Abstract
Determining the effect of an invasive species on enzootic pathogen dynamics is critical for understanding both human epidemics and wildlife epizootics. Theoretical models suggest that when a naive species enters an established host–parasite system, the new host may either reduce (‘dilute’) or increase (‘spillback’) pathogen transmission to native hosts. There are few empirical data to evaluate these possibilities, especially for animal pathogens. Buggy Creek virus (BCRV) is an arthropod-borne alphavirus that is enzootically transmitted by the swallow bug (Oeciacus vicarius) to colonially nesting cliff swallows (Petrochelidon pyrrhonota). In western Nebraska, introduced house sparrows (Passer domesticus) invaded cliff swallow colonies approximately 40 years ago and were exposed to BCRV. We evaluated how the addition of house sparrows to this host–parasite system affected the prevalence and amplification of a bird-associated BCRV lineage. The infection prevalence in house sparrows was eight times that of cliff swallows. Nestling house sparrows in mixed-species colonies were significantly less likely to be infected than sparrows in single-species colonies. Infected house sparrows circulated BCRV at higher viraemia titres than cliff swallows. BCRV detected in bug vectors at a site was positively associated with virus prevalence in house sparrows but not with virus prevalence in cliff swallows. The addition of a highly susceptible invasive host species has led to perennial BCRV epizootics at cliff swallow colony sites. The native cliff swallow host confers a dilution advantage to invasive sparrow hosts in mixed colonies, while at the same sites house sparrows may increase the likelihood that swallows become infected.
Keywords: arbovirus, Buggy Creek virus, cliff swallow, house sparrow, pathogen transmission, virus ecology
1. Introduction
A major question in the study of infectious disease dynamics is what effect an increased number of potential host species may have on pathogen transmission [1–6]. One way that host diversity may increase is through introduction of invasive species [7]. However, the effect of an invasive host species on established host–pathogen systems is difficult to predict. With vector-borne viruses, less competent invasive species may dampen transmission through the dilution effect, in which invasives deflect vector meals away from more competent native hosts [1,5,8]. The idea behind the dilution effect goes back to early suggestions that the presence of cattle may reduce malarial and viral infections in humans [9,10]. Recent work has shown negative correlations between viral pathogen prevalence and species diversity of vertebrate hosts [11–14], and that an invasive host can reduce transmission of pathogens among native hosts [15].
Alternatively, if a vertebrate host entering a new environment proves to be a more competent amplifying host than native species, transmission of pathogens among enzootic maintenance hosts and vectors can increase through ‘spillback’ from the invasive to the native host [6]. This scenario has not been definitively documented in any natural system to date, although considerable indirect evidence indicates that it may be a common way in which invasive hosts impact native host–parasite transmission dynamics [6].
Buggy Creek virus (BCRV, Togaviridae, Alphavirus) is an arthropod-borne virus (arbovirus) that occurs in enzootic cycles involving its avian host, the cliff swallow (Petrochelidon pyrrhonota), and its only known vector, the ectoparasitic swallow bug (Hemiptera: Cimicidae: Oeciacus vicarius). Unlike most arboviruses that have multiple enzootic vertebrate hosts and insect vectors [16–20], BCRV has been isolated only from swallow bugs and birds associated directly with cliff swallow nests [21–26]. Within the last century, introduced house sparrows (Passer domesticus) have moved into cliff swallow nesting colonies in many parts of North America, and in the process sparrows have encountered swallow bugs that feed on them as an alternative source of blood meals. These bugs have exposed sparrows to BCRV and therefore have enabled the virus to exploit a novel and taxonomically distinct avian host. BCRV occurs in two different lineages in the Great Plains that differ by up to 6 per cent at the nucleotide level [27,28]; one of these lineages is selectively transmitted to vertebrate hosts, while the other circulates predominately in the vectors [29,30].
BCRV offers a rare opportunity to compare and contrast the roles of a natural versus an introduced host in virus amplification and to examine how co-occurrence of these two hosts potentially either enhances or reduces transmission of the bird-adapted lineage in this relatively simple host–pathogen system. Our specific objectives are to compare for each avian host: (i) age-related infection prevalence, (ii) level of viraemia, (iii) relative exposure to vectors, and (iv) the extent to which prevalence of virus infection in bugs predicts that in the vertebrate hosts.
2. Materials and methods
(a). Study organisms and study area
Cliff swallows are highly colonial, migratory passerines that breed across much of western North America [31]. They build gourd-shaped mud nests on the sides of cliff faces, inside highway and railroad culverts, and underneath bridges. The nests persist from year to year and are frequently re-used by cliff swallows for multiple seasons [32]. Swallows arrive in our study area in early to mid May and typically raise a single brood, with most nestlings fledging by mid July. Individual colonies are highly synchronous and are quickly vacated by swallows after the nestlings fledge. Nestlings are in the nest for about 26 days before fledging [31].
House sparrows were introduced repeatedly into North America beginning in the 1850s [33] and are now widely dispersed and found mainly in peridomestic settings. Sparrows are semi-colonial, often forming aggregations of 2–20 nests in close proximity. They are sedentary, remaining at or near breeding sites year-round [34]. House sparrows are multi-brooded, with nesting in our study area beginning in late April and ending in late July; peak egg-laying periods are in mid May, late June and late July. New broods start soon after earlier ones fail or fledge. Nestlings fledge at 14–17 days of age [34]. Sparrows evict cliff swallows from their mud nests or occupy abandoned nests in colonies where cliff swallows are either present or absent.
The swallow bug is a haematophagous nest-based ectoparasite, and as many as 2600 bugs have been found in a single cliff swallow nest [32]. Swallow bugs are long-lived and can survive without a blood meal for up to 3 years [35,36]. Bugs feed on birds mostly at night and cluster on the outside of active nests during the day after blood feeding.
