Abstract
The major histocompatibility complex (MHC) is an extraordinarily diverse cluster of genes that play a key role in the immune system. MHC gene products are also found in various body secretions, leading to the suggestion that MHC genotypes are linked to unique individual odourtypes that animals use to assess the suitability of other individuals as potential mates or social partners. We investigated the relationship between chemical odour profiles and genotype in a large, naturally reproducing population of mandrills, using gas chromatography–mass spectrometry and MHC genotyping. Odour profiles were not linked to the possession of particular MHC supertypes. Sex influenced some measures of odour diversity and dominance rank influenced some measures of odour diversity in males, but not in females. Odour similarity was strongly related to similarity at the MHC, and, in some cases, to pedigree relatedness. Our results suggest that odour provides both a cue of individual genetic quality and information against which the receiver can compare its own genotype to assess genetic similarity. These findings provide a potential mechanism underlying mate choice for genetic diversity and MHC similarity as well as kin selection.
Keywords: semiochemicals, olfaction, MHC odourtype, mate choice, honest signalling, kin recognition
1. Introduction
The major histocompatibility complex (MHC) is an extraordinarily diverse cluster of genes that play a critical role in the immune system [1]. MHC genes are also linked to key reproductive and social behaviours, including mate choice and kin selection [2–5]. Owing to its role in the immune system, mate choice for MHC genotype may offer several, non-exclusive fitness advantages, by providing offspring with genes that are associated with immunity to particular pathogens [6] or increased (or optimal) MHC diversity and thus disease resistance, or by providing a ‘moving target’ against infection by rapidly evolving parasites [7,8]. For example, choice for MHC-disparate partners or those with an optimal set of MHC dissimilar alleles has been reported in taxa as diverse as birds, fishes and lizards [9–11], as well as non-human primates [12]. The question of whether our own species' mate choice is related to MHC has been a source of controversy, but recent reviews have concluded that the mixed results may reflect context-dependent preference expression [13,14].
Mate choice for MHC dissimilarity may also allow animals to avoid inbreeding, by increasing genome-wide genetic diversity as well as MHC diversity in offspring [7,8]. While animals should avoid mating with close relatives, they should bias their social behaviour towards them [15]. Kin selection requires that individuals be able to discriminate between conspecifics with different degrees of genetic relatedness. This discrimination may be based on familiarity, but kin may also be unfamiliar. For example, maternal kin in Cercopithecine primates are raised in close matrilineal groups, and are familiar with one another from birth. However, paternal kin may be equally related to one another [16], but are raised in different matrilines, and are thus unfamiliar. Nevertheless, paternal kin bias positive social behaviour towards one another relative to unrelated individuals [17]. Evidence from mice suggests that MHC may be involved in such kin discrimination. Female mice prefer to nest with partners that share MHC alleles [18] and retrieve pups that are genetically identical to themselves in preference to those differing only at the MHC [19].
This evidence for a key role for MHC in behaviours linked to reproductive success begs the question of how animals are able to recognize and assess the MHC genotype of conspecifics. One possible answer lies in the fact that MHC gene products are also found in bodily secretions. This observation, in combination with the extreme variability of MHC genes, led to the hypothesis that MHC genotypes are linked to unique individual odourtypes—complex mixtures of volatile chemicals present in secretions [20]. Although MHC odourtypes may not be the only signals of olfactory individuality, which may also be mediated via various other polymorphic gene products in odour, including non-volatile MHC class I peptides [2,21], volatile odortypes alone are sufficient to convey MHC haplotype information in mice [22,23]. Furthermore, choice experiments have shown that many species, including humans, are able to differentiate between the odour of individuals on the basis of MHC genotype (reviews in Beauchamp & Yamazaki [2] and Penn [7]). However, these behavioural assays shed little light on the link between MHC genotype and odourtype.
If odour signals MHC genotype, then individuals should possess stable odourtypes and MHC similarity should be reflected in similarity of odour profiles. Studies investigating this relationship have focused on mouse urine and suggest that individual odourtypes are the product of a complicated interaction between MHC and other genes, with numerous compounds expressed differentially according to the MHC genotype (e.g. [24–26]). Volatile components of human urine are also associated with the MHC genotype [27]. Related, or genetically similar, individuals are likely to possess similar MHC genotypes, and a relationship between kinship or overall genetic distance and similarity in odour profile has been reported for some vertebrate species [28–32]. Crucially, however, no study, to our knowledge, has yet combined the investigation of MHC similarity, overall relatedness and odour profiles in a non-model species.
Here, we integrate genetic and chemical data to investigate the relationship between MHC genotype, pedigree relatedness and odour profiles in a large, naturally reproducing population of mandrills (Mandrillus sphinx, Cercopithecinae). Both male and female mandrills possess a sternal gland, unlike most Old World monkeys. Volatile odour profiles of the sternal gland secretion differ according to both variable (age, dominance rank in males) and fixed (sex, possibly individual identity) features of the signaller [33]. MHC-diverse males have higher reproductive success, and mandrills reproduce preferentially with MHC dissimilar individuals [34]. One possible mechanism underlying these patterns is pre-copulatory mate selection based on MHC genotype. Moreover, mandrills are able to discriminate paternal kin from non-kin [35], despite their polygynandrous mating system. If more closely related individuals, which are more similar at the MHC, possess more similar odour phenotypes, then they may identify these unfamiliar relatives by comparing their odour with self or known relatives (‘phenotype matching’; [17]).
