Abstract
Fire may have been a crucial component in the evolution of the Cape flora of South Africa, a region characterized by outstanding levels of species richness and endemism. However, there is, to date, no critical assessment of the age of the modern fire regime in this biome. Here, we exploit the presence of two obligate post-fire flowering clades in the orchid genus Disa, in conjunction with a robust, well-sampled and dated molecular phylogeny, to estimate the age by which fire must have been present. Our results indicate that summer drought (winter rainfall), the fire regime and the fynbos vegetation are several million years older than currently suggested. Summer drought and the fynbos vegetation are estimated to date back to at least the Early Miocene (ca 19.5 Ma). The current fire regime may have been established during a period of global cooling that followed the mid-Miocene Climatic Optimum (ca 15 Ma), which led to the expansion of open habitats and increased aridification. The first appearance of Disa species in the grassland biome, as well as in the subalpine habitat, is in striking agreement with reliable geological and palaeontological evidence of the age of these ecosystems, thus corroborating the efficacy of our methods. These results change our understanding of the historical mechanisms underlying botanical evolution in southern Africa, and confirm the potential of using molecular phylogenies to date events for which other information is lacking or inconclusive.
Keywords: fire, Cape flora, Orchidaceae, Disa, palaeoecology
1. Introduction
Fire influences global ecosystem patterns and processes and must have had a pronounced effect on the evolution of these biotas [1,2]. Without fire, the distribution of ecosystems around the world would be substantially different [3,4]. Vast areas of flammable biomes such as Mediterranean ecosystems, savannas and humid grasslands would turn into forest, particularly in Africa [4]. Although fossil charcoal appears in the geological record soon after the emergence of terrestrial plants [5,6], the age of the current fire regimes is largely unknown [5], but is presumed to be not much older than 6–8 million years (Ma) [4].
Palaeoclimatological and palaeoecological reconstructions are mostly based on plant and animal fossil data (e.g. [7–9]). Although geochemical and geomorphological information is also used (e.g. [10,11]), this comes mainly from marine basins and the link between marine and terrestrial realms is not always clear [12]. For southern Africa the Neogene fossil record is remarkably poor and does not allow for confident reconstruction of the climate, vegetation [13], or the age of the current fire regimes [4]. Other methodologies and independent lines of evidence would thus be welcome to augment our patchy understanding of past ecosystems. Here, we use the evolutionary history of the orchid genus Disa (subfamily Orchidoideae) based on neontological data, to reconstruct past climates and environments.
A reconstruction of the palaeoenvironments of the Cape floristic region (CFR) of southern Africa is particularly interesting since it constitutes, with 9000 species in an area of 90 000 km2 [14,15], the second most important centre of endemism richness in the world [16]. Most of this richness is found in fynbos, a shrubland ecosystem similar to chaparral in California and kwongan in southwest Australia, characterized by a Mediterranean climate and recurrent fires every 5–50 years [17]. Palaeoclimatic changes have been proposed to have triggered [18–20] and sustained [21,22] plant radiations in the CFR. However, climate alone cannot explain species diversity in the CFR, as the region contains more than twice as many species as expected by global environmental models [23]. A consensus is therefore emerging that the history of diversification in the Cape, possibly the combination of a long history of speciation [24,25] with the existence of long-term stable environment [25,26] might be key to the exceptional modern diversity. Thus, in order to understand the origins of this remarkable diversity, it is essential to correlate speciation history of its elements with the evolution of the relevant modern environments, including fire regime, climate and edaphic conditions [27,28].
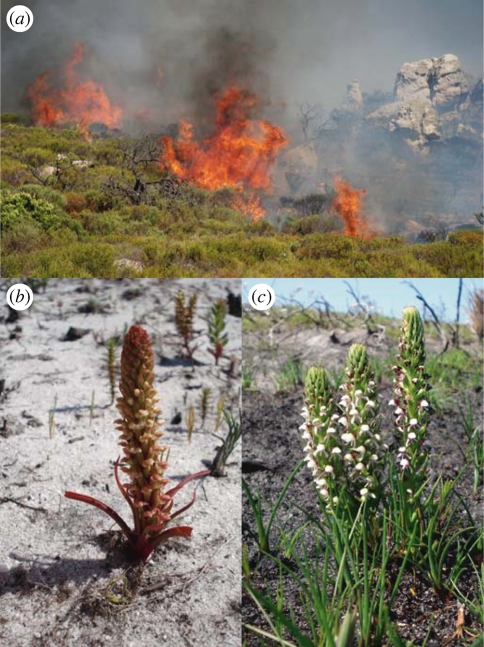
Almost half of the species richness in the CFR is accounted for by only 33 ‘Cape floral clades’ [13], of which the large orchid genus Disa is one. This is a particularly charismatic group of orchids, and Disa uniflora, the ‘Pride of Table Mountain’, is well known and often used as an emblem (e.g. of the Mountain Club of South Africa). Of 180 species [29], 100 occur in the CFR and 86 are restricted to it [15]. The various species can be found from the coastal sands to the summits of the highest mountains, yet they are never common [30]. Most are geophytes, well adapted to a fire-prone environment. Indeed, many species are obligate fire-dependent and will only flower in the first year after fire (figure 1), although the mechanism or potential triggers for this are not understood. Disa is an ideal clade with which to explore the evolution of Cape environments, as a robust phylogeny of the genus is available, which contains 70 per cent of all species, representing all taxonomic sections, geographical areas and major habitats ([31]; see also electronic supplementary material, figure S1).
Figure 1.
Fire constitutes an essential element of the Cape floristic region (CFR). Like many other plants in this ecosystem, some Disa species flower only the first year after a fire swept through the landscape. (a) Fynbos fire at Slangkop, South Africa; (b) D. conferta (section Monadenia) and (c) D. obtusa (section Disella), both obligate fire-dependent species, endemic to the CFR.