BCRV is a single-stranded, positive-sense RNA alphavirus in the western equine encephalomyelitis virus (WEEV) antigenic complex [23,27,28]. This virus is ecologically distinct from other alphaviruses in that it is transmitted by swallow bugs rather than mosquitoes [23,25,37,38]. Prevalence of BCRV in swallow bugs averages approximately 25 per cent of bug pools over our study area and across different years [24,26,39]. The two lineages (A and B) of the virus are ecologically distinct, with lineage A more likely to be found at colony sites containing only house sparrows or at mixed-species colonies, and lineage B at sites with only cliff swallows [29,40].
Our study area is a 60 × 200 km area largely contiguous with the North and South Platte rivers in western Nebraska, USA, and is centred at the Cedar Point Biological Station (41°13′ N, 101°39′ W) in Keith County [32]. About 170 cliff swallow colony sites in this study area are occupied to varying degrees by only cliff swallows, cliff swallows and house sparrows together, or only house sparrows. In the summers of 2006–2008, we studied cliff swallows and house sparrows at colonies situated in concrete culverts beneath highways or railroads and on the sides of bridges. We determined, by checking nest contents, whether each colony site studied contained both species or only one. Information on construction dates of bridges or culverts used as nesting sites was provided by the Nebraska Department of Roads or by our knowledge of when structures first appeared and were first used by sparrows and/or swallows.
(b). Field sampling
Adult and fledged juvenile cliff swallows and house sparrows were captured in mist nets at 22 colony sites during the summers of 2006–2008. No colony was visited (and thus no bird was sampled) more than once per 4 day interval. All netted birds and nestlings taken from nests (see below) were banded with a United States Geological Survey band and bled by either brachial (2006) or jugular (2007–2008) venipuncture with a 29-gauge insulin syringe; 0.1 ml of blood was placed in 0.4 ml of BA-1 virus diluent [26]. Birds were either released or returned to their nest after sampling. Blood samples were stored on wet ice in the field, returned to the laboratory, clarified by centrifugation and the supernatant stored at −70°C until analysis. Adult birds recaptured during or between seasons were re-sampled.
In 2006–2007, cliff swallow and house sparrow nests were examined for eggs in 31 colonies. Nests containing eggs were identified and numbered, then visited every 2–4 days to determine hatch date and then nestling age. In 2008, we sampled only house sparrow nestlings and used our prior experience with nestlings to estimate age. Nestlings of both species aged 4–17 days were bled one to two times during the nestling period. To examine potential age effects on infection prevalence, we classified nestlings aged 4–6 days as ‘young’ and those 7–17 days as ‘old’, because in both species nestlings at about 7 days start to preen (C. Brown & V. O'Brien 1983–2010, personal observation), and thus they might be exposed to fewer swallow bugs (and virus) once this behaviour begins.
We counted swallow bugs clustering on the outside of active cliff swallow and house sparrow nests as a measure of bug parasitism; this was done by visually examining the exterior of each nest with a flashlight [41]. We collected bugs for virus testing from active cliff swallow nests by brushing bugs off the nest exterior into a wide-mouthed collecting jar. Too few bugs were found on the outside of sparrow nests to provide appropriate pool sizes (greater than or equal to 50 bugs) for virus testing. Instead, for house sparrow nests (that contained nestlings we had sampled), we removed the nest from the substrate, fostered the nestlings to an adjacent nest, placed the entire nest into a plastic bag and later picked through the nest chunks with forceps to harvest bugs. Bugs brushed off the outside of nests and found in collected nests were sorted into pools of 100 while alive and the pools stored at −70°C until processing [25,26].
(c). Virus detection and determining titres
Processing of swallow bugs and bird sera for virus detection is described in Brown et al. [25] and O'Brien et al. [42]. RNA was extracted, and RT–PCR performed on each sample using the methods of Moore et al. [26]. Samples that were initially BCRV-positive by RT–PCR were subjected to plaque assay in Vero cells, as described by Brown et al. [43]. Viraemia titres were determined for bird sera by serial 10-fold dilution. Titres were measured only for birds that had become infected (or had hatched) within the previous 4 days, these individuals having been negative for BCRV at earlier ages. Plaques as evidence of cytopathic effect (CPE) were scored on day 3 after Vero cell infection and titre expressed as plaque-forming units per microlitre (PFU ml−1). Samples that did not show CPE were subjected to re-extraction and RT–PCR to confirm the presence of viral RNA in the sample.
(d). Serology
Swallow and sparrow sera were screened for antibodies to BCRV with an enzyme immunoassay (EIA) developed for BCRV using the methods of Chiles & Reisen [44]. EIA-positives were identified using Nebraska BCRV, isolated from a swallow bug pool and passaged once in Vero cells, as antigen. Positive EIAs had a ratio of the mean optical density of two antigen-positive wells divided by an antigen-negative well greater than 2.0. EIA-positive samples were confirmed with a plaque reduction neutralization test (PRNT) on Vero cell culture. PRNT90 titres greater than or equal to 20 were considered to indicate previous exposure to BCRV.
(e). Data analysis
For analysing prevalence, samples were considered BCRV-positive if they met either of the two criteria: (i) RT–PCR-positive on initial screening and confirmed by plaque assay (≥1.7 log10 PFU ml−1), or (ii) RT–PCR-positive on initial screening, negative by plaque assay and positive by RT-PCR on second screening. For individuals sampled more than once, if virus-negative, the bird was included in each age category at which it was sampled; if virus-positive, it was included only for the age at which it was first positive and not included at all for later ages. Mean virus titres were calculated from log-transformed values for descriptive comparisons. Analyses of prevalence classified each nest as either positive (greater than or equal to 1 nestling-positive at some time) or negative (no nestlings ever positive), given that infection of one nestling in a brood led to a significantly higher likelihood of other nestlings in the same nest also being positive for BCRV [45]. Nest prevalence data were aggregated by colony site and means across sites presented.
