Abstract
Inverse correlations between genetic variability and parasitism are important concerns for conservation biologists. We examined correlations between neutral genetic variability and the presence of antibodies to canine distemper virus (CDV) and feline parvovirus (FPV) in a free-ranging population of raccoons. Over 3 years there was a strong relationship between age and seroprevalence rates. Most young animals were seronegative to CDV and FPV, but the oldest age class was greater than 80 per cent seropositive to both viruses. CDV-seropositive animals had greater heterozygosity and lower measures of inbreeding compared with CDV-seronegative animals. This relationship was strongest among the youngest animals and did not occur during a 1 year CDV epidemic. In contrast, FPV-seropositive animals only had significantly lower measures of inbreeding in 1 year, perhaps because FPV-associated mortality is relatively low or primarily occurs among very young individuals that were under-represented in our sampling. These results suggest that even in large outcrossing populations, animals with lower heterozygosity and higher measures of inbreeding are less likely to successfully mount an immune response when challenged by highly pathogenic parasites.
Keywords: genetic variability, heterozygosity, inbreeding, raccoon, canine distemper virus, parvovirus
1. Introduction
Heterogeneities in the extent of parasitism among individuals in a population are a virtual given [1]. A central theme in disease ecology is attempting to understand why infections are aggregated across hosts, with some individuals more likely to become infected than others. The contribution of an individual's general level of genetic variability to the likelihood of contracting a pathogen and overcoming exposure is unclear. The level of genetic variability possessed by an individual is closely associated with its fitness [2,3], and recent attention has focused on links between genetic variability and parasitism or disease resistance as a mechanism underlying this association. Individuals with high coefficients of inbreeding or reduced heterozygosity may have increased parasite loads, and inbred individuals may be more likely to die of disease than outbred individuals [4–14].
Many of these studies have assessed levels of genetic variability using neutral loci such as microsatellites, which are most often found in non-coding regions and are unlikely to directly affect pathogen resistance. Recently, studies have found that variance in the inbreeding coefficient (f) within a population may affect correlations between genetic variability and fitness. The association is strong in more inbred individuals with increased homozygosity [15] and weaker in relatively outbred individuals. This ‘general effect hypothesis’ predicts that any populations with low variance in f will have weak or non-existent correlations between heterozygosity and fitness [16–18].
Such correlations have received extensive attention owing, in part, to their potentially profound importance in fields such as conservation biology [19]. Much of the work that forms the basis for our understanding of relationships between genetic variability and parasitism derives from contrasting highly inbred individuals or populations with outbred (control) individuals or populations, captive experiments or from island populations or populations that have gone through bottlenecks [4–9,20,21]. These studies show that extreme loss of variability is associated with increased susceptibility to parasites.
We examined genetic and demographic correlates of canine distemper virus (CDV) and feline (=raccoon) parvovirus (FPV) seroconversion in free-ranging raccoons (Procyon lotor) to test the hypothesis that correlations between genetic variability and parasitism also occur in large outcrossing populations if the pathogen has the potential to strongly influence fitness. Raccoons are among the most common mid-sized mammals of North America, and CDV and FPV infections are important non-anthropogenic sources of mortality for raccoons [22–24]. Young emerge from the den by around two months of age, at which time they can be exposed to either or both of these pathogens. CDV is primarily spread through aerosol droplets and FPV is primarily spread through contact with an infected animal's body fluids or faeces. We addressed whether genetic variability may be mediating mortality by examining relationships between genetic variability and survival from exposure to CDV and FPV. Under an assumption that levels of neutral genetic variation are surrogates for measures of adaptive genetic variation owing to a subset of the target loci being linked to functional loci [25,26], we tested (i) whether genetic variability differed between animals that have not been exposed to CDV or FPV and those that have successfully seroconverted (i.e. survived infection), and (ii) whether these associations were best observed in the context of general heterozygosity, the extent of inbreeding, or the extent of outbreeding. We predicted adults that lack antibodies for these pathogens will have lower genetic variability than those that have antibodies. The underlying justification here is that CDV and FPV can cause relatively high levels of mortality (greater than 25%) among many carnivore species including raccoons [27–31], and that infected individuals with low levels of genetic variability would be less likely to mount successful immune responses.
2. Material and methods
We collected blood samples from raccoons across 10 sites in Missouri, USA during 2005–2007 as part of a broader examination of raccoon disease ecology [32,33]. All sites were located on state, federal, or university conservation or research areas within 60 km of Columbia, MO, USA. Sites consisted of second growth oak (Quercus spp.) and hickory (Carya spp.) forest with a maple (Acer spp.) and cedar (Juniperus virginiana) understory. Based on telemetry and recapture data on over 500 individuals, no animals have ever been observed to move between sites [34], but given their proximity to one another and a lack of major geographical barriers such as rivers, gene flow among the populations is likely [35]. Disease owing to viruses (excluding raccoon rabies virus that does not currently exist in Missouri) and severe winter weather appear to be the primary non-anthropogenic causes of raccoon mortality in the region.