Here, we apply the results from a recent study on the ages of major orchid clades [32], based on all reliable orchid fossils described so far, to calibrate the rate-corrected phylogeny of Disa and so infer ages for ancestral states of the biomes, rainfall regimes and habitats in which Disa occurs. We focus particularly on those attributes for which no independent dates are available, such as the age of the fynbos vegetation, winter rainfall and current fire regime, to evaluate how these dates affect our interpretation of the evolutionary history of the Cape flora. We test the assumption that the ecological evolution in the genus is phylogenetically conservative, and therefore suitable for a reconstruction of Neogene African environments. Finally, we test our methods by determining whether the molecular dates of the first occupation of various habitats in Africa fit the known ages of these habitats (e.g. alpine Drakensberg and grassland).
2. Material and methods
Phylogenetic relationships were inferred for seven outgroup and 136 ingroup taxa, representing 70 per cent of all recognized Disa species and infraspecific taxa. One nuclear and two plastid gene regions were sequenced and combined in a data matrix containing 4094 characters. In a parsimony analysis, 87 nodes of 142 (61%) were supported with a bootstrap support value of 75 per cent or higher, whereas the topology resulting from a Bayesian inference analysis had 101 (71%) nodes with a posterior probability of 0.95 or above (see [31] for details).
For the molecular dating analysis, we applied two widely used algorithms: penalized likelihood implemented in r8s [33] and a Bayesian relaxed clock implemented in BEAST [34]. In both cases, topological and branch length uncertainty was taken into account. A single calibration point was used: the crown age of Disa, whose median age was estimated to 19.45 Ma (95% highest posterior density, HPD: 10.2–30 Ma) by Gustafsson et al. [32]. In that study, the dating analysis of Ramirez et al. [35], based on a fossil from Dominican amber, was extended with the inclusion of two newly described macrofossils assigned to genera Earina and Dendrobium from New Zealand [36]. In addition, in Gustafsson et al. [32] a Bayesian relaxed clock was employed, and the age of fossil Liliacites was not used as a maximum age constraint for crown Asparagales, an assumption made by Ramirez et al. [35] that received no support in the cladistic analysis of Doyle et al. [37]. To take into account the compound error associated with secondary calibrations, in the BEAST analysis the age prior for crown Disa was set as a normally distributed subset of the age range obtained for the same node in Gustafsson et al. [32].
Disa species were coded for occurrence in biomes, rainfall seasonality and habitat (20 states in total; see electronic supplementary material for details). For the character optimization analysis, we used binary coding (absence/presence) to allow for polymorphic states at internal nodes [38]. Maximum likelihood optimization of ancestral states was performed in Mesquite v. 2.6 [39]. Initially, all characters were optimized using these assumptions over the maximum a posteriori tree. To take into account phylogenetic uncertainty, each character was then optimized over a sample of 1000 penalized likelihood chronograms, and average frequencies across trees calculated for each node of the maximum a posteriori tree.
We tested for phylogenetic conservative characters by calculating the number of steps each character required for a parsimony reconstruction, and comparing this to the distribution of minimum lengths for the same character reshuffled 1000 times using Mesquite, while keeping the proportions of the states constant. If the number of steps of the observed distribution was outside 95 per cent of the randomized state distributions, the Null hypothesis that the character states were randomly distributed was rejected. See electronic supplementary material for a detailed description of the methods used.
3. Results
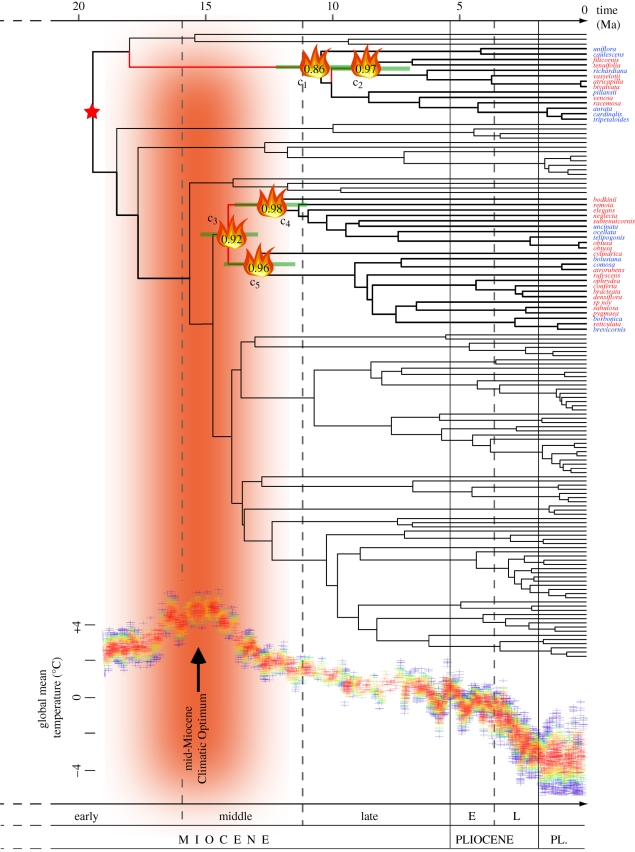
Chronograms inferred by penalized likelihood and BEAST are presented in electronic supplementary material, figures S2 and S3, respectively. In general, median ages estimated by BEAST tended to be younger than those estimated by penalized likelihood, as observed in other studies (e.g. [40]). However, the 95% confidence intervals (CI)/HPD were mostly overlapping for any given node. We report penalized likelihood estimates in the text, but for key nodes we indicate mean ages and CIs from both methods in table 1.