3. Results
(a). Virus prevalence
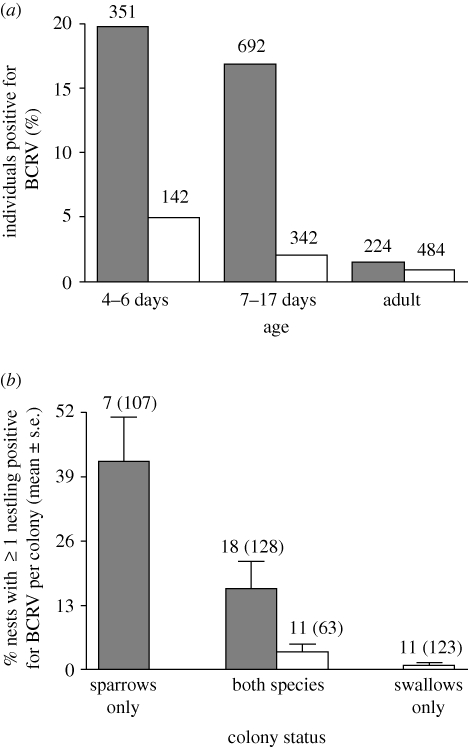
A total of 968 cliff swallow and 1267 house sparrow serum samples were tested for BCRV in 2006–2008. Overall, BCRV prevalence by individuals across all colonies and years was 1.9 per cent in cliff swallows and 14.9 per cent in house sparrows (figure 1a). There was age structure in virus prevalence, with nestlings of both cliff swallows (χ21 = 5.6, p = 0.02) and house sparrows (χ21 = 37.0, p < 0.0001) more likely to be BCRV-positive than fledged juveniles and adults, who were rarely infected (figure 1a). However, virus prevalence did not differ between young and old nestlings (4–6 days versus 7–17 days) for either cliff swallows (χ21 = 3.0, p = 0.09) or house sparrows (χ21 = 1.34, p = 0.25), and therefore further analyses of nestlings do not include age.
Figure 1.
(a) Buggy Creek virus (BCRV) prevalence in individual house sparrows and cliff swallows of different ages in all colonies (n = 43 colonies) and (b) virus prevalence by nest (greater than or equal to 1 nestling positive per nest) per colony site (mean ± s.e. across sites) at colonies containing only sparrows, only swallows and both species. Samples were collected in the summers of 2006–2008 in western Nebraska. In (a), sample size (shown above bars) is the number of birds sampled; in (b), the number of colonies is shown with the total number of nests in parentheses. In (a), juveniles (birds that had fledged) are included with adults (one juvenile sparrow was positive). In (b), only colonies with greater than one nest sampled were included. Shaded bar, house sparrow; open bar, cliff swallow.
Species composition of a colony had an effect on BCRV prevalence in nestlings (figure 1b). House sparrow nests per colony site had significantly higher BCRV prevalence (measured as the percentage of nests with greater than or equal to 1 nestling positive per nest) than did cliff swallow nests per site in single-species colonies (Wilcoxon test; Z = 3.03, p = 0.002) but not when the species occurred in mixed-species colonies (Z = 1.03, p = 0.30; figure 1b). Virus prevalence per site was significantly lower in house sparrow nests in mixed-species colonies than in sparrow nests in single-species colonies (Wilcoxon test; Z = 1.97, p = 0.045); for cliff swallows, prevalence by nest per site was five times higher in mixed-species colonies than in single-species colonies, but the difference was not significant (Z = 1.13, p = 0.26; figure 1b).
(b). Seroprevalence
For evidence of past exposure, we tested 394 cliff swallows from 2006 for BCRV antibodies, including older nestlings (n = 97), fledged juveniles (n = 65) and adults (n = 232). Of these, 13(3.3%) were positive by EIA (eight nestlings, two juveniles and three adults), but only seven were confirmed by PRNT. We tested 181 samples from 154 adult and 11 juvenile house sparrows captured in 2008; overall seroprevalence for house sparrows was 20.6 per cent (29 adults and one juvenile). Most EIA-positive sparrow samples (93.8%, n = 32) were confirmed by PRNT. House sparrows were significantly more likely to show BCRV antibodies whether we considered all EIAs as true-positives (χ21 = 45.2, p < 0.0001) or restricted the analysis to only PRNT-positives (χ21 = 51.4, p < 0.0001).
Species composition of a colony did not affect seroprevalence in either species. The percentage of seropositive cliff swallows in five single-species colonies (4.4%, n = 113) did not differ significantly from the percentage in three mixed-species colonies (2.9%, n = 279; χ21 = 0.49, p = 0.48). The percentage of seropositive house sparrows in six single-species colonies (21.3%, n = 89) did not differ significantly from the percentage in eight mixed-species colonies (15.5%, n = 90; χ21 = 1.07, p = 0.30).
(c). CPE and virus titres
Nestling house sparrow sera positive for BCRV by RT–PCR were more frequently cytopathic in Vero cells (77.7% CPE, n = 180) than BCRV-positive cliff swallow sera (35.7% CPE, n = 14; χ21 = 12.2, p < 0.0001). The frequency with which sera were cytopathic in house sparrows did not vary significantly with whether the colony was single-species (77.2%, n = 101) or mixed-species (78.5%, n = 79; χ21 = 0.04, p = 0.84), nor did this frequency in nestling cliff swallows vary with whether the colony was single-species (40.0%, n = 5) or mixed-species (33.3%, n = 9; χ21 = 0.06, p = 0.80). No sera from BCRV-positive adult/juvenile house sparrows (n = 4) or cliff swallows (n = 4) were cytopathic.
The mean (±s.e.) and maximum virus titres in nestling house sparrows (n = 132) were 4.6 (±0.2) and 9.1 log10 PFU ml−1, respectively, and in nestling cliff swallows (n = 5) 3.3(±0.8) and 5.6 log10 PFU ml−1, respectively. Because very few RT–PCR-positive cliff swallow samples exhibited plaque growth, we could not statistically compare titres between species. Among house sparrows, mean titre (4.9 ± 0.2, n = 70) among those in single-species colonies did not differ significantly from that (4.3 ± 0.2, n = 62) in mixed-species colonies (Wilcoxon test, Z = –1.41, p = 0.16). In analysing mean virus titres in serum, we assumed that the sampling point in time following infection was random, and therefore means represent a comparative measure of viral load.