Trapping occurred at all 10 areas in 2005 but we only included data from six of the areas in 2006 and 2007, because the behaviour of raccoons at four sites was altered by the placement of feeding stations, which may have influenced parasite prevalence and abundance [34,36,37]. Traps (Tomahawk no. 207.5, Tomahawk Live Trap Co., Tomahawk, WI, USA) were baited with mackerel and checked daily. Raccoons were immobilized with an injection of ketamine hydrochloride and xylazine [38], marked with metal ear tags, weighed, sexed, measured and aged into one of five age classes based on the tooth wear patterns described by Grau et al. [39] as amended by Monello & Gompper [32]: kits = 0–5 m; I = 6–14 m; II = 15–38 m; III = 39–57 m; and IV = over 58 m. Because kits represented only a small portion of the total sampled population, these individuals were lumped with age class I individuals. A blood sample was collected from each individual via femoral venipuncture. Animals were released at the site of capture following recovery from anaesthesia.
We tested each individual for exposure to CDV and FPV using Biogal Immunocomb ‘dot’-ELISA kits (Biogal-Galad Labs, Kibbutz Galad, Israel) for IgM and IgG antibodies. Based on manufacturer's instructions, results are quantified in S units on a scale of 0–6. While these kits provide quantitative titre evaluations, we treat the results as presence–absence data because of the potential for an animal's titre to fluctuate over time [40]. Cut-off values for a positive response for IgG and IgM analyses occurred at S2 for both FPV and CDV (ca ≤ 1 : 32 IgG titre using a haemagglutination inhibition test or a serum neutralization test, respectively). IgM antibodies appear early in the course of an infection, and thus an IgM-positive (IgM+) individual was assumed currently infected or recently re-exposed. In the absence of IgM antibodies, an IgG-positive (IgG+) result indicated an individual had overcome viral exposure. An IgM-negative/IgG-negative (IgM−/IgG−) individual was assumed not to have been exposed to the virus. Individuals who are IgM+/IgG+ included those who have previously generated a successful immune response but have recently been re-exposed as well as those who had recently been exposed for the first time but for whom the outcome of this exposure was unresolved.
Total genomic DNA was extracted from blood samples using DNeasy Tissue Kits (Qiagen, Valencia, CA, USA). Individuals were genotyped using 12 unlinked nuclear microsatellite loci [41]. For each individual we used program IRmacroN3 (W. Amos, Cambridge University) to calculate three measures of genetic variability: observed heterozygosity (Ho), internal relatedness (IR) and heterozygosity weighted by locus (HL). Observed heterozygosity is the proportion of heterozygous loci and represents general levels of genetic variability. Internal relatedness is a measure of microsatellite diversity that weights homozygotes for rare alleles more heavily than homozygotes for common alleles since the former are more likely derived from related parents, and thus gives a measure of the extent of inbreeding [42,43]. A similar and perhaps more accurate measure of inbreeding may be HL, which weights more informative loci in proportion to their allelic variability [44].
Seroprevalence (% of examined individuals diagnosed as seropositive) to each pathogen was calculated using one sample from each individual. We randomly selected one sample from recaptured individuals with more than one antibody test. We contrasted these measures of seroprevalence to the age and sex of sampled animals with X2-tests. The exposure status of each individual was assessed in the year in which it was captured. However, the exposure event may have occurred in years prior to the capture year. Therefore, we did not examine (age)×(year) or (sex)×(year) interactions. Because individual raccoons were potentially related to one another, and thus potentially genetically non-random, we used randomization tests to assess the statistical relationships between measures of disease exposure and measures of genetic variability. We calculated differences in means of genetic parameters for seropositive and seronegative individuals and then conducted 10 000 randomizations of the data using program RT v. 2.1 (West Inc., Cheyenne, WY, USA) and quantified the per cent of the randomizations that were greater than the observed mean.
3. Results
(a). Viral seroprevalence
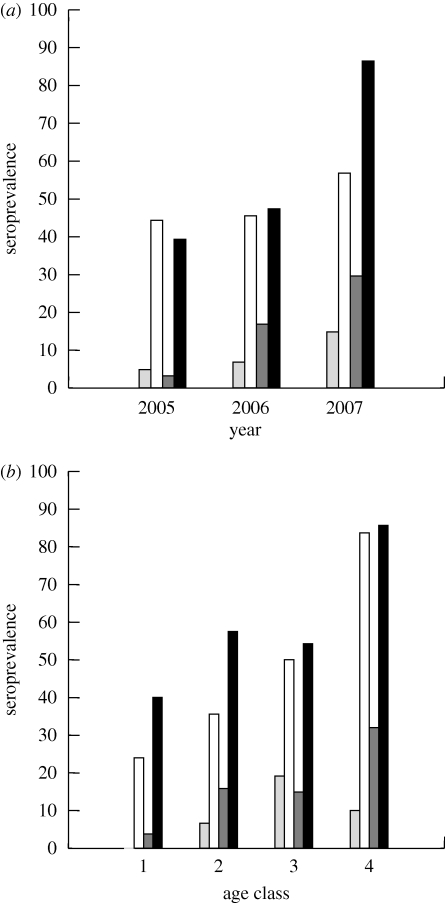
Across all individuals and all years, IgG+ seroprevalence to CDV and FPV was 61.2 and 50 per cent, respectively (n = 197). There was considerable between-year variance, especially for CDV, as an outbreak occurred in 2007 resulting in an approximate doubling of seroprevalence of IgM and IgG antibodies relative to the previous years (figure 1a). The overall proportion of the population that was IgM+ at the time of capture was lower (FPV: 9.4%; CDV: 17.8%; n = 202) than for IgG, although with similar between-year variability (figure 1a). This IgM+ cohort included individuals who were IgM+ /IgG− (FPV: 2.6%; CDV 4.1% over all years, n = 196) as well as those who were IgM+/IgG+ (FPV: 7.1%; CDV 13.8%). The per cent of animals who were never exposed to FPV or CDV (IgM−/IgG−) varied across years from 38.2 to 55.7 per cent for FPV and from 8.6 to 59.0 per cent for CDV (over all years: FPV = 47.4%; CDV = 34.7%).
