Abstract
Most models of animal foraging and consumer choice assume that individuals make choices based on the absolute value of items and are therefore ‘economically rational’. However, frequent violations of rationality by animals, including humans, suggest that animals use comparative valuation rules. Are comparative valuation strategies a consequence of the way brains process information, or are they an intrinsic feature of biological decision-making? Here, we examine the principles of rationality in an organism with radically different information-processing mechanisms: the brainless, unicellular, slime mould Physarum polycephalum. We offered P. polycephalum amoebas a choice between food options that varied in food quality and light exposure (P. polycephalum is photophobic). The use of an absolute valuation rule will lead to two properties: transitivity and independence of irrelevant alternatives (IIA). Transitivity is satisfied if preferences have a consistent, linear ordering, while IIA states that a decision maker's preference for an item should not change if the choice set is expanded. A violation of either of these principles suggests the use of comparative rather than absolute valuation rules. Physarum polycephalum satisfied transitivity by having linear preference rankings. However, P. polycephalum's preference for a focal alternative increased when a third, inferior quality option was added to the choice set, thus violating IIA and suggesting the use of a comparative valuation process. The discovery of comparative valuation rules in a unicellular organism suggests that comparative valuation rules are ubiquitous, if not universal, among biological decision makers.
Keywords: rationality, slime moulds, foraging, content-dependent, transitivity, independence of irrelevant alternatives
1. Introduction
How do individuals make decisions when choosing between items that vary in two or more attributes, particularly when these attributes are in conflict? A common example involves choosing a restaurant: should we select the higher priced, but higher quality restaurant or the lower priced, lower quality restaurant? Many models of human and animal decision-making assume that individuals use absolute valuation rules by weighing the value of each item's attributes separately and then summing them to arrive at the item's absolute value. The decision maker then selects the item with the highest valuation index [1]. Value is therefore an intrinsic property of the item, and should not change if other options are present. By contrast, a comparative decision-making mechanism might involve ranking each item's attributes separately, and then summing these to arrive at an overall ranking. The relative value of an item therefore depends on other options in the choice set [2]. Although they do not conform to most models of economic rationality, comparative valuation mechanisms might be favoured by natural selection because these methods often produce similar results to absolute valuation, but are computationally efficient [2–4]. Nevertheless, the assumption of absolute valuation underpins many models of human and animal decision-making despite evidence that humans [5,6] bees [2,7] and birds [7–9] seem to use comparative, rather than absolute valuation rules. Do these violations of rationality indicate that comparative valuation rules are the norm for biological decision makers, or do they occur as a consequence of neuron-based decision-making systems?
Here, we examine valuation rules in the food choices of an organism with radically different information-processing mechanisms to all other organisms studied thus far: plasmodia of the acellular slime mould, P. polycephalum (Supergroup: Amoebozoa). Unlike previously studied organisms, slime moulds lack a brain, and all information processing occurs via highly decentralized processes. During its ‘plasmodium’ life-stage P. polycephalum consists of a single, multi-nucleate cell that searches for food by moving through its environment in an amoeboid manner. The use of comparative valuation rules by these simple, unicellular organisms would strongly suggest that comparative valuation rules are intrinsic to biological decision-making and that economic and behavioural models based on absolute valuation are untenable.
Absolute valuation should cause preferences to be consistent across contexts, a concept encapsulated in two major principles: transitivity [5] and independence of irrelevant alternatives (IIA; [10]). Weak stochastic transitivity requires that preferences have a consistent, internally coherent ordering: for example, if option A is preferred to option B, and option B is preferred to option C, then weak transitivity implies that option A should be preferred to option C. Strong stochastic transitivity requires the strength of preferences to be consistent such that the strength of preference between options on the extremes of the preference scale (A versus C in the above example) will be equal to or stronger than between items adjacent in the preference ordering (A versus B or B versus C). Violations of transitivity are inconsistent with absolute valuation and suggest the use of comparative valuation rules.
