Graphical abstract
Keywords: Inhibition, Model compound, Transition state, Silanol, Toxicity, Silicone
Abstract
Three stable silanetriols with increasing steric protection of the silicon atom have been tested for inhibition of acetylcholinesterase (AChE). For all tested silanetriols we found reversible inhibition of the AChE activity at a 100 μM concentration. The highest inhibition rate was found for the sterically least hindered cyclohexylsilanetriol with 45% inhibition relative to galanthamine hydrobromide for which an IC50 value of 121 ± 3 μM was determined as well. The cytotoxicity of the silanetriols used was found to be negligible at concentrations relevant for inhibition.
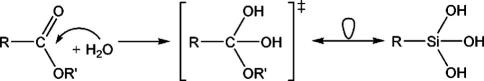
The dominance of the element carbon in the chemistry of the living world contrasting the prevalence of silicon in the inanimate lithosphere has inspired chemists to search for bioactive silicon compounds. One general access to bioactive silicon compounds is the replacement of selected carbon atoms with silicon in known pharmaceuticals which is also referred to as carbon/silicon switch strategy.1,2 Siladrugs, pro-drugs and odorants have been developed following this approach.1–6 Recently, also silicon compounds mimicking transition states occurring in the metabolism of organic substrates have been very successfully employed for the inhibition of hydrolytic enzymes.7–13 Interestingly, the silanediols (R2Si(OH)2) used for this purpose normally have no stable organic counterpart (R2C(OH)2) according to Erlenmeyers rule.14,15 Therefore they are touching the border between bioactive silicon compounds derived from organic lead structures and those for which there are no carbon analogs like, for example, silatranes16–19 and the phtalocyanine Pc4.20,21
Inspired by the work of Sieburth and co-workers concerned with silanediols, it seems obvious that silanetriols (R–Si(OH)3), in which even more silanol functions are attached to the same silicon atom, could be a mimic for hydrated transition states occurring in the hydrolytic cleavage of carboxylic acid derivatives (Scheme 1). In an earlier report the functional group –Si(OH)3 has already been tested for inhibition of a serine hydrolase but no significant activity had been observed.22 One issue raised by the authors conducting this study was the instability of the specific silanetriol under investigation toward condensation, which might be the actual reason for its poor activity. Based on our own experience with the chemistry of silanetriols,23–27 we wondered whether an improved stability of the silanetriol might also give rise to its inhibitory activity. Consequently, we investigated the activity of some stable silanetriols (R–Si(OH)3, R = i-pentyl, c-hexyl, 2,6-dimesitylphenyl) as novel inhibitors for the hydrolytic enzyme acetylcholinesterase (AChE). There is general interest in choline esterase inhibitors because they may be used in the treatment of Alzheimer’s disease.28
Scheme 1.
Isolobal resemblance of a silanetriol with the tetrahedral hydrolysis intermediate of a carboxylic ester.
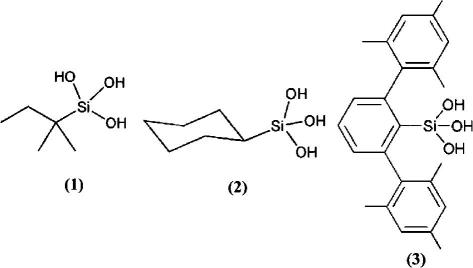
Three simple silanetriols (R–Si(OH)3, R = i-pentyl (1),29 c-hexyl (2), 2,6-dimesitylphenyl (3),23 Scheme 2) differing in their steric environment and flexibility around the functional group have been tested for their inhibitory activity using a modified version of the colorimetric method of Ellman.30 The enzyme acetylcholinesterase hydrolyzes the substrate acetylthiocholine with formation of thiocholine. The thiocholine reacts with Ellman’s reagent (5,5′-dithio-bis(2-nitrobenzoic acid; DTNB) to produce 2-nitrobenzoate-5-mercaptothiocholine and 5-thio-2-nitrobenzoate which can be detected photometrically at 405 nm.
Scheme 2.
Chemical structures of silanetriols 1–3 used in this inhibition study.