Figure 1.
Seroprevalence (% exposed) of CDV and FPV subdivided by IgM and IgG antibody production among raccoons (a) over 3 years (IgM: n = 202; IgG: n = 197), and (b) over all 3 years combined and subdivided by age class (IgM: n = 199; IgG: n = 193). Light grey bar, FPV-IgM; open bar, FPV-IgG; dark grey bar, CDV-IgM; black bar, CDV-IgG.
(b). Age and seroconversion
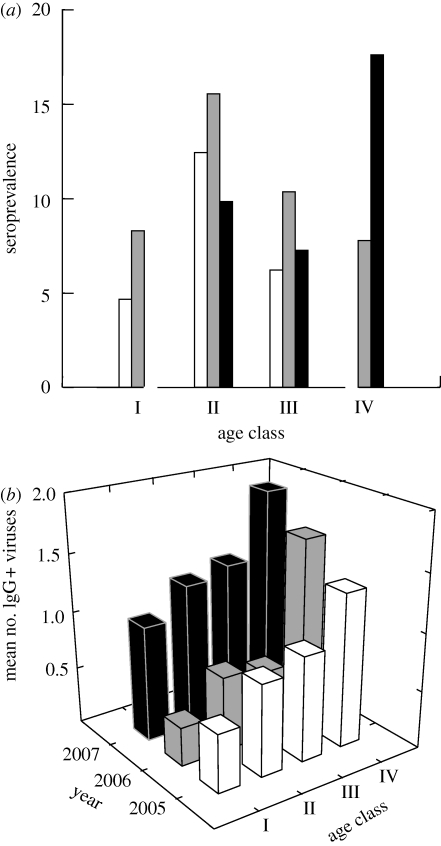
There was a strong relationship between age and seroconversion (FPV: X2 = 35.468, d.f. = 5.000, p < 0.001; CDV: X2 = 19.792, d.f. = 5.000, p = 0.001). Most young animals were seronegative to FPV and CDV, but the oldest age class was greater than 80 per cent seropositive for both viruses (figure 1b). When data on FPV and CDV were combined, individuals in the youngest age class were never found to have contracted or overcome exposure to both viruses (figure 2a). In contrast, among older age classes, most animals had been exposed to at least one of the viruses, and for age class IV + , no individuals were found who had not been exposed to at least one of the viruses, and most had been exposed to both viruses (figure 2a). This pattern held for each of the 3 years of examination, although values were higher in each age class in 2007, reflecting the CDV epizootic that occurred that year (figure 2b). There were no significant differences in the seroconversion of males and females (FPV: X2 = 0.21, d.f. = 1, p = 0.886; CDV: X2 = 2.632, d.f. = 1, p = 0.105).
Figure 2.
(a) Per cent of raccoons (n = 193), subdivided by age class, who were seropositive for zero (23.3% of animals across all years), one (42.0%) or two (34.7%) viruses (CDV and FPV). (b) Mean number of IgG+ seroconversions for raccoons, subdivided by age class and year of capture. (a) Open bar, zero virus; grey bar, one virus; black bar, two viruses.
(c). Genetic variability and seroconversion
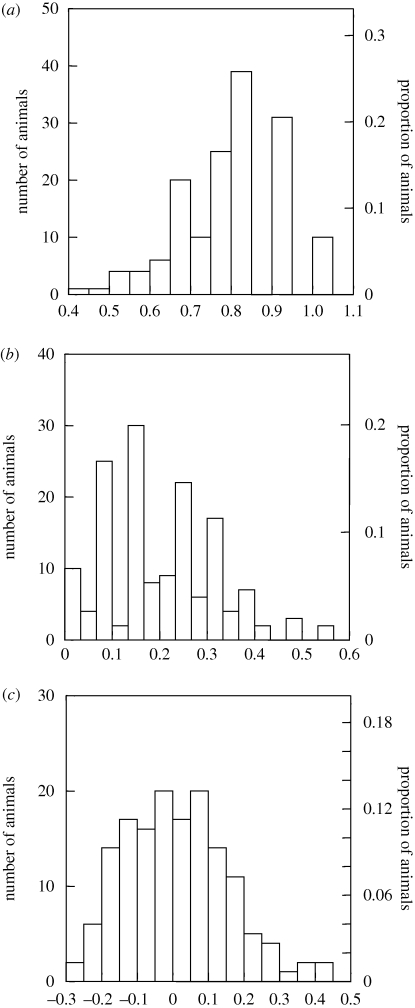
Measures of genetic variability were calculated for 151 individuals. Overall, populations showed high levels of heterozygosity and outbreeding, and low levels of inbreeding (figure 3), but with considerable variability (mean ± s.d.: Ho = 0.789 ± 0.123; IR = 0.010 ± 0.145; HL = 0.201 ± 0.119). Heterozygosity was negatively skewed while the other measures were positively skewed, indicating the presence of some individuals whose genotypes reflected lower heterozygosity and higher levels of inbreeding (figure 3).