We examined the hypotheses that odour signals MHC genotype, and that odour similarity reflects genetic similarity, testing the following predictions:
— odour profiles differentiate reliably between animals possessing particular MHC genotypes,
— individual odour diversity is related to MHC diversity. Because this is the first investigation of odour profile diversity in mandrills, we also examined the influence of sex, age and rank on diversity, and
— chemical distance in odour profile is related to MHC dissimilarity and pedigree relatedness at the dyadic level.
2. Material and Methods
(a). Subjects
The mandrill colony at the Center International de Recherches Médicales, Franceville (CIRMF), Gabon, was established in 1983–1984 with 15 unrelated animals in a 6.5 ha forest enclosure (E1). A second group was established in a smaller enclosure (E2, 3.5 ha) in 1994 by transferring 17 mandrills from E1. All subsequent changes in group size have been owing to natural reproduction, deaths and occasional removals. The mandrills forage freely and receive monkey chow, fruit and vegetables daily. Group sizes during the study were 75 animals in E1 (45 females, 30 males) and 72 animals in E2 (34 females, 25 males).
We collected odour samples directly from anaesthetized individuals captured in March and October 2004 and March 2005, with additional opportunistic sampling when animals were captured for other reasons. We extracted the age and pedigree [36] of all study subjects from long-term colony records and determined dominance ranks for both sexes from daily records of avoidance interactions. Female dominance ranks were stable during the study period, male ranks changed periodically [37].
We collected odour samples in two ways: (i) by rubbing a sterile cotton swab against the sternal gland 10 times vertically and 10 times horizontally, using steady pressure and (ii) by collecting hairs from the sternal gland. We exposed control swabs to the air during sampling to identify any volatile compounds that did not derive from the mandrills. We transferred swabs, hair samples and control swabs to separate sterile vials, froze them immediately in liquid nitrogen and stored them at −80°C. We obtained 88 swab samples (59 samples from 27 males, 29 samples from 18 females, one to four replicates per individual, mean 2), and 89 samples of sternal gland hair from 43 individuals (60 samples from 26 males aged 6.2–17.3 years, mean 10.7 years; 29 samples from 17 females aged 6.5–26.4 years, mean 14.8 years; one to four replicates per individual, mean 2).
(b). Odour analyses
We have detailed our extraction and gas chromatography–mass spectrometry (GC–MS) methods elsewhere [33]. Briefly, we subjected swab samples to dynamic headspace extraction and hair samples to solid-phase microextraction, followed by GC–MS. We standardized peak retention times using an internal standard (α pinene) and identified eluted compounds by comparison with the NIST mass spectral database, v. 5.0 (Agilent Technologies, Santa Clara, CA, USA). We determined the relative amounts of compounds by integrating the areas of the corresponding peaks in the total ion current profile and calculated percentages with respect to the total area. We retained peaks that comprised at least 0.05 per cent of the total area of the chromatogram and analysed all samples in a short period of time to minimize inter-assay variability. We identified a total of 47 distinct peaks that were not present in the control swabs in 88 swab samples, and 59 distinct peaks in the volatile chemical composition of hair samples (see [33] for details).
(c). MHC genotyping
We used DNA from blood collected during captures to genotype 40 study animals (24 males and 16 females) for MHC-DRB [34,38]; insufficient DNA was available for the remaining three males and three females for whom we had odour samples. We PCR-amplified MHC-DRB sequences and analysed products using denaturing gradient gel electrophoresis and direct sequencing [38] and repeated all genotyping experiments to confirm the assigned genotypes.
Ideally, studies should survey a larger region of the MHC than MHC-DRB, but this requires a level of knowledge of MHC structure that is lacking for most non-model organisms. However, the MHC region is characterized by strong linkage disequilibrium [39], meaning that relatively small segments of the MHC provide valuable information about the larger complex.
We use the number of different sequences present in an individual as a measure of MHC diversity, without making assumptions about the number of loci involved. We also used MHC-DRB sequences to determine 11 MHC-DRB supertypes [34]. Supertypes are groups of sequences that share peptide-binding motifs and are therefore functionally similar. They also collapse large numbers of sequences, each present in only a few animals, into a smaller number of variables, more suitable for use in statistical analyses.
(d). Statistical analyses
We used principal component analysis (PCA) to reduce the chemical compounds in mandrill odour samples to a smaller number of uncorrelated principal components (PCs) that explained most of the variance. We retained PCs with eigenvalues greater than 1 (15 PCs for swabs, explaining a total 79.3% of the variance; 18 PCs for hair samples, explaining a total 76.8% of the variance; [33]). We used these PCs as covariates in discriminant function analysis (DFA) to examine the relationship between odour and MHC genotype, grouping samples using possession of individual supertypes (present/absent for each).
