemodynamic variables and oxygen transport after the Norwood procedure. Heart. 2006;92:1678–1685. doi: 10.1136/hrt.2005.087270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toet MC, Lemmers PMA. Brain monitoring in neonates. Early Hum Dev. 2009;85:77–84. doi: 10.1016/j.earlhumdev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Van Bel F, Lemmers P, Naulaers G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfall. Neonatology. 2008;94:237–244. doi: 10.1159/000151642. [DOI] [PubMed] [Google Scholar]
- Ilves P, Lintrop M, Talvik I, Muug K, Asser K, Veinla M. Developmental changes in cerebral and visceral blood flow velocity in healthy neonates and infants. J Ultrasound Med. 2008;27:199–207. doi: 10.7863/jum.2008.27.2.199. [DOI] [PubMed] [Google Scholar]
- Romagnoli C, Giannantonio C, Pia De Carolis M, Gallini F, Zecca E, Papacci P. Neonatal color Doppler US study: normal values of cerebral blood flow velocities in preterm infants in the first month of life. Ultrasound Med Biol. 2006;32:321–331. doi: 10.1016/j.ultrasmedbio.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Naulaers G, Morren G, Van Huffel S, Casaer P, Devliegar H. Cerebral tissue oxygenation index in very premature infants. Arch Dis Child Fetal Neonatal Ed. 2002;87:F189–F192. doi: 10.1136/fn.87.3.F189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitani K, Kawaguchi M, Tatsumi K, Kitaguchi K, Furuya H. A comparison of the INVOS 4100 and the NIRO 300 near-infrared spectrophotometers. Anesth Analg. 2002;94:586–590. doi: 10.1097/00000539-200203000-00020. [DOI] [PubMed] [Google Scholar]
- Cleary GM, Higgins ST, Merton DA, Cullen JA, Gottlieb RP, Baumgart S. Developmental changes in renal artery blood flow velocity during the first three weeks of life in preterm neonates. J Pediatr. 1996;129:251–257. doi: 10.1016/s0022-3476(96)70250-0. [DOI] [PubMed] [Google Scholar]
- Pezzati M, Danesi G, Pozzessere A, Cosenza EB, Rubaltelli FF. Renal blood flow velocity in preterm and term neonates during the fourth day of life: changes in relation to gestational age and birth weight. Biol Neonate. 1998;73:19–23. doi: 10.1159/000013955. [DOI] [PubMed] [Google Scholar]
- Martinussen M, Brubakk A-M, Linker DT, Vik T, Yao AC. Mesenteric blood flow velocity and its relation to circulatory adaptation during the first week of life in healthy term infants. Pediatr Res. 1994;36:334–339. doi: 10.1203/00006450-199409000-00011. [DOI] [PubMed] [Google Scholar]
- Hou X, Ding H, Teng Y, Zhou C, Tang X, Ding H. Research on the relationship between brain anoxia at different regional oxygen saturations and brain damage using near-infrared spectroscopy. Physiol Measures. 2007;28:1251–1265. doi: 10.1088/0967-3334/28/10/010. [DOI] [PubMed] [Google Scholar]
- Kurth CD, Levy WJ, McCann J. Near-infrared spectroscopy cerebral oxygen saturation thresholds for hypoxia-ischemia in piglets. J Cerebral Blood Flow and Metab. 2002;22:335–341. doi: 10.1097/00004647-200203000-00011. [DOI] [PubMed] [Google Scholar]
- Dent CL, Spaeth JP, Jones BV, Schwartz SM, Glauser TA, Hallinan B, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006;131:190–197. doi: 10.1016/j.jtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Steinhorn DM, Kane JM. Lack of irrefutable validation does not negate clinical utility of near-infrared spectroscopy monitoring: learning to trust new technology. J Crit Care. 2008;24 (3:472.e1–477.e1. doi: 10.1016/j.jcrc.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Kishi K, Kawaguchi M, Yoshitani K, Nagahata T, Furuya H. Influence of patient variables and sensor location on regional cerebral oxygen saturation measured by INVOS 4100 near-infrared spechtrophotometers. J Neurosurg Anesthesiol. 2003;15:302–306. doi: 10.1097/00008506-200310000-00002. [DOI] [PubMed] [Google Scholar]
- Moriyama I, Hino K, Ninomiya Y, Nabuchi K, Kato Y, Tsuji Y, et al. The problem of hemoglobin switching in premature infants and IUGR infants. Asia Oceania J Obstet Gynaecol. 19898;15:87–92. doi: 10.1111/j.1447-0756.1989.tb00158.x. [DOI] [PubMed] [Google Scholar]