Abstract
Immune defences are an important component of fitness. Yet susceptibility to pathogens is common, suggesting the presence of ecological and evolutionary limitations on immune defences. Here, we use structural equation modelling to quantify the direct effects of resource quality and selection history, and their indirect effects mediated via body condition prior to an immune challenge on encapsulation and melanization immune defences in the tobacco hornworm, Manduca sexta. We also investigate allocation trade-offs among immune defences and growth rate following an immune challenge. We found considerable variation in the magnitude and direction of the direct effects of resource quality and selection history on immune defences and their indirect effects mediated via body condition and allocation trade-offs. Greater resource quality and evolutionary exposure to pathogens had positive direct effects on encapsulation and melanization. The indirect effect of resource quality on encapsulation mediated via body condition was substantial, whereas indirect effects on melanization were negligible. Individuals in better condition prior to the immune challenge had greater encapsulation; however, following the immune challenge, greater encapsulation traded off with slower growth rate. Our study demonstrates the importance of experimentally and analytically disentangling the relative contributions of direct and indirect effects to understand variation in immune defences.
Keywords: immune responses, structural equation model, resource quality, domestication, allocation trade-off, Manduca sexta
1. Introduction
Pathogens (broadly defined sensu Stock et al. [1]) negatively impact host survival and reproduction, such that host immune defences are an important determinant of overall host fitness [2–4]. Although all plants and animals possess some form of immune defence [5], susceptibility to pathogens is common [6]. Given the importance of immune defences for host fitness, the prevalence of susceptible phenotypes seems paradoxical. Why is there variation in immune defences?
Immune defence is an evolved trait. Although it is clear that selection imposed by pathogens, including bacteria, fungi, viruses and parasitoid natural enemies, has played an important role in shaping immune defences over long evolutionary time scales [7,8], the short-term dynamics of contemporary selection on host immune defences are less clear. Because there is considerable spatial and temporal variation among populations in their exposure to pathogens (e.g. [9–11]), the question becomes, to what extent are immune defences limited by variability in exposure to agents of natural selection? If contemporary selection is important for maintaining immune defences, what are the consequences of relaxing selection on immune defences? Laboratory domestication of model organisms [12] provides an ideal opportunity to address this question: selection on immune defences is often relaxed for domesticated laboratory populations since they are relatively protected from pathogens compared with their wild population counterparts. Reduced immune defences of domesticated versus wild populations would suggest contemporary selection is important for maintaining immune defences.
In addition, ecological factors, operating within a generation, may limit immune defences. Resource quality and availability largely determine maximum potential allocation to immune defences, and immune defences are generally improved with greater resource quality and availability (both in the laboratory [13–15] and in the field [16]). However, competing demands of growth, maintenance and reproduction prevent sole allocation of resources to immune defences [17]. As a result, resource allocation can impose ecological limits either on immune defences or aspects of growth, maintenance and reproduction, depending on the current needs of the host [18–20]. An important distinction is that while resources may directly affect immune defences, resources may also act indirectly on immune defences. In the latter case, the effects of resources on immune defences are mediated via non-immune defence aspects of the host's biology. For example, resource quality can impact both immune defences (see above) and body condition [21], but body condition can also impact immune defences [22–28]. Yet very few studies are able to distinguish the direct effects of resources on immune defences from their indirect effects mediated via other aspects of the host's biology. A recent exception is a study by Smilanich et al. [29] on immune defences in a nymphalid caterpillar, where structural equation modelling (SEM) was used to reveal significant direct effects of plant allelochemicals on immune defences and indirect effects mediated through host metabolism. The extent to which indirect effects of resources mediate overall immune defences is largely unknown, but may provide important insight into ecological limitations on immune defences.
In this study, we examine the relative importance of the direct effects of resource quality and selection history (population-level differences in evolutionary exposure to pathogens), and their indirect effects mediated by body condition prior to an immune challenge and allocation trade-offs following an immune challenge on immune defences in the tobacco hornworm, Manduca sexta. To accomplish this, we take advantage both of a recent host plant shift in M. sexta from a typical high-quality host plant (tobacco; Nicotiana tabacum) onto a novel low-quality host plant (devil's claw; Proboscidea louisianica), and the laboratory domestication of M. sexta over the past 35 years (more than 260 generations) where we can directly compare two laboratory populations with the wild field population from which they were originally derived. We examine two major components of innate (non-specific) immune defences—encapsulation, involving the layering of haemocytes (immune cells) around invaders to form a protective capsule, and melanization, involving the deposition of melanin, a cytotoxic molecule [30,31]—in response to an abiotic immune challenge. Encapsulation and melanization represent functional consequences of immune activation, and are often strong predictors of survival and performance following an immune challenge: for example, in M. sexta, experimental reductions in melanization and encapsulation result in significantly lower survival against biotic immune challenges [32,33]. Our experimental and analytical approach (see figure 1 for a complete conceptual map) allows us to examine: (i) the direct effects of resource quality (tobacco versus devil's claw) and selection history (wild versus domesticated populations) on two immune defences, melanization and encapsulation, (ii) the indirect effects of resource quality and selection history on immune defences as mediated by body condition, estimated using growth rate prior to an immune challenge, and (iii) allocation trade-offs between immune defences and growth rate following an immune challenge.
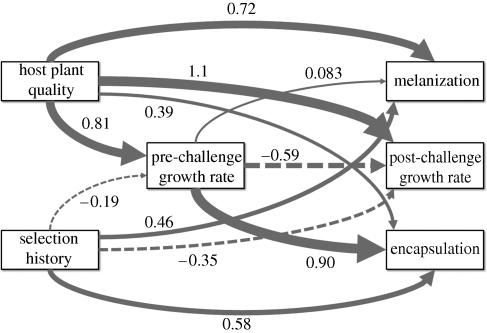
Figure 1.