(d). Parasitism by bug vectors
Counts of swallow bugs on the nests differed strongly depending on the species occupying the nest; mean (±s.e.) bugs per nest per site was 150.8(±30.7) bugs for cliff swallow nests (range 6.4–567.3 bugs, 20 colony sites), when compared to 5.8(±2.6) bugs per nest per site for house sparrow nests (range 0–37.5 bugs, 15 colony sites). Mean bugs on cliff swallow nests at 12 mixed-species colonies (231.0 ± 54.7 bugs) was significantly higher than that on cliff swallow nests at eight single-species colonies (97.3 ± 28.3 bugs; Wilcoxon test, p = 0.019). Mean bugs on house sparrow nests at nine mixed-species colonies (4.1 ± 1.8 bugs) did not differ significantly from that on house sparrow nests at six single-species sites (8.3 ± 6.0 bugs; Wilcoxon test, p = 0.67).
For 11 colony sites containing house sparrows that were studied in 2006–2008, we determined when the site was first used by sparrows based on road construction history or personal observations. Dates ranged from 2 to 41 years ago, with a mean (±s.e.) time since first occupancy by house sparrows of 16.6(±4.1) years.
(e). Virus in vectors in relation to vertebrate hosts
Overall, BCRV prevalence in house sparrows at a colony site was positively related to BCRV prevalence in bug pools collected from cliff swallow nests at the site (rs = 0.74, p = 0.004, n = 13 colonies), whereas BCRV prevalence in cliff swallows was unrelated to virus prevalence in bug pools from cliff swallow nests at the site (rs = 0.22, p = 0.36, n = 19 colonies). For entire house sparrow nests (n = 24) collected from 13 colonies, in which all bugs in the nest were harvested and tested for BCRV, 20(83.3%) had the same BCRV infection status in bugs (greater than or equal to 1 pool positive or all negative) as did the nestling sparrows from the nest; this association was significant (χ21 = 10.5, p = 0.001). At eight colonies where bugs from both species' nests were tested for BCRV, the mean (±s.e.) virus prevalence in bug pools from house sparrow nests per site was 48.4 per cent (±36.7), significantly greater than the prevalence of 15.2 per cent (±19.1) for bug pools from cliff swallow nests (paired t-test; t = 2.93, p = 0.019).
4. Discussion
Strong differences were observed for this arbovirus in infection prevalence between the natural reservoir swallow host and the recently introduced sparrow host. Even though house sparrows were exposed to far fewer swallow bugs than cliff swallows, on average sparrows were almost eight times more likely to be detected as infected than were cliff swallows, and the sparrows' higher seroprevalence indicated higher levels of past infection among surviving birds. Colony composition influenced BCRV prevalence, with sparrows showing significantly reduced prevalence, and swallows tending towards increased prevalence, when colonies contained both species relative to colonies with either species alone. The few swallows that produced detectable viraemias had lower titres than sparrows, and the reduced levels of cytopathicity in virus samples from swallows too may have reflected low titres that were below the plaque-assay detection threshold. The concordance between BCRV detected in bugs at a site with virus prevalence in house sparrows there but not with that in swallows, and the higher virus prevalence in bugs from sparrow nests than from swallow nests at the same site, indicates that house sparrows routinely amplify BCRV to levels that can horizontally infect bug vectors, whereas cliff swallows do not.
This host–parasite system is complicated by the co-occurrence of two virus lineages in the study area and at times in the same colonies [29]. However, because BCRV lineage A is strongly associated with sites containing house sparrows and is preferentially transmitted to birds [30], our comparisons of virus prevalence in house sparrows in single-species versus mixed colonies, and in sparrows versus swallows in mixed colonies apply to this one lineage. Some of the reduction in virus prevalence in cliff swallows at single-species colonies could be because lineage B predominates at those sites; this lineage apparently circulates mostly in bugs and does not replicate well in avian hosts [29,40] and thus is likely irrelevant to vector–host transmission dynamics.
A consequence of the introduction of a highly competent host such as the house sparrow into an enzootic arbovirus system is that this host may select for horizontally transmitted virus genotypes adapted to replication in vertebrates [46–48]. The recent divergence of the two BCRV lineages dates to about when sparrows first began occupying swallow colonies, implicating the highly competent sparrow as a driver of this genetic change in the virus [28,29].
The suitability of the house sparrow as an amplifying host for BCRV presumably reflects the sparrow's very recent encountering of this pathogen and its inherent susceptibility to alphaviruses [49,50]. Sparrows have been present in Nebraska since about 1900 [51], but they are exposed to BCRV only when occupying cliff swallow nests, which probably began in our study area in the 1960s as swallows switched to nesting on human-built nest attachment sites, such as bridges and culverts near towns or cities where sparrows occur [32]. Some of our study colonies had been first occupied by house sparrows only 2 years earlier. At sites with only house sparrows and even with relatively low exposure to bugs there, virus prevalence in broods of sparrows was over 40 per cent and caused BCRV to become focally epizootic at such sites. Sparrows frequently succumb to virus infection, especially at younger ages [45], with virus typically isolated from brain tissue [52].
House sparrows benefited from co-occupancy of a colony site with cliff swallows: BCRV prevalence in sparrow nests was about 60 per cent less at such sites than at sparrow-only colonies. The presence of cliff swallows represents a dilution effect for sparrows both because (i) bugs prefer to parasitize swallows when available, as indicated by our counts of bugs on the two host species' nests in mixed colonies, and (ii) the less-competent swallows deflect blood meals away from the more competent sparrows, reducing transmission of virus to other bugs and making maintenance at epizootic levels less likely. On the other hand, cliff swallows may facilitate BCRV epizootics in house sparrows by providing a majority of the blood meals for bugs at a site, creating a form of parasite-mediated competition, where a species that is not the main amplifying host provides major sustenance for the vector and allows vector persistence and pathogen transmission to the more susceptible host when the preferred host is unavailable or in low numbers [3,53,54]. That virus prevalence in nestling sparrows was directly related to BCRV in the bug vectors collected on cliff swallow nests suggests that the extent to which swallows sustain infected bugs will influence the likelihood of house sparrows being infected.