Independence of irrelevant alternatives holds that a decision maker's preference for a particular option should not change when a new option of lesser value is added to the choice set. One version of IIA, called the constant ratio rule, states that the relative proportion of choices made between two options should be the same regardless of whether they are presented on their own or in the presence of a third, less preferred option [10]. Violations of the constant-ratio rule can occur for two reasons: either because the organism uses comparative, rather than absolute valuation mechanisms, or because the organism violates Luce's axiom, which states that the probability of selecting an option is proportional to the ratio between the value of that option and the sum of the values of the other available options [10]. For example, the random dilution effect is a rational choice mechanism that can, nevertheless, result in violations of the constant-ratio rule [3]. An individual could use a decision rule such that it first allocates a fixed proportion of its choices to a preferred option, and then randomly allocates the remaining preference between the non-preferred options. The random dilution effect occurs because the addition of a new item to the choice set dilutes the effect of the random choices, resulting in a change in the relative preference for the focal item. Although violation of the constant-ratio rule is suggestive of comparative valuation mechanisms, it does not rule out the possibility of effects such as the random dilution effect. A stronger version of IIA, known as the principle of ‘regularity’ provides stronger evidence of absolute valuation mechanisms. Regularity is violated if the addition of a new alternative to a choice set causes an increase in absolute preference for one of the original options. Violations of regularity can only occur if an organism uses a comparative valuation mechanism [3].
2. Material and Methods
Our original P. polycephalum culture was obtained from Southern Biological Supplies. We reared the culture on 30 × 20 cm plastic tubs containing 2 per cent agar. Cultures were maintained at 24°C in the dark. We fed cultures on flakes of rolled oats (Carmen's Organic, Australia), which were liberally sprinkled across the surface of the agar daily. We sub-cultured plasmodia into new tubs every 2 days.
One day prior to the experiment, laboratory cultures of P. polycephalum were randomly assigned to a starved or non-starved treatment group. Plasmodia to be used in our starved treatment were sub-cultured onto tubs containing only agar and no food, while those to be used in our non-starved treatment were sub-cultured onto agar sprinkled with oatmeal.
Plasmodial fragments were obtained for the experiment by cutting small pieces from either the starved or non-starved cultures. The mean weight of plasmodial fragments was 0.01 ± 0.0009 g (n = 649). Plasmodial fragments become fully functional individuals within minutes of being separated from the main cell [11]. Food disks were made by mixing differing amounts (3%, 5% or 10%) of finely ground oats into the liquid of 2 per cent agar, and then pouring the agar into 2.5 mm (diameter) holes cut out of a base of 2 per cent agar. In the binary choice trials, food sources were side by side with approximately 5 mm space of clear agar between them. In the ternary experiments, food sources were arranged into a triangle, with approximately 5 mm space between options.
Our first goal was to determine whether or not plasmodia meet the requirements for transitivity. It has been suggested that many apparent violations of rationality may be owing to inadvertent changes in an animal's state caused by training regimes [12]. We examined this hypothesis by testing for transitivity and IIA in both starved and non-starved plasmodia. We offered plasmodia a choice between two food disks that differed in the concentration of nutrients (oatmeal) and exposure to light. In P. polycephalum exposure to UV interferes with cellular processes, causes nuclear degeneration [13] and induces sporulation [14]. We used three levels of oatmeal concentration (3%, 5% and 10%) and two illumination levels: Light (‘L’, 750 lux) and Dark (‘D’, 43 lux). Forty-three lux of illumination was achieved by shading the food disk with black construction paper. By exposing the food patch to ambient laboratory light from fluorescent bulbs mounted approximately 5 m above the laboratory bench, 750 lux was achieved. Combining each level of oatmeal concentration with each level of illumination resulted in six food options: 3L, 3D, 5L, 5D, 10L, 10D, where the number indicates the oatmeal concentration. Each food option was paired against every other option for a total of 15 binary choice experiments. Starved (n = 15) and non-starved plasmodia (n = 15) were assigned to each binary choice experiment. A single plasmodium was placed in the centre of the arena so that it was in direct contact (and thus aware of) with all food options. The plasmodium's final choice was recorded after 24 h. Since the plasmodium is amoeboid, it is possible for it to select two or more food sources simultaneously; these six events were classified as ‘split decisions’ and were omitted from the analyses. Since slime moulds leave behind a mucous trail as they move, we were able to rule out the possibility that slime moulds moved between multiple options during the course of the experiment.