Figure 3.
Histograms of genetic variability for 151 raccoons from 10 populations in Missouri, USA. Measures of genetic variability are expressed as (a) observed heterozygosity (Ho), (b) heterozygosity weighted by locus (HL), and (c) internal relatedness (IR).
Of the 151 individuals with genetic data, viral exposure data were available for 147 individuals, a small number of whom were sampled in multiple years, resulting in pathogen × genetic data for 56 individuals in 2005, 51 in 2006 and 54 in 2007. For FPV, there were no significant differences in any genetic measures between seropositive and seronegative individuals captured in 2005 and 2006 (table 1). In 2007, however, IR and HL were both greater in seronegative individuals. There were no significant differences in any of the genetic measures when the data were subdivided by age class (table 1). In contrast to FPV, CDV showed differences across the 3 year study, with this pattern principally driven by differences that occurred in 2005 and 2006, but not in 2007. In 2005 and 2006, animals that were CDV seropositive had greater mean Ho (+0.06 to +0.08) and reduced mean IR (−0.07 to −0.10) and HL (−0.06 to −0.08) than animals that were CDV seronegative (table 2). When the data were examined by age class, differences were most apparent in the youngest individuals, where CDV-seropositive individuals had greater mean Ho and CDV-seronegative individuals had lower levels of IR and HL.
Table 1.
Differences in observed mean measures of genetic variability between feline parvovirus (FPV) seronegative and FPV-seropositive raccoons, subdivided by year and age class. Positive values indicate seronegative animals had greater means for the metric of interest. Values in parentheses represent the per cent of 10 000 randomization trials that resulted in mean differences greater than the observed differences. Values in bold represent those greater than expected by chance (α < 0.05).
| sample size (n) | obs. ΔHo (% >obs.) | obs. ΔIR (% >obs.) | obs. ΔHL (% >obs.) | |
|---|---|---|---|---|
| year | ||||
| 2005 | 56 | 0.0051 (42.05) | 0.0113 (38.67) | −0.0001(50.30) |
| 2006 | 51 | −0.0343 (83.76) | 0.0403 (16.06) | 0.0335 (15.90) |
| 2007 | 54 | −0.0119 (60.46) | 0.0747 (3.74) | 0.0642 (3.25) |
| all years | 147 | −0.0187 (81.82) | 0.0289 (11.66) | 0.0217 (13.95) |
| age | ||||
| I | 18 | 0.0001 (51.21) | 0.0088 (45.53) | 0.0012 (49.20) |
| II | 60 | −0.0385 (87.87) | 0.0624 (5.33) | 0.0415 (9.87) |
| III | 37 | 0.0120 (39.10) | −0.0026 (52.65) | −0.0045 (54.65) |
| IV | 37 | −0.0347 (76.81) | 0.0238 (33.22) | 0.0258 (28.81) |
Table 2.
Differences in observed mean measures of genetic variability between canine distemper virus (CDV) seronegative and CDV-seropositive raccoons, subdivided by year and by age class. Positive values indicate seronegative animals had greater means for the metric of interest. Values in parentheses represent the per cent of 10 000 randomization trials that resulted in mean differences greater than the observed differences. Values in bold and italics represent those greater than expected by chance at α < 0.05 and < 0.01, respectively.
| sample size (n) | obs. ΔHo (% >obs.) | obs. ΔIR (% >obs.) | obs. ΔHL (% >obs.) | |
|---|---|---|---|---|
| year | ||||
| 2005 | 56 | −0.0596 (96.77) | 0.0728 (3.34) | 0.0611 (2.81) |
| 2006 | 51 | −0.0781 (99.26) | 0.1003 (0.44) | 0.0782 (0.64) |
| 2007 | 54 | −0.0119 (60.46) | 0.0124 (41.62) | 0.0152 (36.85) |
| all years | 147 | −0.0366 (96.45) | 0.0443 (3.43) | 0.0371 (3.08) |
| age | ||||
| I | 18 | −0.1422 (97.27) | 0.1772 (1.62) | 0.1402 (3.08) |
| II | 60 | 0.0190 (26.61) | −0.0145 (65.92) | −0.0194 (74.55) |
| III | 37 | −0.0663 (92.92) | 0.0764 (7.83) | 0.0612 (8.14) |
| IV | 37 | −0.0414 (76.92) | 0.0582 (18.89) | 0.0521 (17.57) |
4. Discussion
In many raccoon populations as well as populations of other species in the order Carnivora, viral parasites such as parvoviruses, morbilliviruses and rabies virus may be important non-anthropogenic causes of mortality [30,45]. This is especially true for distemper and paroviruses, which are often enzootic and can cause high rates of juvenile mortality [46,47]. While it is unclear what portion of young raccoons were exposed to these pathogens in this study, ca 25 per cent of age class I raccoons (6–14 months) were seropositive to FPV, and ca 40 per cent were seropositive to CDV suggesting relatively high rates of early exposure, since many young individuals probably do not survive the initial exposure event. In general, neonatal mortality of raccoons is high (e.g. Gehrt & Fritzell [24] report neonate survival of ca 52–65% and partial or complete mortality for 18 of 33 litters), and although the sources of this mortality are usually unknown, studies of other carnivore species suggest that pathogen exposure may be important [48,49]. However, pathogen exposure is not limited to young individuals; the per cent of the population that had successfully seroconverted increased with age, and among the oldest age groups, the vast majority of individuals were seropositive for both pathogens. Thus in this system, animals are likely to eventually be exposed to both pathogens and must either seroconvert or die.