Table 1.
Comparison of age estimates (Ma) for key nodes in the Disa phylogeny, depending of the molecular dating method used.
| clade in figure 2 | taxonomic section | penalized likelihood |
BEAST |
||||
|---|---|---|---|---|---|---|---|
| mean | lower | upper | mean | lower | upper | ||
| C1 | Disa | 10.5 | 9.01 | 12.2 | 9.68 | 7.41 | 12.1 |
| C2 | — | 8.62 | 6.95 | 10.1 | 8.12 | 5.95 | 10.4 |
| C3 | Disella + Monadenia | 14.0 | 12.9 | 15.2 | 10.9 | 8.51 | 13.4 |
| C4 | Disella | 12.5 | 11.0 | 13.9 | 9.09 | 6.69 | 11.4 |
| C5 | Monadenia | 12.9 | 11.5 | 14.3 | 9.32 | 6.88 | 12.0 |
The relative likelihood values for the character state optimization over all nodes of the maximum a posteriori tree are reported in the electronic supplementary material, table S1. Attribute shifts supported by an average (n = 1000 trees) of greater than or equal to 0.95 for biome, rainfall seasonality and habitat are shown in electronic supplementary material, figures S4–S6, respectively. Shifts supported by an average relative likelihood greater than or equal to 0.70 but less than 0.95 are indicated with an asterisk. The root node of Disa was unambiguously optimized to fynbos biome and to winter rainfall, both with a mean relative likelihood (λm) of 0.99. The obligate fire-dependent clades Monadenia (node C5 in figure 2; 11.5 Ma, lower bound of the 95% CI of the crown group age) and Disella (node C4 in figure 2; 11 Ma, lower bound of the 95% CI of the crown group age) both optimized to fire (λm = 0.96 and 0.98, respectively; figure 2). The most recent common ancestor of these two sister clades (node C3 in figure 2; 12.9 Ma, lower bound of the 95% CI of the crown group age) was most probably also fire-dependent, given its high likelihood (λm = 0.92) and because this scenario would require only a single habitat transition instead of two. Significance tests showed that biomes, rainfall seasonality (except all year rainfall) and most of the habitats (except subalpine and southeast cloud zone) are phylogenetically conservative. The null hypothesis that these states were randomly distributed across the tree was rejected (electronic supplementary material, table S2). Detailed results are reported in electronic supplementary material.
Figure 2.
Temporal evolution of Disa and earliest optimizations of fire. Maximum a posteriori tree from the Bayesian analysis of 136 taxa, with branch lengths proportional to mean ages estimated from a sample of 1000 independently dated chronograms using penalized likelihood. The oldest shifts to fire habitats are outlined by the fire symbol (with relative average likelihood values inside), and the corresponding branches along which shifts occurred highlighted in red. Species marked in red are obligate fire-dependent. Green bars indicate 95% confidence intervals of critical nodes (clades C1–C5; see table 1 for precise values). The red star indicates the calibration point. Temperature curve from Zachos et al. [45]; timescale from Gradstein & Ogg [77].
4. Discussion
Our results suggest that the fynbos biome as a whole, as well as the winter rainfall climate of the western and southern part of the CFR, are far older than has generally been assumed [41–44] and may date back to approximately 19.5 Ma (crown age of Disa from [32]). Our results further suggest that fires in the CFR were sufficiently predictable to select for fire-stimulated flowering by about 12.9 Ma (C5 in figure 2; λm = 0.96; 95% CI = 11.5–14.3 Ma) or even earlier (clade C3, approx. 14 Ma, λm = 0.92, 95% CI = 12.9–15.2 Ma). Interestingly, these ages fall within a period of global climate cooling subsequent to the mid-Miocene Climatic Optimum, approximately 15 Ma [11,45] (see temperature curve in figure 2) and with the start of the radiation of many species-rich clades (e.g. Moraea [21], Satyrium [25], Pelargonium [22], Protea [46] and Muraltia [25,47]) or with a sharp increase in species numbers such as in Bruniaceae [48]. Thus, fire could have provided the environment during the Late Miocene–Pliocene in which the diversification of the current hyperdiverse clades of the Cape flora was initiated.
The development of a fire regime requires at least two preconditions: flammable vegetation (e.g. fynbos, grassland, chaparral, Eucalyptus forest), and a long dry period such as in a winter rainfall climate where summers are hot and dry. Our data confirm that both these conditions were met. That the fynbos biome was already established by approximately 19.5 Ma is not unexpected and is supported by other plant groups. Verboom et al. [25] analysed 17 clades typical of the fynbos and identified three where the stem node was even older (Moraea, Iridaceae, 26.3 Ma; Ehrharta, Poaceae 40.9 Ma and Restionaceae, 61.3 Ma). Furthermore, the crown age of the Cape floral clades Crotalarieae p.p., Podalyriae (Fabaceae) and Leucadendriinae (Proteaceae) were estimated to be 46.3, 44.6 and 22–39 Ma, respectively [46,49]. Cape floral elements belonging to Restionaceae, Proteaceae and Ericaceae are already present in the Arnot Pipe deposits of Banke, which are confidently dated back to between 64 and 71 Ma [50]. These three families are also present in the lignite deposits of the Knysna area but the dating of these vary from as old as the Oligocene to as young as the Miocene [51–53]. Thus, many of the characteristic clades of fynbos were already present by the Middle/Late Miocene, indicating that the fynbos vegetation type might also have been present.