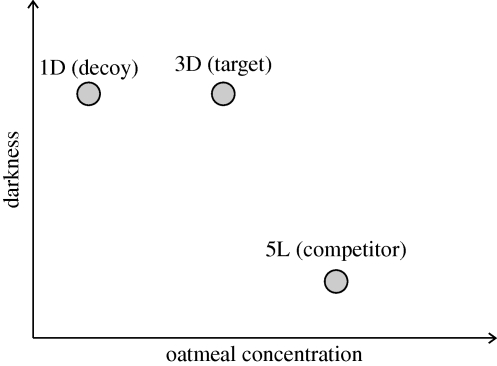
Next, we examined IIA in both starved and non-starved plasmodia using binary and ternary choice trials. In binary trials, the plasmodium was given a choice between a target option (3D) that was high in one attribute (light level), and a competitor that was high in the other attribute (5L) (nutrient concentration). In ternary trials, a decoy (1D) was added to the choice set. In this configuration, 1D is said to be ‘asymmetrically dominated’, a configuration that is known to elicit violations of IIA in humans [15] and other animals [7] (figure 1).
Figure 1.
Testing for the asymmetrically dominated decoy effect. An option is said to be dominated if it is lower in one attribute than an alternative. A decoy is asymmetrically dominated if it is inferior to one option in all attributes, but is only inferior to the other option along one attribute. In our experiment, the decoy (1D) is dominated on both attributes by the target (3D), but is only dominated by the competitor along one attribute (oatmeal concentration).
3. Results
(a). Transitivity
Rank was determined by counting the number of binary competitions ‘won’ by each food option (indicated in brackets). The ranking for non-starved plasmodia was 10D (5) >5D (4) >10L (3) >3D (2) >5L (1) >3L (0). The ranking for starved plasmodia was 10D (5) >10L (4) >5D (3) >3D (2) >(5L = 3L) (one each). Both non-starved and starved plasmodia had linear preference rankings that satisfied weak stochastic transitivity (table 1). To test for violations of strong stochastic transitivity, we divided the preference rankings into four sets of triplets, with each triplet containing three adjacent options (table 2). For each triplet, we examined whether the number of plasmodia selecting the winning option was higher for the adjacent pairs than for the extreme pair using a one-sided Fisher's exact test. For both non-starved and starved plasmodia, the number of plasmodia selecting the wining option at the extremes of the preference rankings was not significantly stronger than for any pair of options adjacent in the rankings (table 2). Thus, both starved and non-starved P. polycephalum plasmodia satisfied the requirements for strong and weak transitivity. While violations of transitivity constitute strong evidence of comparative decision-making strategies, it is important to note that the reverse is not true, and that the slime mould's ability to make transitive decisions does not necessarily imply that the organism uses an absolute valuation process. This is because a comparative decision-making mechanism will lead to intransitivity only under a narrow range of conditions [2].
Table 1.
Results of binomial choice trials on non-starved and starved plasmodia. (Values in bold are for non-starved plasmodia; values in plain text are for starved plasmodia. The food option in each cell is the winner (significantly greater than 50%) of that binary competition. The number in brackets shows the percentage of plasmodia that selected the winning option (excluding split decisions, those that died, and those that selected neither option). The number underneath is the p-value for the binomial test. n = 15 (unless marked with an asterisk). *2 plasmodia selected both and were omitted. n = 13, **1 plasmodium selected both and was omitted, 1 died. n = 13, ***2 plasmodia selected both and were omitted. n = 13, ****1 plasmodium selected both and was omitted. n = 14.)