Given that individuals must seroconvert to survive, the results presented here support the hypothesis that the level of genetic variability found in an individual is fundamentally important in predicting the likelihood that the individual will successfully seroconvert when exposed to highly pathogenic parasites. While variability at neutral microsatellite loci is unlikely to affect the ability of an individual to seroconvert, the level of variability may predict levels of functional genetic variation needed to mount an immune response to these diseases (e.g. [26]). The CDV results reveal that those individuals who have survived previous exposure had greater genetic variability (Ho) and lower levels of inbreeding (IR and HL) than those individuals who were seronegative (and who therefore had yet to be exposed to CDV). We interpret these results as indicating that more inbred individuals with lower levels of genetic variability are less likely to survive exposure to CDV.
Interestingly, however, similar patterns were not as clear for FPV. A significant relationship was observed between FPV seroconversion and measures of inbreeding (both IR and HL), but not measures of general heterozygosity, for only 1 of the 3 years of the study. Little is known about the dynamics of parvoviruses in free-ranging carnivore populations, and that which is known derives principally from a single intensively studied population of wolves (Canis lupus). In wolves, canine parvovirus (CPV) is a newly emerged pathogen that can limit population size via high pup mortality [47,48]. High pup mortality owing to CPV in unvaccinated domestic dog populations may also occur [29,50]. While FPV is known to kill raccoons [29], if much of the mortality among newly exposed animals is manifested in juvenile individuals with waning levels of maternal antibodies (i.e. 2–4 months; [51]), we may have missed the opportunity to explicitly test for a link between FPV and genetic variability because kits were under-represented in our sampling.
Seroconversion to CDV was correlated with heterozygosity and measures of inbreeding for 2 of the 3 years of the study. CDV is a highly virulent pathogen of domestic and wild carnivores [28], including raccoons [22,23]. While clinical CDV disease is best understood in dogs where it is predominantly seen in young individuals [31], in raccoons all age classes may be affected [22]. Nonetheless, the pattern of CDV seropositive individuals having higher Ho and lower IR and HL was most apparent among the youngest individuals (age 6–14 months). This pattern is similar to that reported by Rijks et al. [12] who observed differences in heterozygosity, IR and HL for juvenile, but not adult, harbour seals (Phoca vitulina) who were infected by either of two species of highly pathogenic lungworms, compared with those individuals who were not infected. Only in 2007 when the rise in IgG values and doubling of IgM values indicate a CDV epidemic occurred in the raccoon population did these differences not occur. An explanation for the lack of differences associated with 2007's increased exposure is not apparent. These between year differences should be treated with caution, however, because animals (excepting age class I) may have been exposed to CDV in years prior to when they were assayed. Furthermore, multiple strains of CDV may potentially circulate within raccoon populations and strains may vary in virulence [52].
Collectively, these results indicate that the link between reduced heterozygosity and increased susceptibility to pathogens is not solely a phenomenon intrinsic to taxa that have reduced genetic variability at a population-wide scale (e.g. populations of conservation concern or bottlenecked populations). Much of the work that forms the basis for our understanding of relationships between genetic variability and parasitism derives from contrasting highly inbred individuals or populations with outbred (control) individuals or populations [6,7,9], captive experiments [5] or from island populations or populations that have gone through bottlenecks [4,20,21]. While those studies show that extreme loss of variability is associated with increased susceptibility to parasites, results of this study suggest that such patterns may be the norm even in large outcrossing populations.
Acknowledgements
Research was carried out under Missouri Department of Conservation permit no.12869 and University of Missouri Animal Care and Use Protocol no.3927.
This work was supported by NSF grants DEB-0347609 (to M.E.G.) and DEB-0841654 (to M.E.G. and L.S.E.), and by Division of Biological Sciences new investigator funding to L.S.E. We thank B. Cook, K. Faries, T. Hamilton, D. Kuschel, K. McBride, T. McVicker, K. Mottaz, E. O'Hara, S. Pennington, K. Purnell, M. Wehtje, A. Wiewel and J. Wisdom for assistance in the field and lab. Comments from M. Ruiz-Lopez and from two anonymous reviewers greatly improved the manuscript.