Based on the presumed intensification of the Benguela cold water upwelling, the modern Benguela current system had been dated to ca 10 Ma [54,55]. This was thought to have led to increased aridification of the Cape and to the inception of a Mediterranean type climate with most rainfall concentrated in winter by about 5.5–5 Ma [20,42,44]. However, the heightened productivity in the Benguela current, which was used to infer increased upwelling, is mirrored by similar increases in other non-upwelling parts of the oceans [56], and is now suggested to be part of global response to palaeoceanographic changes, rather than the result of a localized increase in the upwelling of cold nutrient-rich water. Linder [13] critically reviewed all fossil data and concluded that there is no direct evidence to suggest the establishment of the winter-rainfall regime at the Miocene–Pliocene boundary.
There is ample fossil charcoal (fusain) evidence for the presence of fires, almost since the first land plants were established [5,6]. The many Cretaceous fossil angiosperms preserved as charcoalified remains testify to the early connection between fires and angiosperms. However, fire during the Cenozoic is less well studied and understood [5]. A substantial increase in charcoal in marine sediments indicates that fires became more widespread during the Late Miocene, coinciding with the simultaneous spread of tropical savannas and humid C4 grasslands in Asia, Africa and America approximately 6–10 Ma [4,57]. Fire ecologists recognize different types of fire regimes and fires typically associated with the above vegetation recur on a 1–5 year cycle [57], whereas, for instance, those associated with Australian eucalypt forests occur on a cycle of 10–100 years [58]. The eucalypt fire system dates back to at least the Middle Miocene [59,60]. Mediterranean vegetation fire, such as those in the heathlands of the Cape fynbos, burn on a cycle of 5–50 years [17]. Little information is available on the age of fire in Mediterranean ecosystems. The first positive charcoal evidence of fires in southwestern Australia dates back to the mid-Pliocene. Atahan et al. [61] documented that 3.15 Ma deposits from Yallalie, showing a mix of heath and sclerophyll woodland with some remaining rainforest elements, had a fire frequency of between 5 and 13 years. No information on the age of fire regime in the Cape fynbos exists [13]. Consequently, the minimum date inferred here for fire in fynbos is quite possible, and is consistent with the globally available fossil data.
Eight out of 10 tested habitats are not randomly distributed on the phylogeny, implying phylogenetic niche conservatism for these habitat attributes (electronic supplementary material, table S2). Such niche conservatism has been repeatedly demonstrated [62–66], and in this case implies that the most recent common ancestor of almost all monophyletic sections [31] can be significantly optimized to a characteristic habitat: section Phlebidia, Vaginaria and Coryphaea are typically lithophytic or grow in rocky soil; Disella and Monadenia flower in the first year after fire; Reticulibractea, Trichochila, Stenocarpa, Spirales, Ovalifoliae and Schizodium occur in mature heath vegetation, while Aconitoideae and Micranthae are grassland clades. Section Stenocarpa can further be neatly divided into two clades, one which is fynbos adapted and the other which occurs in grassland. The only ecologically heterogeneous clade is comprised of sections Disa and Atromaculiferae, which is sister to the rest of the genus and cannot be assigned to one particular habitat. Thus, the basal split within the genus seems to have resulted in one small clade that has occupied a variety of habitats, whereas the rest of the genus progressively filled particular niches. Nevertheless, the extent of niche conservatism shown by most sections of Disa gives us confidence in projecting habitat attributes onto ancestral nodes.
Fossil and geological evidence show that the grassland and subalpine habitat were all established before they were occupied by Disa (electronic supplementary material, figure S6) indicating that our optimization methods are reliable. We dated grasslands to be at least 12.4 Ma (λm = 0.98; 95% CI =11.1–13.8 Ma) suggesting they were present in Africa from at least the Middle Miocene. Although the crown Poaceae are estimated to be approximately 96 Ma on the basis of molecular evidence [67] and the oldest, unequivocal African records of grass pollen found in Niger Delta date back to the Palaeocene [68], African grasslands and savannas only became more extensive from the Middle Miocene [8,69]. In the Niger Delta, grass cover expanded at ca 16 Ma and became widespread by the Late Miocene [70]. At Fort Ternan (Kenya), a grassland mosaic that supported Afromontane forest, alpine meadows and marsh was present 14 Ma [71,72]. By the Middle Miocene, Zambezian grassy woodlands were already widespread [42]. Our inferred date of first grassland occupation is thus strikingly consistent with the timing of grassland expansion. In fact, it seems that Disa occupied grassland almost as soon as it became available, which is not surprising as ground orchids in general are almost pre-adapted to this type of habitat. Grasslands lack competing shrubs and trees, and orchids, because of their tubers, are able to survive the regular fires that shape and maintain this vegetation. Similar cases of opportunistic immigration linked to species diversification have been recently proposed for other habitats and taxa in the Southern Hemisphere, as a consequence of the expansion of open habitats following the mid-Miocene Climatic Optimum. These include the orchid genus Hoffmannseggella in eastern South America [73] and Livistona palms in Australia [74]. Since climate cooling appears to have fostered the diversification of these clades in the past, current global warming may constitute an underestimated threat to the long-time conservation of plants in similar environments.
The occupation of the subalpine habitat by Disa is Plio-Pleistocene in age. The first occupation in East Africa by D. stairsii dates back to 4.3 Ma (95% CI = 2.9–5.8 Ma), while the earliest occupation in the Drakensberg (D. fragrans) is 4.1 Ma (95% CI = 2.7–5.6 Ma), both around the Miocene–Pliocene boundary. Most of the subalpine species endemic to the Drakensberg are, however, of Pleistocene origin. The subalpine zone of the Drakensberg was only established between 5 and 3 Ma when the eastern escarpment of southern Africa was uplifted by about 900 m to its current height [75,76]. In eastern Africa, this habitat is also of recent origin and was formed mostly as a result of rifting and volcanic activity during the Pliocene/Pleistocene [75].