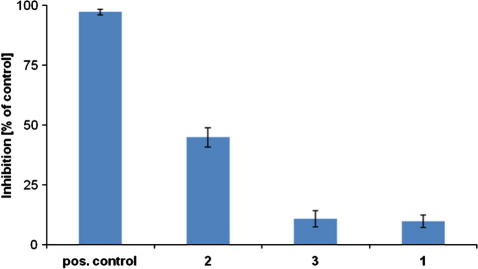
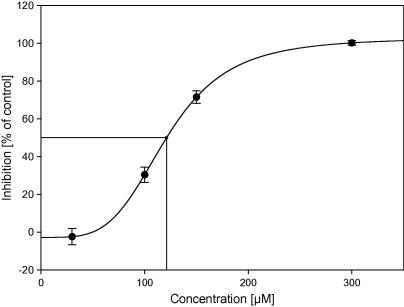
For all tested silanetriols 1–3 we found inhibition of the AChE activity (Fig. 1). The highest inhibition rate was observed for cyclohexyl substituted silanetriol (2) with 45% relative inhibition (100 μM). Significantly lower inhibition rates have been observed for 1 and 3 with 10% and 12% relative inhibition, respectively. Compound 2 as the most active in this series was used for a determination of an IC50 value. With respect to the highest activity after 15 min (vi) we have chosen this point for the determination of the IC50 value. Four different concentrations ranging from 30 to 300 μM were used for establishing a dose response curve and results have been evaluated with the sigmaplot program package. The sigmoidal 4-parameter logistics curve was used for data fitting yielding an IC50 value of 121 ± 3 μM for silanetriol 2. A graphical representation of the IC50 curve clearly showing a dose dependent inhibitory effect is illustrated in Figure 2.
Figure 1.
Comparison of the inhibitory activity of 1–3 in % after 15 min in relation to galanthamine hydrobromide as positive control. Compounds and control were tested at a final concentration of 100 μM. Data are expressed as % of solvent control as mean values ± SEM of at least eight independent experiments.
Figure 2.
Graphical representation of the IC50 determination of the inhibition on acetylcholinesterase of 2. Four different concentrations (ranging from 30 to 300 μM) were tested. Data are represented as inhibition in % of solvent control and are expressed as mean values ± standard deviation of at least eight independent experiments.
Compounds 1–3 are members of a simple yet pharmacologically completely unexplored substance class. Therefore the obtained inhibition values ranging from 10% to 45% are quite promising compared to the positive control galanthamine hydrobromide which was tested at the same concentration (100 μM). Galanthamine is an alkaloid which occurs naturally in various plant species of Galanthus and Narcissus (Amaryllidaceae family). It is a known reversible inhibitor of cholinesterase activity.31 From their chemical structure and their synthetic accessibility and variability silanetriols 1–3 are extremely simple compared to galanthamine. The significantly lower inhibition values for 1 and 3 are particularly interesting since in this study these two silanetriols are the more sterically shielded ones while 2 is the least hindered congener. Consequently there should be an optimum size for the substituent adjacent to the –Si(OH)3 group which renders steric protection to the functional group against condensation, yet leaves room to access the active site of the enzyme.
An important aspect of inhibition of the AChE activity is the reversibility of the inhibition. Certain inhibitors like phosphoric acid derivatives formerly used as insecticides or chemical warfare agents may lead to an irreversible phosphorylation of the serine OH group of the active site of AChE and eventually to the destruction of the enzyme.32–35
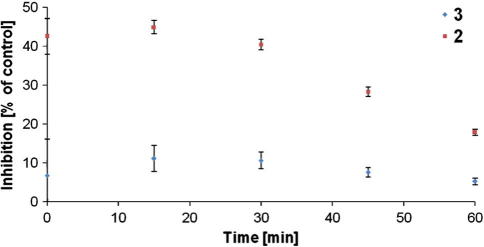
Therefore, we also investigated the time dependence of the inhibition activity (Fig. 3). As it turns out the inhibition of AChE by silanetriols 1–3 is not irreversible. As can be seen in Figure 3 for 2 and 3, there is a continuous decay of the enzyme inhibition with time which rules out an irreversible silylation of the serine OH group as was suggested for a related serine hydrolase.22 The reversible character of this inhibition during which the enzyme remains intact is also an important prerequisite for a potential use of silanetriols as a therapeutic drug.
Figure 3.
Time dependent inhibitory effect on acetylcholinesterase activity for 2 and 3. Activity is referred to solvent control and data are shown as mean values ± SEM of at least eight independent experiments.
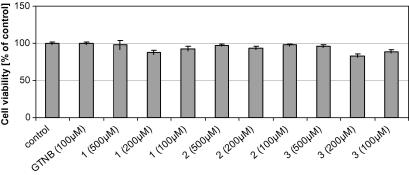
To analyze, whether the tested silanetriols 1–3 show a nonspecific (re)activity in human cells—associated with cytotoxicity—we performed an XTT proliferation assay to investigate the cell viability after treatment with the respective compounds. The human fetal lung fibroblast cell line (MRC-5) was used because it is especially suited for preliminary estimates of cytotoxicity for humans. At three different points in time (1, 15 and 41 h) cell viability was examined by adding XTT solution to measure the metabolic activity of viable cells. This XTT cell proliferation assay is based on the cleavage of the yellow 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide inner salt (XTT) by ubiquitous dehydrogenases leading to the formation of an orange formazan dye. The amount of dye is proportional to the number of metabolic active cells and can be quantified photometrically by differential measurement at two wavelengths. No significant cytotoxicity of compounds 1–3 was found for this human cell line, even after incubation for almost 45 h (data not shown). The cell viability of all tested compounds after 15 h incubation time is displayed in Figure 4.