House sparrows may increase BCRV prevalence in cliff swallows by exposing swallows to more vectors and in so doing represent one of the few instances of pathogen spillback [6] known. The increased numbers of bugs counted on cliff swallow nests in mixed colonies, relative to swallow-only colonies, suggests that swallows encounter more bugs when sparrows are present, and this alone may account for the trend towards higher virus prevalence in nestling swallows in mixed colonies. Bugs are more numerous at sites with sparrows, because the sedentary house sparrows facilitate overwinter survival of virus-infected bugs by allowing the bugs to feed earlier in the spring and later in the summer than would be possible at sites containing only migratory cliff swallows [40]. By serving as an alternative host for bugs, sparrows also promote bug survival when cliff swallows do not return to a given colony site in a summer, resulting in more bugs being present when cliff swallows return there in a later year. Introduced host species provide an alternative source of blood meals for vectors and may thus increase pathogen transmission (by increasing vector abundance) in other host–pathogen systems as well [2,55].
In addition, selection for the more virulent lineage A genotypes in the presence of sparrows, and the sparrows' promoting overwinter survival specifically of lineage A virus [40] could represent spillover of sparrow-adapted virus to cliff swallows and account in part for the higher BCRV infection prevalence we found for swallow nests in mixed colonies. While the number of cliff swallows infected in mixed colonies remains relatively low and the difference in prevalence for swallows in mixed versus single-species colonies was not statistically significant, further work on how their exposure to the more bird-associated BCRV lineage in mixed colonies may result in cliff swallow pathology is warranted.
Enzootic arboviruses often occur in cryptic transmission cycles in which virus prevalence in both vectors and vertebrate hosts is often low, with little pathological effects of infection on the hosts [18–20,56–59]. BCRV appears to be a typical enzootic pathogen in the absence of house sparrows, with low infection prevalence among cliff swallows (e.g. in swallow-only colonies) and no observable morbidity or mortality in cliff swallows [45]. The virus has not been found in any vertebrate host except for cliff swallows and house sparrows occupying swallow nesting colonies [21,22] or in any vector other than the swallow bug. Thus, this virus is restricted to a narrow ecological niche that historically involved only one host and a vector that is largely a specialist on that host.
We do not know how long BCRV has been associated with cliff swallows and their ectoparasites, but the WEEV group of arboviruses collectively arose in the New World tropics about 1900–1300 years ago [60], providing an upper limit on how long BCRV may have been associated with cliff swallows. This time may have been long enough for swallows to evolve resistance to this pathogen, despite their current exposure to the (more recently evolved) bird-adapted BCRV lineage A in mixed colonies. The fact that prevalence of virus in the bug vectors was not related to prevalence in the cliff swallow host indicates a lack of effective BCRV transmission between bugs and swallows. This result could also reflect increased presence of lineage B virus in bugs at sites with only cliff swallows. Either possibility underscores the cliff swallow's unsuitability as an amplifying host, which presumably caused the divergence of BCRV lineage B as a primarily bug-associated virus not dependent on amplification in vertebrates [61]. Cliff swallows appear to be poor amplifying hosts for arboviruses in general: in an experimental infection study of St Louis encephalitis virus, the cliff swallow was the only completely refractory species tested [56]. With their greater exposure to horizontally transmitted parasites, highly colonial bird species such as cliff swallows may be better able to resist pathogens, in part, as a consequence of their investing more in immune defence than their more solitary relatives [62–64].
House sparrows occupying swallow colonies represent predictable spatial foci that contain many BCRV-susceptible nestlings each summer. All nestlings produced are probably susceptible to the virus, because frequent turnover among adult house sparrows at cliff swallow colony sites from year to year results in the continual immigration of breeders, not previously exposed to BCRV into these sites [45]. Even if a few infected nestlings survive to breed, there is no evidence that effective maternal antibodies to arboviruses are transmitted to nestling house sparrows [65,66]. As long as house sparrows continue to occupy cliff swallow colonies and come in contact with swallow bugs and BCRV, the large numbers of sparrow nestlings produced each year should sustain perennial BCRV epizootics at these sites. Our results, and those of others [49,67,68], highlight the potential importance of nestling house sparrows in the population ecology of some arboviruses.
Our study shows that the addition of a susceptible vertebrate host species greatly increases overall prevalence of this vector-borne pathogen in its natural ecosystem. The regular BCRV epizootics in house sparrows may result in this virus being more likely to spillback into its native host or (if BCRV adapts to infecting more vagile blood-feeding arthropods such as mosquitoes) to spillover to other potential host species or different environments. The cliff swallow clearly confers a dilution advantage to sparrows that occupy mixed colonies, while at the same time sparrows may increase the likelihood that swallows become infected.
Acknowledgements
Procedures for the capture and blood sampling of wild birds were approved by the Institutional Animal Care and Use Committee of the University of Tulsa.
We thank Jillian Blackwell, Ananda Ellis, Ying Fang, Sandra Garcia, Allison Johnson, Sarah Knutie, Kristen Lear and Sara Robinson for field or laboratory assistance, and Martin Pfeffer and two anonymous reviewers for comments on the manuscript. The University of Nebraska-Lincoln allowed us to use the facilities of the Cedar Point Biological Station. This work was funded by the National Institutes of Health (AI057569), the National Science Foundation (DEB-0514824) and the American Ornithologists' Union.