We described the semiochemical complexity of mandrill sternal gland secretions using two diversity indices. Richness is the total number of compounds in the odour profile regardless of relative abundance. The Shannon index (H) takes into account how the diversity is distributed among the different compounds, and is higher when diversity is more even [40].
We compared diversity indices in males and females using a general linear mixed model (GLMM), including individual identity as a random factor to account for the use of multiple samples for the same individual, diversity as the dependent variable and sex as the independent variable. We examined the relationships between rank and age and diversity indices separately for the two sexes using similar GLMMs. Finally, we used further GLMMs to investigate the relationship between MHC diversity (number of sequences and number of supertypes possessed, in separate analyses) and diversity indices.
We used multi-dimensional scaling to create distance matrices for odour profiles, calculated as squared Euclidean distances for each dyad for both swabs and hairs. Where we had more than one sample for an individual, we used the mean distance to each other animal. We used the complete microsatellite pedigree for the colony [36] to create relatedness matrices, using the relatedness coefficient, f, for each possible dyad. We constructed MHC dissimilarity matrices using the number of (i) different MHC sequences and (ii) different MHC supertypes possessed by each dyad.
We compared the two matrices of chemical distance (for swab and hair samples) with each other and with matrices of f and MHC dissimilarity using Kendall's τ matrix correlations with 2000 permutations, permuting rows and columns independently. We used partial matrix correlations to examine the relationship between chemical distance and MHC dissimilarity, controlling for f. We ran analyses for (i) all animals together, (ii) same-sex dyads, and (iii) cross-sex dyads. In the latter case, we permuted columns only because the matrices were not square. Age and dominance rank influence male odour profiles [33] and are strongly correlated in our dataset, so we also partialled out the influence of rank difference in males. Replacing the rank difference matrix with age difference did not alter the significance of our results. MHC supertype dissimilarity was highly positively correlated with MHC sequence dissimilarity (all dyads: τ = 0.656, p-right = 0.0005), and results for the two MHC dissimilarity estimates did not differ qualitatively, so we present only those for MHC sequence dissimilarity.
We conducted PCA, DFA and multi-dimensional scaling using SPSS 15.0 for Windows, and matrix correlations using Matman 1.1 [41], set α < 0.05 and corrected for multiple testing where appropriate.
3. Results
(a). Odour profiles and possession of supertypes
Mandrills in this study possessed two to seven MHC sequences (mean ± s.e.m.: 4.3 ± 0.2) and two to six MHC supertypes (mean ± s.e.m.: 3.6±0.2). MHC diversity based on sequences and supertypes were highly positively correlated (r = 0.883, p < 0.001, n = 40). DFA did not differentiate between odour profiles of mandrills that possessed and did not possess any of the 11 MHC supertypes (see the electronic supplementary material), despite the fact that inclusion of repeat samples for some individuals increases the risk of type I error (but retains information concerning intra-individual variation). While these analyses were limited by the fact that some supertypes occurred in only a few individuals, while others occurred in most (range 2–31 individuals, mean ± s.e.m. 15.1 ± 2.6 individuals), they suggest that odour does not signal the possession of particular supertypes.
(b). Comparison of odour diversity with sex, rank, age and MHC diversity
Male odour profiles were more diverse than female profiles for H in hair samples, but not for Richness (table 1). H in swab samples was significantly related to male rank (table 2), with dominant males more diverse than subordinates. However, there was no relationship between male rank and odour diversity in hair samples. Odour diversity was not significantly related to rank in females, or to age in either sex.
Table 1.
Results of GLMM investigating sex differences in the chemical diversity of odour profiles in mandrills. (Within-individual variation where individuals contributed more than one sample: range 0–20 (Richness), 0–1.6 (H).)
| mean ± s.e.m. |
|||||
|---|---|---|---|---|---|
| male | female | f | p | ||
| swabs (d.f. = 1,84) | Richness | 18 ± 1 | 17 ± 1 | 1.59 | 2.11 |
| H | 1.5 ± 0.1 | 1.4 ± 0.1 | 0.91 | 0.345 | |
| hairs (d.f. = 1,93) | Richness | 22 ± 1 | 21 ± 1 | 1.48 | 0.227 |
| H | 2.0 ± 0.1 | 1.8 ± 0.1 | 4.52 | 0.036 | |
Table 2.
Results of GLMMs investigating relationships between chemical diversity and rank, age and MHC diversity in male and female mandrills. (H, Shannon index. d.f.: male swabs 1,50; male hairs 1,54; female swabs 1,25; females hairs 1,27. We examined the influence of age and rank separately in males, because these two variables were collinear.)