| 3D | 5L | 5D | 10L | 10D | ||
|---|---|---|---|---|---|---|
| 3L | 3D (100%) p < 0.0001 3D (100%) p < 0. 0001 | 5L (80%) p = 0.0165L (57%) p = 0.52* | 5D (100%) p < 0.0001 5D (100%) p < 0.0001 | 10L(100%) p < 0.0001 10L (100%) p < 0.0001 | 10D (100%) p < 0.0001 10D (100%) p < 0.0001 | |
| 3D | 3D (78%) p = 0.027 3D (77%) p = 0.046** | 5D (92%) p = 0.0009*** 5D (80%) p = 0.016 | 10L (100%) p < 0.0001 10L (80%) p = 0.016 | 10D (100%) p < 0.0001 10D (94%) p = 0.0001 | ||
| 5L | 5D (100%) p < 0.0001**** 5D (100%) p < 0.0001 | 10L (86%) p = 0.0027 10L (100%) p < 0.001 | 10D (100%) p < 0.0002**** 10D (100%) p < 0.001 | |||
| 5D | 5D (93%) p = 0.0002 10L (100%) p < 0.0001*** | 10D (100%) p < 0.0001; 10D (86%) p = 0.0027 | ||||
| 10L | 10D (86%) p = 0.0027 10D (100%) p < 0.0001 | |||||
| 10D |
Table 2.
Results of tests for strong stochastic transitivity. (The preference order was broken into triplets, and the extreme parings of each triplet were compared with the adjacent parings using a one-sided Fisher's exact test. p-values < 0.05 do not satisfy strong stochastic transitivity. *5L and 3L were tied in ranking, so a triplet was constructed using both values.)
| triplet | extreme pair | adjacent pair1 | p, adjacent1 versus extreme | adjacent pair 2 | p, adjacent2 versus extreme |
|---|---|---|---|---|---|
| non-starved | |||||
| 10D,5D,10L | 10D versus 10L | 10D versus 5D | 0.26 | 5D versus 10L | 0.50 |
| 5D,10L,3D | 5D versus 3D | 5D versus 10L | 0.72 | 10L versus 3D | 0.46 |
| 10L,3D,5L | 10L versus 5L | 10L versus 3D | 0.24 | 3D versus 5L | 0.26 |
| 3D,5L,3L | 3D versus 3L | 3D versus 5L | 1.0 | 5L versus 3L | 1.0 |
| starved | |||||
| 10D,10L,5D | 10D versus 5D | 10D versus 10L | 0.24 | 10L versus 5D | 0.32 |
| 10L,5D,3D | 10L versus 5D | 10L versus 5D | 0.17 | 5D versus 3D | 0.67 |
| 5D,3D,5L* | 5D versus 5L | 5D versus 3D | 1.00 | 3D versus 5L | 1.0 |
| 5D,3D,3L* | 5D versus 3L | 5D versus 3D | 0.11 | 3D versus 3L | 1.0 |
(b). Independence of irrelevant alternatives
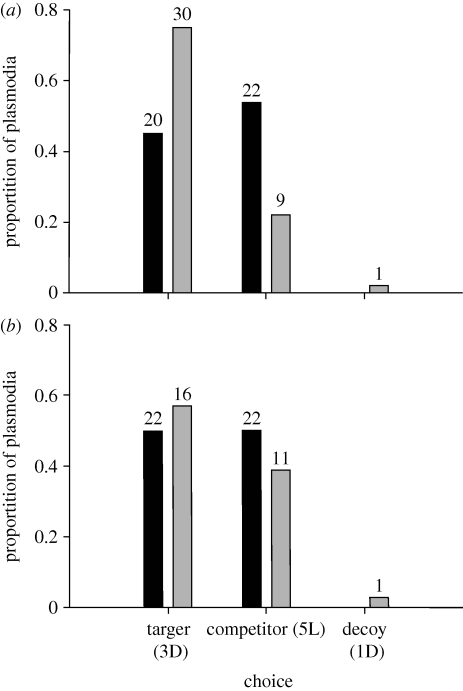
In the IIA experiment, 39 plasmodia (19%) made split decisions by choosing two or more options; these were omitted from the general analysis. Split decisions were always between 3D and 5L, and never included 1D. We examined the effect of context (ternary, binary) and starvation on the probability of making a ‘split decision’ using a multiple nominal logistic model. Context had a significant effect on split decision such that plasmodia in the ternary trials were more likely to make a split decision than those in the binary trials (p = 0.0003, χ2 = 13.33, n = 196). Starved plasmodia made more split decisions than did non-starved plasmodia (p = 0.005, χ2 = 7.85, n = 196).