5. Conclusions
The dating on the first occurrence of Disa in the alpine and grassland habitat is congruent with independently obtained geological and palaeoclimatological data. Indeed, here the fit of events is quite remarkable. The remaining optimizations are not in conflict with independent data, largely because there is none. Here, it provides new insights, namely that the radiation of many of the clades of the modern Cape flora was initiated in an environment with winter rainfall, and that diversification of these clades correlates with the establishment of a modern fire regime during a period of global temperature decrease, subsequent to the mid-Miocene Climatic Optimum.
There remains a serious lack of knowledge about the fundamental role of fire in global ecosystem patterns and processes [1]. Fossil data may be frustratingly rare, as it is the case of the Cape flora, and is almost always time-consuming to assemble. We believe that this investigation, as well as Simon et al. [2], has shown that mapping ecological characters on to robust, dated phylogenies may be a useful technique to supplement palaeontological information in understanding the development of fire adapted biomes globally.
Acknowledgements
We would like to thank Chloé Galley, Timo van der Niet and André Simões for helpful discussions, William Liltved for the fire photo, as well as Susanne Renner and two anonymous reviewers for comments that helped to improve the manuscript. B.B. was financially supported by the National Research Foundation (South Africa) and the Swiss Orchid Foundation at the herbarium Jany Renz (Basel, Switzerland).
References
- 1.Bowman D. M. J. S., et al. 2009. Fire in the Earth system. Science 324, 481–484 10.1126/science.1163886 (doi:10.1126/science.1163886) [DOI] [PubMed] [Google Scholar]
- 2.Simon M. F., Grether R., de Queiroz L. P., Skema C., Pennington R. T., Hughes C. E. 2009. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl Acad. Sci. USA 106, 20359–20364 10.1073/pnas.0903410106 (doi:10.1073/pnas.0903410106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond W. J., Keeley J. E. 2005. Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 20, 387–394 10.1016/j.tree.2005.04.025 (doi:10.1016/j.tree.2005.04.025) [DOI] [PubMed] [Google Scholar]
- 4.Bond W. J., Woodward F. I., Midgley G. F. 2005. The global distribution of ecosystems in a world without fire. New Phytol. 165, 525–538 10.1111/j.1469-8137.2004.01252.x (doi:10.1111/j.1469-8137.2004.01252.x) [DOI] [PubMed] [Google Scholar]
- 5.Scott A. C. 2000. The pre-quaternary history of fire. Paleogeogr. Paleoclimatol. Paleoecol. 164, 281–329 10.1016/S0031-0182(00)00192-9 (doi:10.1016/S0031-0182(00)00192-9) [DOI] [Google Scholar]
- 6.Scott A. C., Glasspool I. J. 2006. The diversification of Paleozoic fire systems and fluctuations in atmospheric oxygen concentration. Proc. Natl Acad. Sci. USA 103, 10861–10865 10.1073/pnas.0604090103 (doi:10.1073/pnas.0604090103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Hammen T., Hooghiemstra H. 2000. Neogene and quaternary history of vegetation, climate, and plant diversity in Amazonia. Quat. Sci. Rev. 19, 725–742 10.1016/S0277-3791(99)00024-4 (doi:10.1016/S0277-3791(99)00024-4) [DOI] [Google Scholar]
- 8.Jacobs B. 2004. Palaeobotanical studies from tropical Africa: relevance to the evolution of forest, woodland and savannah biomes. Phil. Trans. R. Soc. B 359, 1573–1583 10.1098/rstb.2004.1533 (doi:10.1098/rstb.2004.1533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs B. F., Herendeen P. S. 2004. Eocene dry climate and woodland vegetation in tropical Africa reconstructed from fossil leaves from northern Tanzania. Paleogeogr. Paleoclimatol. Paleoecol. 213, 115–123 [Google Scholar]
- 10.Diester-Haass L., Meyers A. P., Rothe P. 1992. The Benguela current and associated upwelling on the southwest African margin: a synthesis of the Neogene-Quaternary sedimentary record at DSDP sites 362 and 532. In Upwelling systems: evolution since the Early Miocene vol. 64 (eds Summerhayes C. P., Prell W. L., Emeis K. C.), pp. 331–342 London, UK: Geological Society Special Publication [Google Scholar]
- 11.Zachos J., Pagani M., Sloan L., Thomas E., Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 10.1126/science.1059412 (doi:10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- 12.Bobe R. 2006. The evolution of arid ecosystems in eastern Africa. J. Arid Environ. 66, 564–584 10.1016/j.jaridenv.2006.01.010 (doi:10.1016/j.jaridenv.2006.01.010) [DOI] [Google Scholar]
- 13.Linder H. P. 2003. The radiation of the Cape flora, southern Africa. Biol. Rev. 78, 597–638 10.1017/S1464793103006171 (doi:10.1017/S1464793103006171) [DOI] [PubMed] [Google Scholar]
- 14.Goldblatt P., Manning J. 2000. Cape plants. A conspectus of the Cape flora of South Africa. Strelitzia Cape Town, South Africa: National Botanical Institute [Google Scholar]