| swab samples |
hair samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Richness |
H |
Richness |
H |
||||||
| f | p | f | p | f | p | f | p | ||
| males | rank | 0.69 | 0.409 | 6.88 | 0.012 | 3.23 | 0.078 | 1.79 | 0.187 |
| age | 0.61 | 0.44 | 0.43 | 0.517 | 2.11 | 0.153 | 0.29 | 0.592 | |
| MHC sequencesa | 0.13 | 0.718 | 1.06 | 0.308 | 0 | 0.992 | 2.71 | 0.106 | |
| MHC supertypesa | 0.27 | 0.609 | 1.07 | 0.360 | 0.04 | 0.840 | 5.36 | 0.024 | |
| females | rank | 1.85 | 0.186 | 0.35 | 0.559 | 1.02 | 0.321 | 0.36 | 0.559 |
| age | 3.98 | 0.057 | 0.20 | 0.597 | 0.09 | 0.761 | 1.41 | 0.246 | |
| MHC sequences | 3.23 | 0.084 | 0.84 | 0.369 | 0.29 | 0.595 | 0.10 | 0.760 | |
| MHC supertypes | 2.15 | 0.155 | 0.77 | 0.387 | 0.81 | 0.382 | 0.09 | 0.768 | |
aIncluding rank as a covariate in the model.
H, but not Richness, increased with the number of MHC supertypes possessed by a male for hair samples, but not swab samples (table 2). There were no significant relationships between odour diversity and number of MHC sequences in males, or between odour diversity and MHC diversity in females (table 2).
(c). Chemical distance, MHC similarity and relatedness
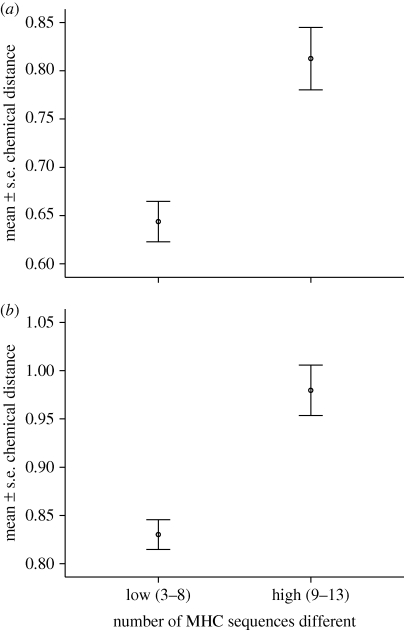
Chemical distances between dyads calculated using hair and swab samples were highly positively and significantly correlated (τ = 0.305, p-right < 0.001). MHC dissimilarity increased with chemical distance for all dyads (table 3 and figure 1), male–male dyads, female–female dyads (swab samples only) and cross-sex dyads (hair samples only; see the electronic supplementary material, S2).
Table 3.
Results of matrix correlations comparing chemical distance, MHC dissimilarity, pedigree relatedness and rank difference in mandrills. (n = 780.)
| matrix correlation |
partial matrix correlation |
|||||||
|---|---|---|---|---|---|---|---|---|
| first matrix (x) | second matrix (y) | p-right | p-left | τxy | control matrix (z) | p-right | p-left | τxyz |
| MHC | swab | 0.024 | 0.977 | 0.142 | f | 0.017 | 0.983 | 0.148 |
| hair | <0.001 | 1 | 0.175 | f | <0.001 | 1 | 0.178 | |
| f | swab | 0.417 | 0.585 | 0.010 | MHC | 0.072 | 0.823 | 0.041 |
| hair | 0.586 | 0.417 | −0.007 | MHC | 0.164 | 0.836 | 0.031 | |
Figure 1.
Relationship between MHC distance and chemical distance in (a) swab and (b) hair samples. Sexes combined and distance measured at the dyadic level with number of MHC sequences split into high and low.
Values of f ranged from 0 (unrelated) to 0.5 (parent–offspring or full siblings) in dyads for whom we had odour samples. f was not significantly correlated with either swab or hair distances in all dyads (table 3) or male–male dyads (see the electronic supplementary material, S2). It was, however, negatively related to chemical distance in hair samples in male–female dyads. Surprisingly, it was positively correlated with chemical distance in hair samples in female–female dyads (see the electronic supplementary material, S2).
Partialling out any effect of f, MHC sequence dissimilarity increased with chemical differences in all analyses (table 3 and electronic supplementary material, S2). In the reverse analysis, partialling out the effect of MHC dissimilarity, we found a significant positive relationship between f and chemical distance in male–male and female–female dyads, but not in all dyads or male–female dyads, suggesting that the relationship between odour distance and MHC dissimilarity was stronger than that between odour distance and f.
4. Discussion
We conducted, to our knowledge, the first comprehensive study of the relationships between MHC genotype and chemical odour profiles for a non-model organism (i.e. an organism other than humans or rodents). Our results suggest that mandrill odour profiles signal MHC genotype in addition to signalling valuable information concerning sex and male rank. Although we were unable to discriminate reliably between individuals possessing particular MHC supertypes based on odour profiles, odour diversity reflected MHC diversity in males, while odour similarity in dyads was also strongly related to similarity at the MHC, and in some cases to pedigree relatedness. Thus, odour provides a signal of both individual genetic diversity and information against which the receiver can compare its own genotype to assess genetic similarity. The positive relationship between odour dissimilarity and MHC dissimilarity in cross-sex dyads provides a potential means by which mandrills could achieve the MHC-disassortative patterns of reproduction that we have previously reported [34]. Moreover, our findings also identify a mechanism by which mandrills may be able to assess the relatedness of unfamiliar individuals by assessing the odour similarity of other animal to their own odour, or to that of known close relatives. This may underlie their ability to discriminate paternal kin from non-relatives [35], and facilitate kin selection. Finally, our findings contribute to an increasing body of evidence suggesting that odour cues play a much greater role in communication in anthropoid primates, including humans, than previously assumed [42,43].