Figure 4.
Cell viability of MRC-5 cells measured by using XTT proliferation assay after incubation with different concentrations of the respective compounds or control for 15 h. Conversion into the measured dye is allowed to proceed for 4 h. Viability is referred to cells treated with solvent solely (control). Data are shown as mean values ± SEM of at least six independent experiments.
In summary, three stable silanetriols 1–3 with increasing steric protection of the silicon atom have been tested for inhibition of acetylcholinesterase (AChE). For all tested silanetriols we found inhibition of the AChE activity at a 100 μM concentration. The highest inhibition rate was found for the sterically least hindered (2) with 45% inhibition in relation to galanthamine hydrobromide for which an IC50 value of 121 ± 3 μM was determined. The cytotoxicity of these compounds at the inhibition concentration was assessed using a human fetal lung fibroblast cell line and was negligible even at substantially higher silanetriol concentrations. Apart from their potential use in pharmaceutical therapy, the activity of silanetriols in the inhibition of hydrolytic enzymes is generally relevant because they may occur as metabolites of cyclic siloxanes like D4 in mammals.36 Although such cyclic siloxanes are widely used in personal care products etc. detrimental effects of their metabolites are not likely owing to the extremely low concentrations that can be expected, based on a recent assessment of the maximum plausible daily exposure of the population in Canada to cyclic siloxanes.37,38 According to the latter report, the concentration of silanetriol metabolites can be expected to be by orders of magnitude lower than the final concentrations of 1–3 used in our inhibition experiments which were 100 μM. Furthermore, the reversible binding to the active site of the enzyme will prevent irreversible enzyme damage. In future work we will aim to improve the inhibitory effect by structural variation of the adjacent organic substituent of the silanetriol.
Acknowledgment
The authors would like to thank the Austrian Science Fund (FWF) for financial support (Grants P17882-N11 and P20575-N19).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2010.10.139.
Supplementary data
Experimental details.
References and notes
- 1.Tacke R., Metz S. Chem. Biodivers. 2008;5:920. doi: 10.1002/cbdv.200890105. [DOI] [PubMed] [Google Scholar]
- 2.Tacke R., Becker B. Main Group Met. Chem. 1987;10:169. [Google Scholar]
- 3.Bains W., Tacke R. Curr. Opin. Drug Discovery Dev. 2003;6:526. [PubMed] [Google Scholar]
- 4.Tacke R. Angew. Chem., Int. Ed. 1999;38:3015. [PubMed] [Google Scholar]
- 5.Tacke R., Linoh H. In: Patai S., Rappoport Z., editors. Vol. 2. 1989. p. 1143. (Chemistry of Organic Silicon Compounds). [Google Scholar]
- 6.Tacke R., Wannagat U. Top. Curr. Chem. 1979;84:1. doi: 10.1007/BFb0048522. [DOI] [PubMed] [Google Scholar]
- 7.Sieburth S.M., Nittoli T., Mutahi A.M., Guo L. Angew. Chem., Int. Ed. 1998;37:812. doi: 10.1002/(SICI)1521-3773(19980403)37:6<812::AID-ANIE812>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Chen C.-A., Sieburth S.M., Glekas A., Hewitt G.W., Trainor G.L., Erickson-Viitanen S., Garber S.S., Cordova B., Jeffry S., Klabe R.M. Chem. Biol. 2001;8:1161. doi: 10.1016/s1074-5521(01)00079-5. [DOI] [PubMed] [Google Scholar]
- 9.Kim J., Glekas A., Sieburth S.M. Bioorg. Med. Chem. Lett. 2002;12:3625. doi: 10.1016/s0960-894x(02)00804-1. [DOI] [PubMed] [Google Scholar]
- 10.Kim J., Sieburth S.M. J. Org. Chem. 2004;69:3008. doi: 10.1021/jo049929i. [DOI] [PubMed] [Google Scholar]
- 11.Kim J., Sieburth S.M. Bioorg. Med. Chem. Lett. 2004;14:2853. doi: 10.1016/j.bmcl.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Juers D.H., Kim J., Matthews B.W., Sieburth S.M. Biochemistry. 2005;44:16524. doi: 10.1021/bi051346v. [DOI] [PubMed] [Google Scholar]
- 13.Sieburth S.M., Chen C.-A. Eur. J. Org. Chem. 2006;2:311. [Google Scholar]
- 14.Erlenmeyer E. Lehrbuch der Organischen Chemie. Leipzig u Heidelberg; C. F. Winters Verlagsbuchhandlung: 1867. [Google Scholar]
- 15.Exceptions with neighboring electron withdrawing groups like: chloral hydrate, ninhydrine, etc.