In the binary choice trials, neither non-starved nor starved plasmodia showed a preference for the target or the competitor (Binomial test (probability of 0.5): p = 0.54, n = 42; p = 1.0, n = 44, respectively)). The absolute number of non-starved plasmodia that selected the target was significantly affected by context (binary or ternary), such that plasmodia were more likely to choose the target in the ternary trials (figure 2; χ2-test: χ2 = 7.76, d.f. = 1, p = 0.005, n = 84). Non-starved plasmodia therefore violate the principle of regularity. In starved plasmodia, context had no significant effect on the absolute number of plasmodia selecting the target (χ2 = 0.351, p = 0.533, d.f. = 1, n = 72), thus satisfying regularity.
Figure 2.
The proportion of plasmodia selecting the target, competitor or the decoy. (a) Non-starved plasmodia. (b) Starved plasmodia. The numbers above the bars indicate the number of plasmodia in each group. (a,b) Grey bars, binary; black bars, ternary.
To examine relative preference (the constant-ratio rule), we omitted plasmodia that selected the decoy in the ternary trials. In non-starved plasmodia, the relative proportion of plasmodia choosing the target was significantly affected by context, such that the relative proportion choosing the target was higher in the ternary trials (χ2-test: χ2 = 8.7, d.f. = 1 p = 0.003, n = 83). This violates the constant-ratio rule. Among starved plasmodia, context did not affect the relative proportion of plasmodia selecting the target (χ2-test: p = 0.44, d.f. = 1, χ2 = 0.57, n = 71). Starved plasmodia therefore satisfy the constant-ratio rule.
4. Discussion
Starved and non-starved P. polycephalum satisfied both weak and strong transitivity by having consistent linear preference rankings. However, non-starved plasmodia violated IIA by increasing both absolute and relative preference for a target option when an inferior decoy was added to the choice set. Violations of regularity are incompatible with absolute valuation mechanisms and instead suggest that P. polycephalum uses a comparative valuation process. The shift in preference cannot be explained by incomplete information or training effects because each plasmodium initially touched all three food sources (see the electronic supplementary material). Rather, it is probably a consequence of P. polycephalum's underlying decision-making process. Violations of IIA have now been observed in several widely separate taxa including hummingbirds [16], starlings [17], humans [18] and honeybees [7]. The discovery of comparative valuation rules in an organism taxonomically distant from animals suggests that these valuation rules may be a common feature of biological decision-making. We therefore suggest that the assumption of absolute valuation in models of human and animal choice behaviour is untenable.
Why is comparative valuation so common among biological decision makers? Comparative decision-making processes are generally less computationally intensive than absolute decision-making mechanisms; under most conditions, they will yield results similar to those reached via an absolute process [2]. Natural selection could favour computationally efficient comparative strategies over the more accurate, but more intensive absolute decision-making strategies [19]. Alternatively, comparative decision-making strategies may arise as an unavoidable consequence of the way in which living systems process information. Studies on decision-making in organisms with a wider range of information-processing systems (such as other unicellular organisms, fungi, cnidarians, etc.) would help determine to what extent living things share a common underlying information-processing system, and could clarify any intrinsic constraints of biological information processing. It is also important to note that although we have shown that P. polycephalum behaves ‘irrationally’ this does not necessarily imply that its behaviour is maladaptive. The experimental environment is novel to slime moulds, and their behaviour might appear maladaptive in the context of the experiment, but may work well in the environments slime moulds have evolved in [20]. Further, recent work suggests that irrational behaviour can, under certain environmental conditions, be consistent with maximizing an organism's expected pay-off [21].
Physarum polycephalum's preference ranking shows that plasmodia made trade-offs between light exposure and food quality. This is consistent with previous work showing that P. polycephalum can make trade-offs between danger and food quality [22]. Physarum polycephalum's ability to make trade-offs suggests that its decision-making strategy is compensatory such that poor values in one of an option's attributes (for example, light exposure) can be compensated by high values in another attribute (oatmeal concentration). Compensatory decision-making processes require the organism to rank each attribute and are therefore more computationally expensive than non-compensatory strategies [23]. Yet, despite lacking a brain P. polycephalum is capable of making consistent, transitive decisions when choosing between food sources that vary in multiple attributes.