Results for the two types of odour samples were broadly similar, but we did find some differences. MHC diversity correlated with odour diversity in male hair samples, but not in swab samples. MHC dissimilarity in females was related to chemical differences in swab samples, but not in hair samples, and the opposite was true for pedigree relatedness. Finally, in cross-sex dyads, MHC dissimilarity and pedigree relatedness were reflected in chemical differences in hair, but not swab samples. We have discussed differences between the two types of odour samples elsewhere [33]. Briefly, they show a relatively low degree of overlap in chemical composition, probably owing to the different analysis methods used, and because each type of sample may include substances that do not derive directly from the scent gland. However, both types of samples include substances that are transferred to the substrate during scent-marking, and both may contribute to an individual's body odour. Indeed, the real cue to mandrills is likely to be composed of a mixture of these substances.
We found no evidence that odour signals the possession of particular genotypes, which may be either good, associated with immunity to particular pathogens [6], or deleterious, associated with higher susceptibility to parasites (e.g. [44] for lemurs). This contrasts with the degree of facial red colour in male mandrills, which is associated with possession of some of the same MHC supertypes [45], and raises the possibility that visual traits signal the possession of particular genotypes, while odour signals other aspects of the genotype (diversity and similarity). A similar scenario under which visual and olfactory communication act in a complementary fashion has been proposed for humans, in which facial attractiveness appears to signal MHC similarity, while odour signals MHC dissimilarity [46].
The sex differences that we identified in odour signal diversity build on our previous analyses in which we found that we could reliably discriminate male and female odour profiles using DFA [33], and suggest that sex differences in odour profiles are owing to differences in how the chemical diversity is distributed among the different compounds that comprise the signal, rather than to differences in the number of compounds present. This provides an interesting comparison with ring-tailed lemurs, where female odour contains more compounds than male's [28], and reflects differences in the social system of the two species. Male mandrills are dominant over females, while female lemurs are dominant over males [47]. Thus, the socially dominant sex has a more diverse odour profile in both species.
The sex difference in the influence of rank on odour profile (there was no rank difference in odour profile diversity in females) is also expected, as we have previously shown that DFA can discriminate reliably between the odour profile of males, but not females, of different ranks [33]. The relationship between male rank and H, but not Richness in swab samples, suggests that the relative proportions of compounds vary more in dominant males than in subordinates, although they have the same number of compounds. This suggests that dominant males may have higher concentrations of certain compounds, that are likely to be testosterone-related, as in mice [48], since higher ranking male mandrills have higher levels of testosterone [37,49].
The relationship between odour diversity and the number of MHC supertypes in males suggests that odour reliably signals MHC diversity in mandrills. Possession of a larger number of supertypes will allow a male to respond to a greater diversity of pathogens, rendering him fitter than less diverse individuals [50]. This is particularly interesting because more MHC-diverse males have higher reproductive success in our study population [34], suggesting that they have an advantage in either male–male competition, female choice or both. However, we found no significant relationships between variation in male red coloration and MHC diversity [45]. Our new results suggest that male genetic quality may instead be communicated to rival males and potential mates via odour.
The relationship between odour distance and MHC dissimilarity appears to reflect a direct link between odour and the MHC, rather than a relationship between overall genetic similarity and odour. Analyses of the relationship between chemical distance and pedigree relatedness produced more ambiguous results than those for MHC dissimilarity. Chemical distance was significantly negatively correlated with relatedness in male–female dyads, as in same-sex dyads of beavers [31]. Odour profile similarity also correlates with microsatellite allele-sharing in ring-tailed lemurs [28,29]. However, chemical distance was unrelated to relatedness in all dyads and in male–male dyads in our study, and positively correlated with relatedness in female–female dyads—i.e. more related female–female dyads were more dissimilar in odour. Moreover, all relationships between pedigree relatedness and chemical distance were weaker than those for MHC dissimilarity, and partial correlation analyses suggested that the relationships between odour dissimilarity and MHC dissimilarity were stronger than those with relatedness. Future studies should test the relative influence of both overall genetic similarity and MHC similarity on odour similarity.
In conclusion, our results demonstrate that odour encodes complex genotypic information in an Old World monkey. These findings are exciting, because they serve to fill the deep phylogenetic gap between studies of rodents (mainly laboratory strains) and humans. Future studies should (i) attempt to disentangle the relationships among MHC genotype, overall genetic make-up and odour profiles, (ii) investigate the non-volatile components of scent marks, because mandrills may also glean information from non-volatile, higher molecular weight compounds (e.g. [51,52]), (iii) test hypotheses proposed to explain how the MHC might affect odour, as the physiological pathways remain unclear [11], and (iv) address the question of how the information available in mandrill odour is received, and how it influences behaviour.
Acknowledgements
This study was approved by Comité d'Ethique Paris Sud and complied with animal care regulations and applicable national laws in Gabon.