References
- 1.Ostfeld R. S., Keesing F. 2000. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 78, 2061–2078 10.1139/cjz-78-12-2061 (doi:10.1139/cjz-78-12-2061) [DOI] [Google Scholar]
- 2.Gilbert L., Norman R., Laurenson K. M., Reid H. W., Hudson P. J. 2001. Disease persistence and apparent competition in a three-host community: an empirical and analytical study of large-scale, wild populations. J. Anim. Ecol. 70, 1053–1061 10.1046/j.0021-8790.2001.00558.x (doi:10.1046/j.0021-8790.2001.00558.x) [DOI] [Google Scholar]
- 3.Holt R. D., Dobson A. P., Begon M., Bowers R. G., Schauber E. M. 2003. Parasite establishment in host communities. Ecol. Lett. 6, 837–842 10.1046/j.1461-0248.2003.00501.x (doi:10.1046/j.1461-0248.2003.00501.x) [DOI] [Google Scholar]
- 4.Dobson A. 2004. Population dynamics of pathogens with multiple host species. Am. Nat. 164(Suppl.), S64–S78 [DOI] [PubMed] [Google Scholar]
- 5.Keesing F., Holt R. D., Ostfeld R. S. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498 10.1111/j.1461-0248.2006.00885.x (doi:10.1111/j.1461-0248.2006.00885.x) [DOI] [PubMed] [Google Scholar]
- 6.Kelly D. W., Paterson R. A., Townsend C. R., Poulin R., Tompkins D. M. 2009. Parasite spillback: a neglected concept in invasion ecology? Ecology 90, 2047–2056 10.1890/08-1085.1 (doi:10.1890/08-1085.1) [DOI] [PubMed] [Google Scholar]
- 7.Lockwood J. L., Hoopes M. F., Marchetti M. P. 2007. Invasion ecology. Malden, MA: Blackwell [Google Scholar]
- 8.Norman R., Bowers R. G., Begon M., Hudson P. J. 1999. Persistence of tick-borne virus in the presence of multiple host species: tick reservoirs and parasite mediated competition. J. Theor. Biol. 200, 111–118 10.1006/jtbi.1999.0982 (doi:10.1006/jtbi.1999.0982) [DOI] [PubMed] [Google Scholar]
- 9.Hess A. D., Hayes R. O. 1970. Relative potentials of domestic animals for zooprophylaxis against mosquito vectors of encephalitis. Am. J. Trop. Med. Hyg. 19, 327–334 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization 1982. Manual on environmental management for mosquito control: with special emphasis on malaria vectors. WHO Offset Publications no. 66. Geneva, Switzerland: World Health Organization [PubMed] [Google Scholar]
- 11.Ezenwa V. O., Godsey M. S., King R. J., Guptill S. C. 2006. Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk. Proc. R. Soc. B 273, 109–117 10.1098/rspb.2005.3284 (doi:10.1098/rspb.2005.3284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt K. A., Ostfeld R. S. 2001. Biodiversity and the dilution effect in disease ecology. Ecology 82, 609–619 10.1890/0012-9658(2001)082[0609:BATDEI]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[0609:BATDEI]2.0.CO;2) [DOI] [Google Scholar]
- 13.Swaddle J. P., Calos S. E. 2008. Increased avian diversity is associated with lower incidence of human West Nile virus infection: observation of the dilution effect. PLoS ONE 3, e2488. 10.1371/journal.pone.0002488 (doi:10.1371/journal.pone.0002488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzán G., Marcé E., Giermakowski J. T., Mills J. N., Ceballos G., Ostfeld R. S., Armién B., Pascale J. M., Yates T. L. 2009. Experimental evidence for reduced rodent diversity causing increased hantavirus prevalence. PLoS ONE 4, e5461. 10.1371/journal.pone.0005461 (doi:10.1371/journal.pone.0005461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Telfer S., Bown K. J., Sekules R., Begon M., Hayden T., Birtles R. 2005. Disruption of a host–parasite system following the introduction of an exotic host species. Parasitology 130, 661–668 10.1017/S0031182005007250 (doi:10.1017/S0031182005007250) [DOI] [PubMed] [Google Scholar]
- 16.Yuill T. M. 1986. The ecology of tropical arthropod-borne viruses. Ann. Rev. Ecol. Syst. 17, 189–219 10.1146/annurev.es.17.110186.001201 (doi:10.1146/annurev.es.17.110186.001201) [DOI] [Google Scholar]
- 17.Morris C. D. 1988. Eastern equine encephalomyelitis. In The arboviruses: epidemiology and ecology, vol. 3 (ed. Monath T. P.), pp. 1–20 Boca Raton, FL: CRC Press [Google Scholar]
- 18.Scott T. W. 1988. Vertebrate host ecology. In The arboviruses: epidemiology and ecology, vol. 1 (ed. Monath T. P.), pp. 257–280 Boca Raton, FL: CRC Press [Google Scholar]
- 19.Reisen W. K., Monath T. P. 1989. Western equine encephalomyelitis. In The arboviruses: epidemiology and ecology, vol. 5 (ed. Monath T. P.), pp. 89–137 Boca Raton, FL: CRC Press [Google Scholar]
- 20.Day J. F. 2001. Predicting St Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Ann. Rev. Entomol. 46, 111–138 10.1146/annurev.ento.46.1.111 (doi:10.1146/annurev.ento.46.1.111) [DOI] [PubMed] [Google Scholar]
- 21.Hayes R. O., Francy D. B., Lazuick J. S., Smith G. C., Gibbs E. P. J. 1977. Role of the cliff swallow bug (Oeciacus vicarius) in the natural cycle of a western equine encephalitis-related alphavirus. J. Med. Entomol. 14, 257–262 [Google Scholar]
- 22.Scott T. W., Bowen G. S., Monath T. P. 1984. A field study on the effects of Fort Morgan virus, an arbovirus transmitted by swallow bugs, on the reproductive success of cliff swallows and symbiotic house sparrows in Morgan County, Colorado, 1976. Am. J. Trop. Med. Hyg. 33, 981–991 [DOI] [PubMed] [Google Scholar]
- 23.Hopla C. E., Francy D. B., Calisher C. H., Lazuick J. S. 1993. Relationship of cliff swallows, ectoparasites, and an alphavirus in west-central Oklahoma. J. Med. Entomol. 30, 267–272 [DOI] [PubMed] [Google Scholar]