- 15.Goldblatt P., Manning J. C., Snijman D. A. 2005. Cape plants: corrections and additions to the flora. 1. Bothalia 35, 35–46 [Google Scholar]
- 16.Kier G., Kreft H., Lee T. M., Jetz W., Ibisch P. L., Nowicki C., Mutke J., Barthlott W. 2009. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl Acad. Sci. USA 106, 9322–9327 10.1073/pnas.0810306106 (doi:10.1073/pnas.0810306106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebelo A. G., Boucher C., Helme N. A., Mucina L., Rutherford M. C. 2006. Fynbos biome. In The vegetation of South Africa, Lesotho and Swaziland, Strelitzia, vol. 19 (eds Mucina L., Rutherford M. C.), pp. 52–219 Pretoria, South Africa: South African National Biodiversity Institute [Google Scholar]
- 18.Goldblatt P. 1978. An analysis of the flora of southern Africa: its characteristics, relationships, and orgins. Ann. Mo. Bot. Gard. 65, 369–436 10.2307/2398858 (doi:10.2307/2398858) [DOI] [Google Scholar]
- 19.Richardson J. E., Weitz F. M., Fay M. F., Cronk Q. C. B., Linder H. P., Reeves G., Chase M. W. 2001. Rapid and recent origin of species richness in the Cape flora of South Africa. Nature 412, 181–183 10.1038/35084067 (doi:10.1038/35084067) [DOI] [PubMed] [Google Scholar]
- 20.Klak C., Reeves G., Hedderson T. 2004. Unmatched tempo of evolution in Southern African semi-desert ice plants. Nature 427, 63–65 10.1038/nature02243 (doi:10.1038/nature02243) [DOI] [PubMed] [Google Scholar]
- 21.Goldblatt P., Savolainen V., Porteous O., Sostaric I., Powell M., Reeves G., Manning J. C., Barraclough T. G., Chase M. W. 2002. Radiation in the Cape flora and the phylogeny of peacock irises Moraea (Iridaceae) based on four plastid DNA regions. Mol. Phylogenet. Evol. 25, 341–360 10.1016/S1055-7903(02)00235-X (doi:10.1016/S1055-7903(02)00235-X) [DOI] [PubMed] [Google Scholar]
- 22.Bakker F. T., Culham A., Marais E. M., Gibby M. 2005. Nested radiation in Cape Pelargonium. In Plant species-level systematics: new perspectives on pattern and process, Regnum Vegetabile, vol. 143 (eds Bakker F. T., Chatrou L. W., Barbara G., Pelser P. B.), pp. 75–100 Ruggel, Liechtenstein: A.R.G. Gantner Verlag [Google Scholar]
- 23.Kreft H., Jetz W. 2007. Global patterns and determinants of vascular plant diversity. Proc. Natl Acad. Sci. USA 104, 5925–5930 10.1073/pnas.0608361104 (doi:10.1073/pnas.0608361104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adamson R. S. 1958. The Cape as an ancient flora. Adv. Sci. 58, 1–10 [Google Scholar]
- 25.Verboom G. A., et al. 2009. Origin and diversification of the Greater Cape flora: ancient species repository, hot-bed of recent radiation, or both? Mol. Phylogenet. Evol. 51, 44–53 10.1016/j.ympev.2008.01.037 (doi:10.1016/j.ympev.2008.01.037) [DOI] [PubMed] [Google Scholar]
- 26.Linder H. P. 2008. Plant species radiations: where, when, why? Phil. Trans. R. Soc. B 363, 3097–3105 10.1098/rstb.2008.0075 (doi:10.1098/rstb.2008.0075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linder H. P. 2005. Evolution of diversity: the Cape flora. Trends Plant Sci. 10, 536–541 [DOI] [PubMed] [Google Scholar]
- 28.Cowling R. M., Proches S., Partridge T. C. 2009. Explaining the uniqueness of the Cape flora: incorporating geomorphic evolution as a factor for explaining its diversification. Mol. Phylogenet. Evol. 51, 64–74 10.1016/j.ympev.2008.05.034 (doi:10.1016/j.ympev.2008.05.034) [DOI] [PubMed] [Google Scholar]
- 29.Bytebier B., Bellstedt D. U., Linder P. H. 2008. A new phylogeny-based sectional classification for the large African orchid genus Disa. Taxon 57, 1233–1251 [Google Scholar]
- 30.Linder H. P., Kurzweil H. 1999. Orchids of southern Africa. Rotterdam/Brookfield: A.A. Balkema [Google Scholar]
- 31.Bytebier B., Bellstedt D. U., Linder P. H. 2007. A molecular phylogeny for the large African orchid genus Disa. Mol. Phylogenet. Evol. 43, 75–90 10.1016/j.ympev.2006.08.014 (doi:10.1016/j.ympev.2006.08.014) [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson A. L., Verola C., Antonelli A. 2010. Reassessing the temporal evolution of orchids with new fossils and a Bayesian relaxed clock, with implications for the diversification of the rare South American genus Hoffmannseggella (Orchidaceae: Epidendroideae). BMC Evol. Biol. 10, 177. 10.1186/1471-2148-10-177 (doi:10.1186/1471-2148-10-177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanderson M. J. 2002. Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol. Biol. Evol. 19, 101–109 [DOI] [PubMed] [Google Scholar]
- 34.Drummond A., Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214. 10.1186/1471-2148-7-214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez S. R., Gravendeel B., Singer R. B., Marshall C. R., Pierce N. E. 2007. Dating the origin of the Orchidaceae from a fossil orchid with its pollinator. Nature 448, 1042–1045 10.1038/nature06039 (doi:10.1038/nature06039) [DOI] [PubMed] [Google Scholar]