We are grateful to CIRMF and the Primate Center staff, Jean Wickings and Marie Charpentier, for long-term collaboration, John Waterhouse for help with a pilot study and Luca Calamai, Gloriano Moneti and Stefano Turillazzi for help with chemical analyses. We thank three anonymous reviewers for constructive comments. CIRMF is financed by the Gabonese government, Total Gabon and the Ministère Français des Affaires Etrangères. The work presented here was funded by a Leverhulme Trust UK project grant (no. F/01576/B).
References
- 1.Klein J. 1986. The natural history of the major histocompatability complex. New York, NY: Wiley [Google Scholar]
- 2.Beauchamp G. K., Yamazaki K. 2003. Chemical signalling in mice. Biochem. Soc. Trans. 31, 147–151 10.1042/BST0310147 (doi:10.1042/BST0310147) [DOI] [PubMed] [Google Scholar]
- 3.Boehm T., Zufall F. 2006. MHC peptides and the sensory evaluation of genotype. Trends Neurosci. 29, 100–107 10.1016/j.tins.2005.11.006 (doi:10.1016/j.tins.2005.11.006) [DOI] [PubMed] [Google Scholar]
- 4.Jacob S., McClintock M. K., Zelano B., Ober C. 2002. Paternally inherited HLA alleles are associated with women's choice of male odor. Nat. Genet. 30, 175–179 10.1038/ng830 (doi:10.1038/ng830) [DOI] [PubMed] [Google Scholar]
- 5.Penn D., Potts W. 1998. How do major histocompatibility complex genes influence odor and mating preferences? Adv. Immunol. 69, 411–436 10.1016/S0065-2776(08)60612-4 (doi:10.1016/S0065-2776(08)60612-4) [DOI] [PubMed] [Google Scholar]
- 6.Hamilton W. D., Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites. Science 218, 384–387 10.1126/science.7123238 (doi:10.1126/science.7123238) [DOI] [PubMed] [Google Scholar]
- 7.Penn D. J. 2002. The scent of genetic compatibility: sexual selection and the major histocompatibility complex. Ethology 108, 1–21 10.1046/j.1439-0310.2002.00768.x (doi:10.1046/j.1439-0310.2002.00768.x) [DOI] [Google Scholar]
- 8.Penn D. J., Potts W. K. 1999. The evolution of mating preferences and major histocompatibility complex genes. Am. Nat. 153, 145–164 [DOI] [PubMed] [Google Scholar]
- 9.Milinski M. 2006. The major histocompatibility complex, sexual selection, and mate choice. Annu. Rev. Ecol. Evol. Syst. 37, 159–186 10.1146/annurev.ecolsys.37.091305.110242 (doi:10.1146/annurev.ecolsys.37.091305.110242) [DOI] [Google Scholar]
- 10.Piertney S. B., Oliver M. K. 2006. The evolutionary ecology of the major histocompatibility complex. Heredity 96, 7–21 [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki K., Beauchamp G. 2007. Genetic basis for MHC-dependent mate choice. Adv. Genet. 59, 130–145 [DOI] [PubMed] [Google Scholar]
- 12.Setchell J. M., Huchard E. In press The hidden benefits of primate sex: evidence that MHC influences mate choice in complex animal societies. BioEssays. [DOI] [PubMed] [Google Scholar]
- 13.Chaix R., Cao C., Donnelly P. 2008. Is mate choice in humans MHC-dependent? PLoS Genet. 4, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havlicek J., Roberts S. C. 2009. MHC-correlated mate choice in humans: a review. Psychoneuroendocrinology 34, 497–512 10.1016/j.psyneuen.2008.10.007 (doi:10.1016/j.psyneuen.2008.10.007) [DOI] [PubMed] [Google Scholar]
- 15.Hamilton W. D. 1964. The genetical evolution of social behavior. I. J. Theor. Biol. 7, 1–16 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 16.Altmann J., et al. 1996. Behavior predicts genetic structure in a wild primate group. Proc. Natl Acad. Sci. USA 93, 5797–5801 10.1073/pnas.93.12.5797 (doi:10.1073/pnas.93.12.5797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widdig A. 2007. Paternal kin discrimination: the evidence and likely mechanisms. Biol. Rev. Camb. Phil. Soc. 82, 319–334 [DOI] [PubMed] [Google Scholar]
- 18.Manning C. J., Wakeland E. K., Potts W. K. 1992. Communal nesting patterns in mice implicate MHC genes in kin recognition. Nature 360, 581–583 10.1038/360581a0 (doi:10.1038/360581a0) [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki K., Beauchamp G. K., Curran M., Bard J., Boyse E. A. 2000. Parent–progeny recognition as a function of MHC odortype identity. Proc. Natl Acad. Sci. USA 97, 10 500–10 502 10.1073/pnas.180320997 (doi:10.1073/pnas.180320997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas L. 1975. Symbiosis as an immunological problem. In Fourth International Congress of Immunology (eds Neter E., Milgrom F.), pp. 2–11 New York, NY: Karger [Google Scholar]