Our results further show that P. polycephalum uses information about its internal state when making decisions. Starved plasmodia were more willing to accept light exposure in order to obtain a particularly nutritious food patch (10L), while non-starved plasmodia preferred the lower quality, but ‘safer’ alternative (5D). This tendency to forage in a dangerous patch when starved also occurs in animals, where starved individuals are more likely to forage in environments with high predation risk, while non-starved individuals tend to prefer safer habitats [24–26]. Surprisingly, starved plasmodia did not have a significant preference for either 3L or 5L even though the consumption of 5L yields twice as much growth as feeding on 3L (see [22], table 1). Starved plasmodia were also more likely to make a split decision by allocating biomass to two food disks. The starved plasmodia's indifference seems to indicate either decreased selectivity or a starvation-induced reduction in the ability to distinguish between similar food sources.
Owing to the slimy nature of acellular slime moulds, it was not possible to test the transitivity and IIA in individuals, and instead, we relied upon population-level preferences. This can cause problems because, under certain conditions, intransitivity of group preferences can arise even if individual preferences are transitive, resulting in a ‘voting paradox’ (also known as Condorcet's paradox). This happens because individuals within the group may have different preference orders. Designing studies that minimize between-individual variation, as we have done in our study, can reduce the likelihood of these paradoxes [27]. Nevertheless, data showing intransitivity on a group level must always be dealt with cautiously. In this respect, the strongest evidence of comparative valuation processes in P. polycephalum comes from the plasmodia's violation of regularity. Violations of regularity at an aggregate level are not predicted unless at least some individuals within the population truly violate regularity [27].
Given that they lack brains (or any form of centralized information processing), how do slime moulds make decisions? Acellular slime moulds, like insect colonies, are collective decision makers, where the behaviour of the collective is a result of the behaviour of its underlying parts. Each slime mould is made up of many tiny pieces of slime mould, each oscillating at a frequency determined partly by the local environment, and partly by interactions with adjacent oscillators such that each oscillator can entrain those close to it [28]. Contact with attractants increases the oscillation frequency while contact with repellents (i.e. light, salts) decreases the frequency of oscillations. When a plasmodium senses or comes into contact with food, increased oscillation frequencies in the region closest to the food source cause biomass to flow towards the attractant [28]. The behaviour of the organism as a whole results from the collective behaviour of internal oscillators. This relatively simple mechanism apparently allows the plasmodium to process information and make decisions. How exactly these factors tie together to result in a comparative decision process is unknown, but is the focus of current research.
Recent work on rationality in ants has led to the suggestion that organisms using collective decision-making processes may be immune to irrational decisions [29]. In collective decision processes, the group's decision may result from the independent assessments of many individuals [29]. In house hunting ants, for example, each scout is thought to assess a single site before returning information to the colony. Since each ant evaluates a single site, there is no difference (from the ant's point of view) between the binary and ternary situation. As a result, the information the colony receives is not influenced by the addition of new alternatives [29]. Physarum polycephalum, which also has a decentralized decision-making mechanism, violated IIA. This suggests that decentralized decision-making systems may be susceptible to IIA under certain conditions. Unlike the ant system, however, we know very little about how individual slime mould components evaluate options, nor do we fully understand the recruitment process, or how information spreads through the plasmodium. We suggest that differences in the organization of collective decision-making systems probably influence the extent to which the group behaves rationally.
Interestingly, even within a treatment group, slime moulds varied in their choices. This is particularly surprising as we controlled for weight, nutritional state and genetic differences. We suggest that some of the variability we observed arises from slight differences in the experiments initial conditions. Although every attempt was made to ensure that slime moulds were equally in contact with all available options, we cannot control whether some parts of the slime mould began moving faster than others. These small differences in initial condition, combined with feedback via biomass recruitment mechanisms, could ultimately result in the observed variability. This sensitivity to starting conditions is similar to that observed in trail-laying ants, where small differences in the number of ants visiting one of two equal quality feeders ultimately resulted in one feeder being selected over the other [30]. Our results support the suggestion that collective decision makers may be very sensitive to initial conditions.