References
- 1.Wilson K., Bjørnstad O. N., Dobson A. P., Merler S., Poglayen G., Randolph S. E., Read A. F., Skorping A. 2002. Heterogeneities in macroparasite infections: patterns and processes. In The ecology of wildlife diseases (eds Hudson P. J., Rizzoli A., Grenfell B. T., Heesterbeek H., Dobson A. P.), pp. 6–44 Oxford, UK: Oxford University Press [Google Scholar]
- 2.Keller L., Waller D. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241 10.1016/S0169-5347(02)02489-8 (doi:10.1016/S0169-5347(02)02489-8) [DOI] [Google Scholar]
- 3.Chapman J. R., Nakagawa S., Coltman D. W., Slate J., Sheldon B. C. 2009. A quantitative review of heterozygosity–fitness correlations in animal populations. Mol. Ecol. 18, 2746–2765 10.1111/j.1365-294X.2009.04247.x (doi:10.1111/j.1365-294X.2009.04247.x) [DOI] [PubMed] [Google Scholar]
- 4.Paterson S., Wilson K., Pemberton J. M. 1998. Major histocompatibility complex variation associated with juvenile survival and parasite resistance in a large unmanaged ungulate population (Ovis aries L.). Proc. Natl Acad. Sci. USA 95, 3714–3719 10.1073/pnas.95.7.3714 (doi:10.1073/pnas.95.7.3714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penn D. J., Damjanovich K., Potts W. K. 2002. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc. Natl Acad. Sci. USA 99, 11 260–11 264 10.1073/pnas.162006499 (doi:10.1073/pnas.162006499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid J. M., Arcese P., Keller L. F. 2003. Inbreeding depresses immune response in song sparrows (Melospiza melodia): direct and inter-generational effects. Proc. R. Soc. Lond. B 270, 2151–2157 10.1098/rspb.2003.2480 (doi:10.1098/rspb.2003.2480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acevedo-Whitehouse K., Gulland F., Greig D., Amos W. 2003. Disease susceptibility in California sea lions. Nature 422, 35. 10.1038/422035a (doi:10.1038/422035a) [DOI] [PubMed] [Google Scholar]
- 8.Acevedo-Whitehouse K., Spraker T. R., Lyons E., Melin S. R., Gulland F., Delong R. L., Amos W. 2006. Contrasting effects of heterozygosity on survival and hookworm resistance in California sea lion pups. Mol. Ecol. 15, 1973–1982 10.1111/j.1365-294X.2006.02903.x (doi:10.1111/j.1365-294X.2006.02903.x) [DOI] [PubMed] [Google Scholar]
- 9.Valsecchi E., Amos W., Raga J. A., Podestà M., Sherwin W. 2004. The effects of inbreeding on mortality during a morbillivirus outbreak in the Mediterranean striped dolphin (Stenella coeruleoalba). Anim. Conserv. 7, 139–146 10.1017/S1367943004001325 (doi:10.1017/S1367943004001325) [DOI] [Google Scholar]
- 10.Pearman P. B., Garner T. W. J. 2005. Susceptibility of Italian agile frog populations to an emerging strain of ranavirus parallels population genetic diversity. Ecol. Lett. 8, 401–408 10.1111/j.1461-0248.2005.00735.x (doi:10.1111/j.1461-0248.2005.00735.x) [DOI] [Google Scholar]
- 11.Luikart G., Pilgrim K., Visty J., Ezenwa V. O., Schwartz M. K. 2008. Candidate gene microsatellite variation is associated with parasitism in wild bighorn sheep. Biol. Lett. 4, 228–231 10.1098/rsbl.2007.0633 (doi:10.1098/rsbl.2007.0633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rijks J. M., Hoffman J. I., Kuiken T., Osterhaus A. D. M. E., Amos W. 2008. Heterozygosity and lungworm burden in harbour seals (Phoca vitulina). Heredity 100, 587–593 10.1038/hdy.2008.18 (doi:10.1038/hdy.2008.18) [DOI] [PubMed] [Google Scholar]
- 13.Lyons E. J., et al. 2009. Homozygosity and risk of childhood death due to invasive bacterial disease. BMC Med. Genet. 10, 55. 10.1186/1471-2350-10-55 (doi:10.1186/1471-2350-10-55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend A. K., Clark A. B., McGowan K. J., Buckles E. L., Miller A. D., Lovette I. J. 2009. Disease-mediated inbreeding depression in a large, open population of cooperative crows. Proc. R. Soc. B 276, 2057–2064 10.1098/rspb.2008.1852 (doi:10.1098/rspb.2008.1852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coltman D. W., Slate J. 2003. Microsatellite measures of inbreeding: a meta-analysis. Evolution 57, 971–983 [DOI] [PubMed] [Google Scholar]
- 16.Balloux F., Amos W., Coulson T. 2004. Does heterozygosity estimate inbreeding in real populations? Mol. Ecol. 13, 3021–3031 10.1111/j.1365-294X.2004.02318.x (doi:10.1111/j.1365-294X.2004.02318.x) [DOI] [PubMed] [Google Scholar]