- 24.Brown C. R., Komar N., Quick S. B., Sethi R. A., Panella N. A., Brown M. B., Pfeffer M. 2001. Arbovirus infection increases with group size. Proc. R. Soc. Lond. B 268, 1833–1840 10.1098/rspb.2001.1749 (doi:10.1098/rspb.2001.1749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown C. R., Brown M. B., Padhi A., Foster J. E., Moore A. T., Pfeffer M., Komar N. 2008. Host and vector movement affects genetic diversity and spatial structure of Buggy Creek virus (Togaviridae). Mol. Ecol. 17, 2164–2173 10.1111/j.1365-294X.2008.03747.x (doi:10.1111/j.1365-294X.2008.03747.x) [DOI] [PubMed] [Google Scholar]
- 26.Moore A. T., Edwards E. A., Brown M. B., Komar N., Brown C. R. 2007. Ecological correlates of Buggy Creek virus infection in Oeciacus vicarius, southwestern Nebraska, 2004. J. Med. Entomol. 44, 42–49 [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer M., Foster J. E., Edwards E. A., Brown M. B., Komar N., Brown C. R. 2006. Phylogenetic analysis of Buggy Creek virus: evidence for multiple clades in the western Great Plains, United States of America. Appl. Environ. Microbiol. 72, 6886–6893 10.1128/AEM.00868-06 (doi:10.1128/AEM.00868-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padhi A., et al. 2008. Phylogeographical structure and evolutionary history of two Buggy Creek virus lineages in the western Great Plains of North America. J. Gen. Virol. 89, 2122–2131 10.1099/vir.0.2008/001719-0 (doi:10.1099/vir.0.2008/001719-0) [DOI] [PubMed] [Google Scholar]
- 29.Brown C. R., Padhi A., Moore A. T., Brown M. B., Foster J. E., Pfeffer M., O'Brien V. A., Komar N. 2009. Ecological divergence of two sympatric lineages of Buggy Creek virus, an arbovirus associated with birds. Ecology 90, 3168–3179 10.1890/08-1731.1 (doi:10.1890/08-1731.1) [DOI] [PubMed] [Google Scholar]
- 30.Brown C. R., Moore A. T., O'Brien V. A., Padhi A., Knutie S. A., Young G. R., Komar N. 2010. Natural infection of vertebrate hosts by different lineages of Buggy Creek virus (Togaviridae, Alphavirus). Arch. Virol. 155, 745–749 10.1007/s00705-010-0638-8 (doi:10.1007/s00705-010-0638-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown C. R., Brown M. B. 1995. Cliff swallow (Hirundo pyrrhonota). In The birds of North America (eds Poole A., Gill F.). Philadelphia, PA/Washington, DC: Academy of Natural Sciences/American Ornithologists' Union; no. 149 [Google Scholar]
- 32.Brown C. R., Brown M. B. 1996. Coloniality in the cliff swallow: the effect of group size on social behavior. Chicago, IL: University of Chicago Press [Google Scholar]
- 33.Lowther P. E., Cink C. L. 2006. House sparrow (Passer domesticus). In The birds of North America online no. 12 (ed. Poole A.). Ithaca, NY: Cornell Laboratory of Ornithology; See http://bna.birds.cornell.edu.bnaproxy.birds.cornell.edu/bna/species/012 [Google Scholar]
- 34.Anderson T. R. 2006. Biology of the ubiquitous house sparrow. New York, NY: Oxford University Press [Google Scholar]
- 35.Smith G. C., Eads R. B. 1978. Field observations on the cliff swallow, Petrochelidon pyrrhonota (Vieillot), and the swallow bug, Oeciacus vicarius Horvath. J. Wash. Acad. Sci. 68, 23–26 [Google Scholar]
- 36.Rannala B. H. 1995. Demography and genetic structure in island populations, PhD thesis Yale University, New Haven, CT [Google Scholar]
- 37.Rush W. A., Francy D. B., Smith G. C., Cropp C. B. 1980. Transmission of an arbovirus by a member of the family Cimicidae. Ann. Entomol. Soc. Am. 73, 315–318 [Google Scholar]
- 38.Rush W. A., Francy D. B., Bailey R. E. 1981. Seasonal changes in susceptibility of a population of swallow bugs (Hemiptera: Cimicidae) to Fort Morgan virus. J. Med. Entomol. 18, 425–428 [Google Scholar]
- 39.Brown C. R., Brown M. B., Moore A. T., Komar N. 2007. Bird movement predicts Buggy Creek virus infection in insect vectors. Vect. Borne Zoo. Dis. 7, 304–314 10.1089/vbz.2006.0646 (doi:10.1089/vbz.2006.0646) [DOI] [PubMed] [Google Scholar]
- 40.Brown C. R., et al. 2010. Winter ecology of Buggy Creek virus (Togaviridae, Alphavirus) in the central Great Plains. Vect. Borne Zoo. Dis. 10, 355–363 10.1089/vbz.2009.0031 (doi:10.1089/vbz.2009.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown C. R., Brown M. B. 2004. Empirical measurement of parasite transmission between groups in a colonial bird. Ecology 85, 1619–1626 10.1890/03-0206 (doi:10.1890/03-0206) [DOI] [Google Scholar]
- 42.O'Brien V. A., Moore A. T., Huyvaert K. P., Brown C. R. 2008. No evidence for spring re-introduction of an arbovirus by cliff swallows. Wilson J. Ornithol. 120, 910–913 10.1676/08-028.1 (doi:10.1676/08-028.1) [DOI] [Google Scholar]
- 43.Brown C. R., Moore A. T., Knutie S. A., Komar N. 2009. Overwintering of infectious Buggy Creek virus (Togaviridae: Alphavirus) in Oeciacus vicarius (Hemiptera: Cimicidae) in North Dakota. J. Med. Entomol. 46, 391–394 [DOI] [PubMed] [Google Scholar]
- 44.Chiles R. E., Reisen W. K. 1998. A new enzyme immunoassay to detect antibodies to arboviruses in the blood of wild birds. J. Vect. Ecol. 23, 123–135 [PubMed] [Google Scholar]
- 45.O'Brien V. A. 2009. Ecological interactions between arboviruses and their avian hosts, PhD thesis University of Tulsa, Tulsa, OK [Google Scholar]