It is remarkable that P. polycephalum, which belongs to an entirely different kingdom of life and lacks a central nervous system, uses the same comparative decision-making processes as do neurologically sophisticated organisms. The ubiquity of comparative decision-making across taxa regardless of neurological complexity suggests that these processes are not constrained by particular information-processing systems and may be an intrinsic feature of biological decision-making.
Acknowledgements
This work was funded by the Human Frontiers Science Programme. Thank you to members of the Social Insects laboratory for comments on an earlier version of this manuscript. Special thanks to Steve Simpson, Ben Oldroyd and Sharoni Shafir for comments on earlier versions of the manuscript. This manuscript was greatly improved by the input of three anonymous reviewers.
References
- 1.Rapoport A. 1989. Decision theory and decision behaviour. London, UK: Kluwer Academic Publishers [Google Scholar]
- 2.Shafir S. 1994. Intransitivity of preferences in honey bees: support for ‘comparative' evaluation of foraging options. Anim. Behav. 48, 55–67 10.1006/anbe.1994.1211 (doi:10.1006/anbe.1994.1211) [DOI] [Google Scholar]
- 3.Bateson M. 2004. Mechanisms of decision-making and the interpretation of choice tests. Anim. Welfare 13, 115–120 [Google Scholar]
- 4.Gigerenzer G. 1997. Bounded rationality models of fast and frugal inference. Swiss J. Econ. Stat. 133, 201–218 [Google Scholar]
- 5.Tversky A. 1969. Intransitivity of preferences. Psychol. Rev. 76, 31–48 10.1037/h0026750 (doi:10.1037/h0026750) [DOI] [Google Scholar]
- 6.Tversky A. 1969. Substitutability and similarity in binary choices. J. Math. Psychol. 6, 1–12 10.1016/0022-2496(69)90027-3 (doi:10.1016/0022-2496(69)90027-3) [DOI] [Google Scholar]
- 7.Shafir S., Waite T. A., Smith B. H. 2002. Context-dependent violations of rational choice in honeybees (Apis mellifera) and gray jays (Perisoreus canadensis). Behav. Ecol. Sociobiol. 51, 180–187 10.1007/s00265-001-0420-8 (doi:10.1007/s00265-001-0420-8) [DOI] [Google Scholar]
- 8.Bateson M. 2002. Context-dependent foraging choices in risk-sensitive starlings. Anim. Behav. 64, 251–260 10.1006/anbe.2002.3059 (doi:10.1006/anbe.2002.3059) [DOI] [Google Scholar]
- 9.Bateson M., Healy S., Hurly T. A. 2002. Irrational choices in hummingbird foraging behaviour. Anim. Behav. 63, 587–596 10.1006/anbe.2001.1925 (doi:10.1006/anbe.2001.1925) [DOI] [Google Scholar]
- 10.Luce R. D. 1959. Individual choice behavior: a theoretical analysis. New York, NY: Wiley [Google Scholar]
- 11.Yoshimoto Y., Kamiya N. 1978. Studies on contraction rhythm of the plasmodial strand. I. Synchronization of local rhythms. Protoplasma 95, 89–99 10.1007/BF01279697 (doi:10.1007/BF01279697) [DOI] [Google Scholar]
- 12.Schuck-Paim C., Pompilio L., Kacelnik A. 2004. State-dependent decisions cause apparent violations of rationality in animal choice. PLoS Biol. 2, e402. 10.1371/journal.pbio.0020402 (doi:10.1371/journal.pbio.0020402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devi V. R., Guttes E., Guttes S. 1968. Effects of ultraviolet light on mitosis in Physarum polycephalum. Exp. Cell Res. 50, 598. [DOI] [PubMed] [Google Scholar]
- 14.Dove W. F., Rusch H. P. (ed.) 1980. Growth and differentiation in Physarum polycephalum. Princeton, NJ: Princeton University Press [Google Scholar]
- 15.Huber J., Payne J. W., Puto C. 1982. Adding asymmetrically dominated alternatives: violations of regularity and the similarity hypothesis. J. Consum. Res. 9, 90. 10.1086/208899 (doi:10.1086/208899) [DOI] [Google Scholar]