- 17.Slate J., David P., Dodds K. G., Veenvliet B. A., Glass B. C., Broad T. E., McEwan J. C. 2004. Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: theoretical expectations and empirical data. Heredity 93, 255–265 10.1038/sj.hdy.6800485 (doi:10.1038/sj.hdy.6800485) [DOI] [PubMed] [Google Scholar]
- 18.Overall A. D. J., Byrne K. A., Pilkington J. G., Pemberton J. M. 2005. Heterozygosity, inbreeding and neonatal traits in Soay sheep on St Kilda. Mol. Ecol. 14, 3383–3393 10.1111/j.1365-294X.2005.02682.x (doi:10.1111/j.1365-294X.2005.02682.x) [DOI] [PubMed] [Google Scholar]
- 19.Spielman D., Brook B. W., Briscoe D. A., Frankham R. 2004. Does inbreeding and loss of genetic diversity decrease disease resistance? Conserv. Genet. 5, 439–448 10.1023/B:COGE.0000041030.76598.cd (doi:10.1023/B:COGE.0000041030.76598.cd) [DOI] [Google Scholar]
- 20.Coltman D. W., Bowen W. D., Wright J. M. 1998. Birth weight and neonatal survival of harbour seal pups are positively correlated with genetic variation measured by microsatellites. Proc. R. Soc. Lond. B 265, 803–809 10.1098/rspb.1998.0363 (doi:10.1098/rspb.1998.0363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whiteman N. K., Matson K. D., Bollmer J. L., Parker P. G. 2006. Disease ecology in the Galápagos hawk (Buteo galapagoensis): host genetic diversity, parasite load and natural antibodies. Proc. R. Soc. B 273, 797–804 10.1098/rspb.2005.3396 (doi:10.1098/rspb.2005.3396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roscoe D. E. 1993. Epizootiology of canine distemper in New Jersey raccoons. J. Wildl. Dis. 29, 390–395 [DOI] [PubMed] [Google Scholar]
- 23.Schubert C. A., Barker I. K., Rosatte R. C., MacInnes C. D., Nudds T. D. 1998. Effects of canine distemper on an urban raccoon population: an experiment. Ecol. Appl. 8, 379–387 10.1890/1051-0761(1998)008[0379:EOCDOA]2.0.CO;2 (doi:10.1890/1051-0761(1998)008[0379:EOCDOA]2.0.CO;2) [DOI] [Google Scholar]
- 24.Gehrt S. D., Fritzell E. K. 1999. Survivorship of a nonharvested raccoon population in South Texas. J. Wildl. Manag. 63, 889–894 [Google Scholar]
- 25.Hannson B., Westerberg L. 2008. Heterozygosity–fitness correlations within inbreeding classes: local or genome-wide effects? Conserv. Genet. 9, 73–83 [Google Scholar]
- 26.Acevedo-Whitehouse K., Petetti L., Duignan P., Castinel A. 2009. Hookworm infection, anaemia and genetic variability of the New Zealand sea lion. Proc. R. Soc. B. 276, 3523–3529 10.1098/rspb.2009.1001 (doi:10.1098/rspb.2009.1001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamberlain M. J., Hodges K. M., Leopold B. D., Wilson T. S. 1999. Survival and cause-specific mortality of adult raccoons in central Mississippi. J. Wildl. Manag. 63, 880–888 [Google Scholar]
- 28.Deem S. L., Spelman L. H., Yates R. A., Montali R. J. 2000. Canine distemper in terrestrial carnivores: a review. J. Zoo. Wildl. Med. 314, 441–451 [DOI] [PubMed] [Google Scholar]
- 29.Barker I. K., Parrish C. R. 2001. Parvovirus infections. In Infectious diseases of wild mammals (eds Williams E. S., Barker I. K.), pp. 131–146 3rd edn. Ames, IA: Iowa State University Press [Google Scholar]
- 30.Funk S. M., Fiorello C. V., Cleaveland S., Gompper M. E. 2001. The role of disease in carnivore ecology and conservation. In Carnivore conservation (eds Gittleman J. L., Funk S. M., Macdonald D. W., Wayne R. K.), pp. 443–466 Cambridge, UK: Cambridge University Press [Google Scholar]
- 31.Williams E. S. 2001. Canine distemper. In Infectious diseases of wild mammals (eds Williams E. S., Barker I. K.), pp. 50–59 3rd edn. Ames, IA: Iowa State University Press [Google Scholar]
- 32.Monello R. J., Gompper M. E. 2007. Biotic and abiotic predictors of tick (Dermacentor variabilis) abundance and engorgement on free-ranging raccoons (Procyon lotor). Parasitology 134, 2053–2062 [DOI] [PubMed] [Google Scholar]
- 33.Monello R. J., Gompper M. E. 2009. Relative importance of demographics, locale, and seasonality underlying lice and flea parasitism of raccoons (Procyon lotor). J. Parasitol. 95, 56–62 10.1645/GE-1643.1 (doi:10.1645/GE-1643.1) [DOI] [PubMed] [Google Scholar]
- 34.Monello R. J. 2009. Experimentally assessing the influence of resource availability and social aggregation on the parasites of raccoons. PhD thesis, University of Missouri, Columbia [Google Scholar]
- 35.Cullingham C. I., Kyle C. J., Pond B. A., Rees E. E., White B. N. 2009. Differential permeability of rivers to raccoon gene flow corresponds to rabies incidence in Ontario, Canada. Mol. Ecol. 18, 43–53 [DOI] [PubMed] [Google Scholar]