- 21.Hurst J. 2009. Female recognition and assessment of males through scent. Behav. Brain Sci. 200, 295–303 [DOI] [PubMed] [Google Scholar]
- 22.Kwak J., Opiekun M., Matsumura K., Preti G., Yamazaki K., Beauchamp G. 2009. Major histocompatibility complex-regulated odortypes: peptide-free urinary volatile signals. Physiol. Behav. 96, 184–188 10.1016/j.physbeh.2008.10.003 (doi:10.1016/j.physbeh.2008.10.003) [DOI] [PubMed] [Google Scholar]
- 23.Restrepo D., Lin W., Salcedo E., Yamazaki K., Beauchamp G. 2006. Odortypes and MHC peptides: complementary chemosignals of MHC haplotype? Trends Neurosci. 29, 604–609 10.1016/j.tins.2006.08.001 (doi:10.1016/j.tins.2006.08.001) [DOI] [PubMed] [Google Scholar]
- 24.Eggert F., Hoeller C., Luszyk D., Mueller-Ruchholtz W., Ferstl R. 1997. MHC-associated and MHC-independent urinary chemosignals in mice. Physiol. Behav. 61, 957–961 [DOI] [PubMed] [Google Scholar]
- 25.Novotny M., Soini H., Koyama S., Wiesler D., Bruce K., Penn D. 2007. Chemical identification of MHC-influenced volatile compounds in mouse urine. I. Quantitative proportions of major chemosignals. J. Chem. Ecol. 33, 417–434 10.1007/s10886-006-9230-9 (doi:10.1007/s10886-006-9230-9) [DOI] [PubMed] [Google Scholar]
- 26.Willse A., Kwak J., Yamazaki K., Preti G., Wahl J., Beauchamp G. 2006. Individual odortypes: interaction of MHC and background genes. Immunogenetics 58, 967–982 10.1007/s00251-006-0162-x (doi:10.1007/s00251-006-0162-x) [DOI] [PubMed] [Google Scholar]
- 27.Eggert F., Luszyk D., Haberkorn K., Wobst B., Vostrowshy O., Westphal E., Bestmann H. J., Mueller-Ruchholtz W., Ferstl R. 1999. The major histocompatability complex and the chemosensory signalling of individuality in humans. Genetica 104, 265–273 10.1023/A:1026431303879 (doi:10.1023/A:1026431303879) [DOI] [PubMed] [Google Scholar]
- 28.Boulet M., Charpentier M. J., Drea C. M. 2009. Decoding an olfactory mechanism of kin recognition and inbreeding avoidance in a primate. BMC Evol. Biol. 9, 281. 10.1186/1471-2148-9-281 (doi:10.1186/1471-2148-9-281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charpentier M., Boulet M., Drea C. M. 2008. Smelling right: the scent of male lemurs advertises genetic quality and relatedness. Mol. Ecol. 17, 3225–3233 10.1111/j.1365-294X.2008.03831.x (doi:10.1111/j.1365-294X.2008.03831.x) [DOI] [PubMed] [Google Scholar]
- 30.Safi K., Kerth G. 2003. Secretions of the interaural gland contain information about individuality and colony membership in the Bechstein's bat. Anim. Behav. 65, 363–369 10.1006/anbe.2003.2067 (doi:10.1006/anbe.2003.2067) [DOI] [Google Scholar]
- 31.Sun L. X., Muller-Schwarze D. 1998. Anal gland secretion codes for relatedness in the beaver, Castor canadensis. Ethology 104, 917–927 10.1111/j.1439-0310.1998.tb00041.x (doi:10.1111/j.1439-0310.1998.tb00041.x) [DOI] [Google Scholar]
- 32.Sun L. X., Muller-Schwarze D. 1998. Anal gland secretion codes for family membership in the beaver. Behav. Ecol. Sociobiol. 44, 199–208 10.1007/s002650050532 (doi:10.1007/s002650050532) [DOI] [Google Scholar]
- 33.Setchell J., Vaglio S., Moggi-Cecchi J., Boscaro F., Calamai L., Knapp L. A. 2010. Chemical composition of scent-gland secretions in an Old World monkey (Mandrillus sphinx): influence of sex, male status, and individual identity. Chem. Senses 35, 205–220 10.1093/chemse/bjp105 (doi:10.1093/chemse/bjp105) [DOI] [PubMed] [Google Scholar]
- 34.Setchell J. M., Charpentier M. J. E., Abbott K. A., Wickings E. J., Knapp L. A. 2010. Opposites attract: MHC-associated mate choice in an anthropoid primate. J. Evol. Biol. 23, 136–148 10.1111/j.1420-9101.2009.01880.x (doi:10.1111/j.1420-9101.2009.01880.x) [DOI] [PubMed] [Google Scholar]