- 36.Conran J. G., Bannister J. M., Lee D. E. 2009. Earliest orchid macrofossils: early Miocene Dendrobium and Earina (Orchidaceae: Epidendroideae) from New Zealand. Am. J. Bot. 96, 466–474 10.3732/ajb.0800269 (doi:10.3732/ajb.0800269) [DOI] [PubMed] [Google Scholar]
- 37.Doyle J., Endress P., Upchurch G. 2008. Early Cretaceous monocots: a phylogenetic evaluation. Acta Musei Nation. Pragae, Ser. B, Hist. Nat. 64, 59–87 [Google Scholar]
- 38.Hardy C. R., Linder H. P. 2005. Intraspecific variability and timing in ancestral ecology reconstruction: a test case from the Cape flora. Syst. Biol. 54, 299–316 10.1080/10635150590923317 (doi:10.1080/10635150590923317) [DOI] [PubMed] [Google Scholar]
- 39.Maddison W. P., Maddison D. R. 2009. Mesquite: a modular system for evolutionary analysis. Version 2.6. See http://mesquiteproject.org. [Google Scholar]
- 40.Frajman B., Eggens F., Oxelman B. 2009. Hybrid origins and homoploid reticulate evolution within Heliosperma (Sileneae, Caryophyllaceae)—a multigene phylogenetic approach with relative dating. Syst. Biol. 58, 328–345 10.1093/sysbio/syp030 (doi:10.1093/sysbio/syp030) [DOI] [PubMed] [Google Scholar]
- 41.Levyns M. R. 1964. Migrations and origin of the Cape flora. Trans. R. Soc. S. Afr. 37, 85–107 [Google Scholar]
- 42.Axelrod D. I., Raven P. H. 1978. Late Cretaceous and Tertiary vegetation history of Africa. In Biogeography and ecology of southern Africa, Monographiae Biologicae, vol. 31 (ed. Werger M. J. A.), pp. 77–130 Hague, The Netherlands: Dr. W. Junk bv Publishers [Google Scholar]
- 43.Linder H. P., Meadows M. E., Cowling R. M. 1992. History of the Cape flora. In The ecology of fynbos, nutrients, fire and diversity (ed. Cowling R. M.), pp. 114–134 Oxford, UK: Oxford University Press [Google Scholar]
- 44.Goldblatt P., Manning J. C. 2002. Plant diversity of the Cape region of southern Africa. Ann. Mo. Bot. Gard. 89, 281–302 10.2307/3298566 (doi:10.2307/3298566) [DOI] [Google Scholar]
- 45.Zachos J. C., Dickens G. R., Zeebe R. E. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451, 279–283 10.1038/nature06588 (doi:10.1038/nature06588) [DOI] [PubMed] [Google Scholar]
- 46.Sauquet H., Weston P. H., Barker N. P., Anderson C. L., Cantrill D. J., Savolainen V. 2009. Using fossils and molecular data to reveal the origins of the Cape proteas (subfamily Proteoideae). Mol. Phylogenet. Evol. 51, 31–43 10.1016/j.ympev.2008.12.013 (doi:10.1016/j.ympev.2008.12.013) [DOI] [PubMed] [Google Scholar]
- 47.Forest F., Nanni I., Chase M. W., Crane P. R., Hawkins J. A. 2007. Diversification of a large genus in a continental biodiversity hotspot: temporal and spatial origin of Muraltia (Polygalaceae) in the Cape of South Africa. Mol. Phylogenet. Evol. 43, 60–74 10.1016/j.ympev.2006.08.017 (doi:10.1016/j.ympev.2006.08.017) [DOI] [PubMed] [Google Scholar]
- 48.Quint M., Claßen-Bockhoff R. 2008. Ancient or recent? Insights into the temporal evolution of the Bruniaceae. Org. Divers. Evol. 8, 293–304 10.1016/j.ode.2008.03.001 (doi:10.1016/j.ode.2008.03.001) [DOI] [Google Scholar]
- 49.Edwards D., Hawkins J. A. 2007. Are Cape floral clades the same age? Contemporaneous origins of two lineages in the genistoids s.l. (Fabaceae). Mol. Phylogenet. Evol. 45, 952–970 [DOI] [PubMed] [Google Scholar]
- 50.Scholtz A. 1985. The palynology of the upper lacustrine sediments of the Arnot Pipe, Banke, Namaqualand. Ann. S. African Mus. 95, 1–109 [Google Scholar]
- 51.Thiergart F., Frantz U., Raukopf K. 1963. Palynologische Untersuchungen von Tertiärkohlen und einer oberflächen Probe nähe Knysna, Südafrika. Advancing Front. Plant Sci. 4, 151–178 [Google Scholar]
- 52.Coetzee J. A. 1983. Intimations of the tertiary vegetation of southern Africa. Bothalia 14, 345–354 [Google Scholar]
- 53.Thwaites R. N., Jacobs E. O. 1989. The Cenozoic history of the coastal landscape of the southern Cape province, South Africa: a review. Quat. Sci. Rev. 8, 283–293 10.1016/0277-3791(89)90043-7 (doi:10.1016/0277-3791(89)90043-7) [DOI] [Google Scholar]
- 54.Siesser W. G. 1978. Aridification of the Namib Desert; evidence from ocean cores. In Antartic glacial history and world paleoenvironments (ed. Van Zinderen-Bakker E. M.), pp. 105–113 Rotterdam, The Netherlands: A.A. Balkema [Google Scholar]
- 55.Siesser W. G. 1980. Late Miocene origin of the Benguela upwelling system off northern Namibia. Science 208, 283–285 10.1126/science.208.4441.283 (doi:10.1126/science.208.4441.283) [DOI] [PubMed] [Google Scholar]
- 56.Diester-Haass L., Meyers P. A., Vidal L. 2002. The late Miocene onset of high productivity in the Benguela Current upwelling system as part of a global pattern. Mar. Geol. 180, 87–103 10.1016/S0025-3227(01)00207-9 (doi:10.1016/S0025-3227(01)00207-9) [DOI] [Google Scholar]