- 36.Wright A. N., Gompper M. E. 2005. Altered parasite assemblages in raccoons in response to manipulated resource availability. Oecologia 144, 148–156 10.1007/s00442-005-0018-3 (doi:10.1007/s00442-005-0018-3) [DOI] [PubMed] [Google Scholar]
- 37.Monello R. J., Gompper M. E. 2010. Differential effects of experimental increases in sociality on ectoparasites of free-ranging raccoons. J. Anim. Ecol. 79, 602–609 10.1111/j.1365-2656.2010.01663.x (doi:10.1111/j.1365-2656.2010.01663.x) [DOI] [PubMed] [Google Scholar]
- 38.Evans R. H. 2002. Raccoons and relatives (Carnivora, Procyonidae). In Zoological restraint and anaesthesia (ed. Heard D.). Ithaca, NY: International Veterinary Information Service; See http://www.ivis.org/special_books/Heard/evans/ivis.pdf [Google Scholar]
- 39.Grau G. A., Sanderson G. C., Rogers J. P. 1970. Age determination in raccoons. J. Wildl. Manag. 34, 364–372 [Google Scholar]
- 40.Junge R. E., Bauman K., King M., Gompper M. E. 2007. A serologic assessment of exposure to viral pathogens and Leptospira in an urban raccoon (Procyon lotor) population inhabiting a large zoological park. J. Zoo. Wildl. Med. 38, 18–26 10.1638/05-123.1 (doi:10.1638/05-123.1) [DOI] [PubMed] [Google Scholar]
- 41.Siripunkaw C., Kongrit C., Faries K. M., Monello R. J., Gompper M. E., Eggert L. S. 2008. Isolation and characterization of polymorphic microsatellite loci in the raccoon (Procyon lotor). Mol. Ecol. Res. 8, 199–201 10.1111/j.1471-8286.2007.01922.x (doi:10.1111/j.1471-8286.2007.01922.x) [DOI] [PubMed] [Google Scholar]
- 42.Amos W., Wilmer J. W., Fullard K., Burg T. M., Croxall J. P., Bloch D., Coulson T. 2001. The influence of parental relatedness on reproductive success. Proc. R. Soc. Lond. B 268, 2021–2027 10.1098/rspb.2001.1751 (doi:10.1098/rspb.2001.1751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bean K., Amos W., Pomeroy P. P., Twiss S. D., Coulson T. N., Boyd L. L. 2004. Patterns of parental relatedness and pup survival in the grey seal (Halichoerus grypus). Mol. Ecol. 13, 2365–2370 10.1111/j.1365-294X.2004.02199.x (doi:10.1111/j.1365-294X.2004.02199.x) [DOI] [PubMed] [Google Scholar]
- 44.Aparicio J. M., Ortego J., Cordero P. J. 2006. What should we weigh to estimate heterozygosity, alleles or loci? Mol. Ecol. 15, 4659–4665 10.1111/j.1365-294X.2006.03111.x (doi:10.1111/j.1365-294X.2006.03111.x) [DOI] [PubMed] [Google Scholar]
- 45.Murray D. L., Kapke C. A., Evermann J. F., Fuller T. K. 1999. Infectious disease and the conservation of free-ranging large carnivores. Anim. Conserv. 2, 241–254 10.1111/j.1469-1795.1999.tb00070.x (doi:10.1111/j.1469-1795.1999.tb00070.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson W. R., Nettles V. F., Hayes L. E., Howerth E. W., Couvillion C. E. 1992. Diseases diagnosed in gray foxes (Urocyon cinereoargenteus) from the southeastern United States. J. Wildl. Dis. 28, 28–33 [DOI] [PubMed] [Google Scholar]
- 47.Mech L. D., Sagar S. M., Paul W. J., Newton W. E. 2008. Demographic effects of canine parvovirus on a free-ranging wolf population over 30 years. J. Wildl. Dis. 44, 824–836 [DOI] [PubMed] [Google Scholar]
- 48.Johnson M. R., Boyd D. K., Pletscher D. H. 1994. Serology of canine parvovirus and canine distemper in relation to wolf (Canis lupus) pup mortalities. J. Widl. Dis. 30, 270–273 [DOI] [PubMed] [Google Scholar]
- 49.Fiorello C. V., Deem S. L., Gompper M. E., Dubovi E. J. 2004. Seroprevalence of pathogens in domestic carnivores on the border of Madidi National Park, Bolivia. Anim. Conserv. 7, 45–54 10.1017/S1367943003001197 (doi:10.1017/S1367943003001197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mason M. J., Gillet N. A., Muggenburg B. A. 1987. Clinical, pathological, and epidemiological aspects of canine parvoviral enteritis in an unvaccinated closed beagle colony: 1978–1985. J. Am. Vet. Med. Assoc. 177, 779–783 [Google Scholar]
- 51.Reif J. S. 1976. Seasonally, natality and herd immunity in feline panleukopenia. Am. J. Epidemiol. 103, 81–87 [DOI] [PubMed] [Google Scholar]
- 52.Lednicky J. A., et al. 2004. Genetically distant American canine distemper virus lineages have recently caused epizootics with somewhat different characteristics in raccoons living around a large suburban zoo in the USA. Virol. J. 1, 2 See http://www.virologyj.com/content/1/1/2 10.1186/1743-422X-1-2 (doi:10.1186/1743-422X-1-2) [DOI] [PMC free article] [PubMed] [Google Scholar]