- 16.Hurly T. A., Oseen M. D. 1999. Context-dependent, risk-sensitive foraging preferences in wild rufous hummingbirds. Anim. Behav. 58, 59–66 10.1006/anbe.1999.1130 (doi:10.1006/anbe.1999.1130) [DOI] [PubMed] [Google Scholar]
- 17.Schuck-Paim C., Kacelnik A. 2007. Choice processes in multialternative decision making. Behav. Ecol. 18, 541–550 10.1093/beheco/arm005 (doi:10.1093/beheco/arm005) [DOI] [Google Scholar]
- 18.Tversky A., Simonson I. 1993. Context-dependent preferences. Manage. Sci. 39, 1179–1189 10.1287/mnsc.39.10.1179 (doi:10.1287/mnsc.39.10.1179) [DOI] [Google Scholar]
- 19.Hutchinson J. M. C., Gigerenzer G. 2005. Simple heuristics and rules of thumb: where psychologists and behavioural biologists might meet. Behav. Process. 69, 97–124 10.1016/j.beproc.2005.02.019 (doi:10.1016/j.beproc.2005.02.019) [DOI] [PubMed] [Google Scholar]
- 20.Houston A. I., McNamara J. M., Steer M. D. 2007. Do we expect natural selection to produce rational behaviour? Phil. Trans. R. Soc. B 362, 1531. 10.1098/rstb.2007.2051 (doi:10.1098/rstb.2007.2051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waksberg A., Smith A., Burd M. 2009. Can irrational behaviour maximise fitness? Behav. Ecol. Sociobiol. 63, 461–471 10.1007/s00265-008-0681-6 (doi:10.1007/s00265-008-0681-6) [DOI] [Google Scholar]
- 22.Latty T., Beekman M. 2010. Food quality and the risk of light exposure affect patch choice decisions in the acellular slime mould Physarum polycephalum. Ecology 91, 22–27 10.1890/09-0358.1 (doi:10.1890/09-0358.1) [DOI] [PubMed] [Google Scholar]
- 23.Franks N. R., Mallon E. B., Bray H. E., Hamilton M. J., Mischler T. C. 2003. Strategies for choosing between alternatives with different attributes: exemplified by house-hunting ants. Anim. Behav. 65, 215–223 10.1006/anbe.2002.2032 (doi:10.1006/anbe.2002.2032) [DOI] [Google Scholar]
- 24.Godin J., Crossman S. 1994. Hunger-dependent predator inspection and foraging behaviours in the threespine stickleback (Gasterosteus aculeatus) under predation risk. Behav. Ecol. Sociobiol. 34, 359–366 10.1007/BF00197006 (doi:10.1007/BF00197006) [DOI] [Google Scholar]
- 25.Gotceitas V., Godin J. 1991. Foraging under the risk of predation in juvenile Atlantic salmon (Salmo salar L.): effects of social status and hunger. Behav. Ecol. Sociobiol. 29, 255–261 10.1007/BF00163982 (doi:10.1007/BF00163982) [DOI] [Google Scholar]
- 26.Pettersson L., Brönmark C. 1993. Trading off safety against food: state dependent habitat choice and foraging in crucian carp. Oecologia 95, 353–357 10.1007/BF00320988 (doi:10.1007/BF00320988) [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson J. W., Kamakura W. A., Lynch J. G. 2000. Unobserved heterogeneity as an alternative explanation for ‘reversal’ effects in behavioral research. J. Consum. Res. 27, 324–344 10.1086/317588 (doi:10.1086/317588) [DOI] [Google Scholar]
- 28.Durham A. C., Ridgway E. B. 1976. Control of chemotaxis in Physarum polycephalum. J. Cell Biol. 69, 218–223 10.1083/jcb.69.1.218 (doi:10.1083/jcb.69.1.218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards S. C., Pratt S. C. 2009. Rationality in collective decision-making by ant colonies. Proc. R. Soc. B 266, 3655–3661 10.1098/rspb.2009.0981 (doi:10.1098/rspb.2009.0981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumpter D. J. T., Beekman M. 2003. From nonlinearity to optimality: pheromone trail foraging by ants. Anim. Behav. 66, 273–280 10.1006/anbe.2003.2224 (doi:10.1006/anbe.2003.2224) [DOI] [Google Scholar]