- 46.Ewald P. W. 1994. Evolution of infectious disease. Oxford, UK: Oxford University Press [Google Scholar]
- 47.Stewart A. D., Logsdon J. M., Jr, Kelley S. E. 2005. An empirical study of the evolution of virulence under both horizontal and vertical transmission. Evolution 59, 730–739 [PubMed] [Google Scholar]
- 48.Brown N. F., Wickham M. E., Coombes B. K., Finlay B. B. 2006. Crossing the line: selection and evolution of virulence traits. PLoS Path. 2, 346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holden P., Hayes R. O., Mitchell C. J., Francy D. B., Lazuick J. S., Hughes T. B. 1973. House sparrows, Passer domesticus (L.), as hosts of arboviruses in Hale County, Texas. I. Field studies, 1965–1969. Am. J. Trop. Med. Hyg. 22, 244–253 [DOI] [PubMed] [Google Scholar]
- 50.Scott T. W., Edman J. D., Lorenz J. H., Hubbard J. L. 1988. Effects of disease on vertebrates' ability behaviorally to repel host-seeking mosquitoes. Misc. Publ. Entomol. Soc. Am. 68, 9–17 [Google Scholar]
- 51.Robbins C. S. 1973. Introduction, spread, and present abundance of the house sparrow in North America. Ornithol. Monogr. 14, 3–9 [Google Scholar]
- 52.O'Brien V. A., Meteyer C. U., Ip H. S., Long R. R., Brown C. R. 2010. Pathology and virus detection in tissues of nestling house sparrows naturally infected with Buggy Creek virus (Togaviridae). J. Wildl. Dis. 46, 23–32 [DOI] [PubMed] [Google Scholar]
- 53.Hudson P. J., et al. 1995. Persistence and transmission of tick-borne viruses: Ixodes ricinus and louping-ill virus in red grouse populations. Parasitology 111, S49–S58 10.1017/S0031182000075818 (doi:10.1017/S0031182000075818) [DOI] [PubMed] [Google Scholar]
- 54.Hudson P., Greenman J. 1998. Competition mediated by parasites: biological and theoretical progress. Trends Ecol. Evol. 13, 387–390 10.1016/S0169-5347(98)01475-X (doi:10.1016/S0169-5347(98)01475-X) [DOI] [PubMed] [Google Scholar]
- 55.Cecere M. C., Gürtler R. E., Chuit R., Cohen J. E. 1997. Effects of chickens on the prevalence of infestation and population density of Triatoma infestans in rural houses of north-west Argentina. Med. Vet. Entomol. 11, 383–388 10.1111/j.1365-2915.1997.tb00426.x (doi:10.1111/j.1365-2915.1997.tb00426.x) [DOI] [PubMed] [Google Scholar]
- 56.McLean R. G., Bowen G. S. 1980. Vertebrate hosts. In St. Louis encephalitis (ed. Monath T. P.), pp. 381–450 Washington, DC: American Public Health Association [Google Scholar]
- 57.Walton T. E., Grayson M. A. 1989. Venezuelan equine encephalitis. In The arboviruses: epidemiology and ecology, vol. 4 (ed. Monath T. P.), pp. 203–231 Boca Raton, FL: CRC Press [Google Scholar]
- 58.Reeves W. C. (ed.) 1990. Epidemiology and control of mosquito-borne arboviruses in California, 1943–1987. Sacramento, CA: California Mosquito and Vector Control Association [Google Scholar]
- 59.Reisen W. K., Hardy J. L., Reeves W. C., Presser S. B., Milby M. M., Meyer R. P. 1990. Persistence of mosquito-borne viruses in Kern County, California, 1983–1988. Am. J. Trop. Med. Hyg. 43, 419–437 [DOI] [PubMed] [Google Scholar]
- 60.Weaver S. C., Kang W., Shirako Y., Rümenapf T., Strauss E. G., Strauss J. H. 1997. Recombinational history and molecular evolution of western equine encephalomyelitis complex alphaviruses. J. Virol. 71, 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown C. R., Moore A. T., Young G. R., Padhi A., Komar N. 2009. Isolation of Buggy Creek virus (Togaviridae: Alphavirus) from field-collected eggs of Oeciacus vicarius (Hemiptera: Cimicidae). J. Med. Entomol. 46, 375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merino S., Martinez J., Møller A. P., Barbosa A., de Lope F., Rodriguez-Caabeiro F. 2001. Physiological and haemtological consequences of a novel parasite on the red-rumped swallow, Hirundo daurica. Int. J. Parasitol. 31, 1187–1193 10.1016/S0020-7519(01)00243-0 (doi:10.1016/S0020-7519(01)00243-0) [DOI] [PubMed] [Google Scholar]
- 63.Møller A. P., Merino S., Brown C. R., Robertson R. J. 2001. Immune defense and host sociality: a comparative study of swallows and martins. Am. Nat. 158, 136–145 10.1086/321308 (doi:10.1086/321308) [DOI] [PubMed] [Google Scholar]
- 64.Møller A. P., Martin-Vivaldi M., Merino S., Soler J. J. 2006. Density-dependent and geographical variation in bird immune response. Oikos 115, 463–474 [Google Scholar]
- 65.Holden P., Francy D. B., Mitchell C. J., Hayes R. O., Lazuick J. S., Hughes T. B. 1973. House sparrows, Passer domesticus (L.), as hosts of arboviruses in Hale County, Texas. II. Laboratory studies with western equine encephalitis virus. Am. J. Trop. Med. Hyg. 22, 254–262 [DOI] [PubMed] [Google Scholar]
- 66.Nemeth N. M., Oesterle P. T., Bowen R. A. 2008. Passive immunity to West Nile virus provides limited protection in a common passerine species. Am. J. Trop. Med. Hyg. 79, 283–290 [PubMed] [Google Scholar]
- 67.Bowen G. S., Monath T. P., Kemp G. E., Kerschner J. H., Kirk L. J. 1980. Geographic variation among St. Louis encephalitis virus strains in the viremic responses of avian hosts. Am. J. Trop. Med. Hyg. 29, 1411–1419 [DOI] [PubMed] [Google Scholar]
- 68.O'Brien V. A., Meteyer C. U., Ip H. S., Brown C. R. 2010. Prevalence and pathology of West Nile virus in naturally infected house sparrows, western Nebraska, 2008. Am. J. Trop. Med. Hyg. 82, 937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]