- 35.Charpentier M. J. E., Peignot P., Hossaert-McKey M., Wickings E. J. 2007. Kin discrimination in juvenile mandrills, Mandrillus sphinx. Anim. Behav. 73, 37–45 10.1016/j.anbehav.2006.02.026 (doi:10.1016/j.anbehav.2006.02.026) [DOI] [Google Scholar]
- 36.Charpentier M., Peignot P., Hossaert-McKey M., Gimenez O., Setchell J. M., Wickings E. J. 2005. Constraints on control: factors influencing reproductive success in male mandrills (Mandrillus sphinx). Behav. Ecol. 16, 614–623 10.1093/beheco/ari034 (doi:10.1093/beheco/ari034) [DOI] [Google Scholar]
- 37.Setchell J. M., Smith T., Wickings E. J., Knapp L. A. 2008. Social correlates of testosterone and ornamentation in male mandrills. Horm. Behav. 54, 365–372 10.1016/j.yhbeh.2008.05.004 (doi:10.1016/j.yhbeh.2008.05.004) [DOI] [PubMed] [Google Scholar]
- 38.Abbott K. A., Wickings E. J., Knapp L. A. 2006. High levels of diversity characterize mandrill (Mandrillus sphinx) MHC-DRB sequences. Immunogenetics 58, 628–640 10.1007/s00251-006-0132-3 (doi:10.1007/s00251-006-0132-3) [DOI] [PubMed] [Google Scholar]
- 39.Kelley J., Walter L., Trowsdale J. 2005. Comparative genomics of major histocompatibility complexes. Immunogenetics 56, 683–695 10.1007/s00251-004-0717-7 (doi:10.1007/s00251-004-0717-7) [DOI] [PubMed] [Google Scholar]
- 40.Magurran A. E. 1988. Ecological diversity and its measurement. Princeton, NJ: Princeton University Press [Google Scholar]
- 41.de Vries H., Netto W. J., Hanegraaf P. L. H. 1993. MATMAN: a program for the analysis of sociometric matrices and behavioural transition matrices. Behaviour 125, 157–175 10.1163/156853993X00218 (doi:10.1163/156853993X00218) [DOI] [Google Scholar]
- 42.Heymann E. W. 2006. The neglected sense of smell in primate behavior, ecology and evolution. Am. J. Primatol. 68, 514–524 [DOI] [PubMed] [Google Scholar]
- 43.Stevenson R. 2010. An initial evaluation of the functions of human olfaction. Chem. Senses 35, 3–20 10.1093/chemse/bjp083 (doi:10.1093/chemse/bjp083) [DOI] [PubMed] [Google Scholar]
- 44.Schwensow N., Fietz J., Dausmann K. H., Sommer S. 2007. Neutral versus adaptive genetic variation in parasite resistance: importance of major histocompatibility complex supertypes in a free-ranging primate. Heredity 99, 265–277 10.1038/sj.hdy.6800993 (doi:10.1038/sj.hdy.6800993) [DOI] [PubMed] [Google Scholar]
- 45.Setchell J. M., Charpentier M., Abbott K. A., Wickings E. J., Knapp L. A. 2009. Is brightest best? Testing the Hamilton–Zuk hypothesis in mandrills. Int. J. Primatol. 30, 825–844 10.1007/s10764-009-9371-0 (doi:10.1007/s10764-009-9371-0) [DOI] [Google Scholar]
- 46.Roberts S. C., Little A. C., Gosling L. M., Jones B. C., Perrett D. I., Carter V., Petrie M. 2005. MHC-assortative facial preferences in humans. Biol. Lett. 1, 400–440 10.1098/rsbl.2005.0343 (doi:10.1098/rsbl.2005.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jolly A. 1966. Lemur behavior. Chicago, IL: University of Chicago Press [Google Scholar]
- 48.Kimura T., Hagiwara Y. 1985. Regulation of urine marking in male and female mice: effects of sex steroids. Horm. Behav. 19, 64–70 10.1016/0018-506X(85)90006-6 (doi:10.1016/0018-506X(85)90006-6) [DOI] [PubMed] [Google Scholar]
- 49.Setchell J. M., Dixson A. F. 2001. Arrested development of secondary sexual adornments in subordinate adult male mandrills (Mandrillus sphinx). Am. J. Phys. Anthropol. 115, 245–252 10.1002/ajpa.1079 (doi:10.1002/ajpa.1079) [DOI] [PubMed] [Google Scholar]
- 50.Doherty P. C., Zinkernagel R. M. 1975. Enhanced immunological surveillance in mice heterozygous at the H-2 gene complex. Nature 256, 50–52 10.1038/256050a0 (doi:10.1038/256050a0) [DOI] [PubMed] [Google Scholar]
- 51.Belcher A., Epple G., Greenfield K. L., Richards L. E., Kuderling I., Smith A. B. 1990. Proteins: biologically relevant components of the scent marks of a primate (Saguinus fuscicolis). Chem. Senses 15, 431–446 10.1093/chemse/15.4.431 (doi:10.1093/chemse/15.4.431) [DOI] [Google Scholar]
- 52.Nevison C. M., Armstrong S., Beynon R. J., Humphries R. E., Hurst J. L. 2003. The ownership signature in mouse scent marks is involatile. Proc. R. Soc. Lond. B 270, 1957–1963 10.1098/rspb.2003.2452 (doi:10.1098/rspb.2003.2452) [DOI] [PMC free article] [PubMed] [Google Scholar]