- 57.Keeley J. E., Rundel P. W. 2005. Fire and the Miocene expansion of C4 grasslands. Ecol. Lett. 8, 683–690 10.1111/j.1461-0248.2005.00767.x (doi:10.1111/j.1461-0248.2005.00767.x) [DOI] [Google Scholar]
- 58.Gill A. M., Catling P. C. 2002. Fire regimes and biodiversity of forested landscapes of southern Australia. In Flammable Australia. The fire regimes and biodiversity of a continent (eds Bradstock R. A., Williams J. E., Gill A. M.), pp. 351–369 Cambridge, UK: Cambridge University Press [Google Scholar]
- 59.Kershaw A. P., Clark J. S., Gill A. M., D'Costa D. M. 2002. A history of fire in Australia. In Flammable Australia. The fire regimes and biodiversity of a continent (eds Bradstock R. A., Williams J. E., Gill M. A.), pp. 3–23 Cambridge, UK: Cambridge University Press [Google Scholar]
- 60.Hill R. S. 2004. Origins of the southeastern Australian vegetation. Phil. Trans. R. Soc. Lond. B 359, 1537–1549 10.1098/rstb.2004.1526 (doi:10.1098/rstb.2004.1526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atahan P., Dodson J. R., Itzstein-Davey F. 2004. A fine-resolution Pliocene pollen and charcoal record from Yallalie, south-western Australia. J. Biogeogr. 31, 199–205 [Google Scholar]
- 62.Peterson A. T., Soberón J., Sánchez-Cordero V. 1999. Conservatism of ecological niches in evolutionary time. Science 285, 1265–1267 10.1126/science.285.5431.1265 (doi:10.1126/science.285.5431.1265) [DOI] [PubMed] [Google Scholar]
- 63.Patterson T. B., Givnish T. J. 2002. Phylogeny, concerted convergence, and phylogenetic niche conservatism in the core Liliales: insights from rbcL and ndhF sequence data. Evolution 56, 233–252 [DOI] [PubMed] [Google Scholar]
- 64.Ackerly D. D. 2003. Community assembly, niche conservatism, and adaptive evolution in changing environments. Int. J. Plant Sci. 164, S165. 10.1086/368401 (doi:10.1086/368401) [DOI] [Google Scholar]
- 65.Svenning C. 2003. Deterministic Plio-Pleistocene extinctions in the European cool-temperate tree flora. Ecol. Lett. 6, 646–653 10.1046/j.1461-0248.2003.00477.x (doi:10.1046/j.1461-0248.2003.00477.x) [DOI] [Google Scholar]
- 66.Crisp M. D., et al. 2009. Phylogenetic biome conservatism on a global scale. Nature 458, 754–756 10.1038/nature07764 (doi:10.1038/nature07764) [DOI] [PubMed] [Google Scholar]
- 67.Bouchenak-Khelladi Y., Verboom G. A., Savolainen V., Hodkinson T. R. 2010. Biogeography of the grasses (Poaceae): a phylogenetic approach to reveal evolutionary history in geographical space and geological time. Bot. J. Linn. Soc. 162, 543–557 10.1111/j.1095-8339.2010.01041.x (doi:10.1111/j.1095-8339.2010.01041.x) [DOI] [Google Scholar]
- 68.Adegoke O. S., Jan du Chene R. E., Agumane A. E., Ajayi P. O. 1978. Palynology and age of the Keri-Keri Formation, Nigeria. Revista Esp. Micropaleontol. 10, 267–282 [Google Scholar]
- 69.Jacobs B. F., Kingston J. D., Jacobs L. L. 1999. The origin of grass-dominated ecosystems. Ann. Mo. Bot. Gard. 86, 590–643 10.2307/2666186 (doi:10.2307/2666186) [DOI] [Google Scholar]
- 70.Morley R. J., Richards K. 1993. Gramineae cuticle—a key indicator of late Cenozoic climatic-change in the Niger Delta. Rev. Palaeobot. Palynol. 77, 119–127 10.1016/0034-6667(93)90060-8 (doi:10.1016/0034-6667(93)90060-8) [DOI] [Google Scholar]
- 71.Retallack G. J. 1992. Middle Miocene fossil plants from Fort Ternan (Kenya) and evolution of African grasslands. Paleobiology 18, 383–400 [Google Scholar]
- 72.Dugas D. P., Retallack G. J. 1993. Middle Miocene fossil grasses from Fort Ternan, Kenya. J. Paleontol. 67, 113–128 [Google Scholar]
- 73.Antonelli A., Verola C., Parisod C., Gustafsson A. L. 2010. Climate cooling promoted the expansion and radiation of a threatened group of South American orchids (Epidendroideae: Laeliinae). Biol. J. Linn. Soc. 100, 597–607 10.1111/j.1095-8312.2010.01438.x (doi:10.1111/j.1095-8312.2010.01438.x) [DOI] [Google Scholar]
- 74.Crisp M. D., Isagi Y., Kato Y., Cook L. G., Bowman D. M. J. S. 2010. Livistona palms in Australia: Ancient relics or opportunistic immigrants? Mol. Phylogenet. Evol. 54, 512–523 10.1016/j.ympev.2009.09.020 (doi:10.1016/j.ympev.2009.09.020) [DOI] [PubMed] [Google Scholar]
- 75.Partridge T. C., Wood B. A., deMenocal P. B. 1995. The influence of global climatic change and regional uplift on large-mammalian evolution in east and southern Africa. In Paleoclimate and evolution, with emphasis on human origins (eds Vrba E. S., Denton G. H., Partridge T. C., Burckle L. H.), pp. 331–355 New Haven, CT: Yale University Press [Google Scholar]
- 76.McCarthy T., Rubidge B. 2005. The story of earth and life. A southern African perspective on a 4.6-billion-year journey. Cape Town, South Africa: Struik Publishers [Google Scholar]
- 77.Gradstein F. M., Ogg J. G. 2004. Geologic Time Scale 2004: why, how, and where next! Lethaia 37, 175–181 10.1080/00241160410006483 (doi:10.1080/00241160410006483) [DOI] [Google Scholar]