- 16.Frye C.L., Vogel G.E., Hall J.A. J. Am. Chem. Soc. 1961;83:996. [Google Scholar]
- 17.Bien E. Pharmazie. 1971;26:224. [PubMed] [Google Scholar]
- 18.Voronkov M.G. Pure Appl. Chem. 1966;13:35. doi: 10.1351/pac196919030399. [DOI] [PubMed] [Google Scholar]
- 19.Voronkov M.G. Top. Curr. Chem. 1979;84:77. doi: 10.1007/BFb0048523. [DOI] [PubMed] [Google Scholar]
- 20.Anderson C.Y., Freye K., Tubesing K.A., Li Y.-S., Kenney M.E., Mukhtar H., Elmets C.A. Photochem. Photobiol. 1998;67:332. [PubMed] [Google Scholar]
- 21.Miller J.D., Baron E.D., Scull H., Hsia A., Berlin J.C., McCormick T., Colussi V., Kenney M.E., Cooper K.D., Oleinick N.L. Toxicol. Appl. Pharmacol. 2007;224:290. doi: 10.1016/j.taap.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratt R.F., Curley K. J. Am. Chem. Soc. 1997;119:1529. [Google Scholar]
- 23.Pietschnig R., Belaj F., Tirrée J.J. Organometallics. 2004;23:4897. [Google Scholar]
- 24.Pietschnig R., Merz K. Organometallics. 2004;23:1373. [Google Scholar]
- 25.Pietschnig R., Belaj F. Inorg. Chim. Acta. 2005;358:444. doi: 10.1016/j.ica.2011.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spirk S., Nieger M., Belaj F., Pietschnig R. Dalton Trans. 2009:163. doi: 10.1039/b812974f. [DOI] [PubMed] [Google Scholar]
- 27.Spirk S., Belaj F., Baumgartner J., Pietschnig R.Z. Anorg. Allg. Chem. 2009;635:1048. [Google Scholar]
- 28.Lane R.M., Kivipelto M., Greig N.H. Clin. Neuropharmacol. 2004;27:141. doi: 10.1097/00002826-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Analytical data for 1: 1H (D2O): 1.36 (q, 2H, 3JHH = 7.5 Hz), 0.93 (s, 6H), 0.87 (t, 3H, 3JHH = 7.5 Hz). 13C (D2O): 31.37 (CH2), 22.24 (CH3), 20.56 (CH3), 8.61 (Cq). 29Si (D2O): −42.2 (s). IR(KBr): 3411 m, 2960 w, 1470 w, 1087 w, 900 s, 860 m, 818 w cm−1. MS (EI): 150.1 (M+), 79.0, 70.1, 63.0, 55.1, 43.1, 36.0, 27.1. Elemental Anal. Calcd for C5H14O3Si: C, 39.97; H, 9.39. Found: C, 40.30; H, 9.37.
- 30.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. Biochem. Pharmacol. 1961;7:88. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 31.Ingkaninan K., de Best C.M., van der Heijden R., Hofte A.J.P., Karabatak B., Irth H., Tjaden U.R., van der Greef J., Verpoorte R. J. Chromatogr., A. 2000;872:61. doi: 10.1016/s0021-9673(99)01292-3. [DOI] [PubMed] [Google Scholar]
- 32.Sidell F.R., Borak J. Ann. Emerg. Med. 1992;21:865. doi: 10.1016/s0196-0644(05)81036-4. [DOI] [PubMed] [Google Scholar]
- 33.Eyer F., Meischner V., Kiderlen D., Thiermann H., Worek F., Haberkorn M., Felgenhauer N., Zilker T., Eyer P. Toxicol. Rev. 2003;22:143. doi: 10.2165/00139709-200322030-00003. [DOI] [PubMed] [Google Scholar]
- 34.Kovach I.M. J. Phys. Org. Chem. 2004;17:602. [Google Scholar]
- 35.Eckert S., Eyer P., Mueckter H., Worek F. Biochem. Pharmacol. 2006;72:344. doi: 10.1016/j.bcp.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Varaprath S., Salyers K.L., Plotzke K.P., Nanavati S. Drug Metab. Dispos. 1999;27:1267. [PubMed] [Google Scholar]
- 37.Publication after screening assessment of a substance—octamethylcyclotetrasiloxane (D4), CAS No. 556-67-2—Specified on the Domestic Substances List [subsection 77(1) of the Canadian Environmental Protection Act, 1999]; Environment Canada, 2008.
- 38.Enei G., Kenny M., Lloyd K. Can. Gaz. Part I. 2008;142:1510. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details.