Structural equation model (path diagram) for the direct effects of host plant quality and selection history on melanization, encapsulation and post-challenge growth rates, and their indirect effects mediated through pre-challenge growth rate. The levels of the dichotomous variables, host plant quality and selection history were assigned such that devil's claw = 0, tobacco = 1, domesticated laboratory population = 0 and wild field population = 1. The width of the path corresponds with the magnitude of the effect. Positive relationships are indicated by solid lines, and negative relationships by dashed lines. See table 1 for 95% CI of the path coefficient estimates.
2. Material and methods
(a). Study organisms
The tobacco hornworm, M. sexta L. (Sphingidae), is distributed across tropical and temperate regions of the Nearctic [34]. Feeding is generally restricted to plants in the Solanaceae, but M. sexta has adopted non-solanaceous host plants (Proboscidea spp.) belonging to the family Martyniaceae, in the southwestern USA [35,36] where these plants are native, and the southeastern USA [37] where these plants have been recently introduced [38]. Here, we use tobacco (N. tabacum) as a representative of a typical, high-quality solanaceous host plant, and devil's claw (P. louisianica) as a novel, low-quality host plant. In our study area (North Carolina), devil's claw is a relatively recent introduction [38], and we have previously documented significant performance costs associated with feeding on this host plant [39].
We used three different genetic lines (populations) of M. sexta, a wild field population and two domesticated laboratory populations, to assess the consequences of selection history for immune defences. The field population was established with early instar larvae collected from tobacco plants at the North Carolina State University (NCSU) Research Station in Clayton, NC, USA. To minimize parental effects, larvae were reared through one generation on artificial diet in the laboratory before use in the experiments. The Duke University (hereafter, Duke) laboratory population came from a colony maintained under standard larval rearing conditions (artificial diet, constant 25°C, 15 h L : 9 h D photocycle) at Duke University; this population was established by hybridizing long-term mass-reared colonies from the University of Washington, University of Arizona and NCSU in 2002 [40]. The University of North Carolina (hereafter, UNC) laboratory population came from a colony maintained under the same standard larval rearing conditions by Gilbert and colleagues at UNC for over 25 years. Animals in these laboratory colonies are not exposed to natural enemies or their naturally occurring host plants at any stage of their life cycle, and are reared on artificial diet containing antimicrobial and antifungal agents to reduce exposure to pathogens.
To our knowledge, all major laboratory colonies of M. sexta are ultimately derived from mass-rearing facilities in Raleigh, NC, USA [41]. The source population for these laboratory strains, including the Duke and UNC colonies, came from field collections of eggs from the NCSU Research Station in Clayton, NC, USA (see above) during the 1960s.
(b). Experiments and measurements
Tobacco and devil's claw (tobacco, Coker var. 319; devil's claw, International Carnivorous Plant Soc., Inc., Pinole, CA, USA) were grown from seed in the greenhouse and fertilized every two weeks with Peter's Pro Solution. The plants were five weeks old at the start of the experiment. No pesticides were ever applied to these plants. Eggs of each population (field and both laboratory colonies) were randomly assigned to host plant species (either devil's claw or tobacco). Initial sample sizes were sufficient to produce at least 15 viable larvae at the time of the immune challenge (see below); approximately 20–25 hatchling larvae from each population were assigned to the tobacco treatment, and approximately 30–35 hatchling larvae from each population were assigned to the devil's claw treatment. Larvae were housed in growth chambers (Percival model 36-VL) under standard conditions (16 L : 8 D photocycle at a constant 25°C). Larvae were reared singly in plastic enclosures (31 × 16 × 13 cm) with screened lids, and were fed on cut leaves kept in water picks until the beginning of the fourth larval instar.
We assessed two major components of innate immune defences (non-specific defences common to all plants and animals [5]), melanization and encapsulation, through the injection of Sephadex beads (see below) into the host haemocoel. The beads activate the deposition of both cytotoxic melanin and encapsulating layers of haemocytes (cf. [30]), similar to the responses against parasitoid eggs or larvae, and bacterial and fungal pathogens (reviewed in Vilmos & Kurucz [42]).
We chose the fourth (penultimate) larval instar for assessing growth rates and immune defences because aspects of immune function such as haemocyte titre decrease immediately prior to and throughout metamorphosis in M. sexta [43]. All larvae in the experiment were therefore of the same developmental stage, but not necessarily the same age or size. To assay growth rates, we recorded development time to fourth instar and body mass both prior to and following the injection of the immune challenge for each individual larva. Pre-challenge larval growth rate was used to examine the potential condition dependence of immune defences, and defined as: ln(body mass at the beginning of the fourth instar)/development time to the fourth instar. Post-challenge growth rate was used to examine potential allocation trade-offs between immune defences and growth rate, and defined as: ln(body mass after the immune challenge/body mass at the beginning of the fourth instar (when the immune challenge occurred)). The immune challenge interval was constant for all larvae (24 h), so there was no age dependence for post-challenge growth rates.
After moulting into fourth instar (defined by slippage of the head capsule), larvae were weighed and then injected under the base of the left fourth proleg (via Hamilton 7000 series microlitre syringe) with at least 15 (but not more than 20) Sephadex beads (DEAE-Sephadex A-25, Sigma; beads were stained with a 0.1 per cent Congo red solution and dried under UV light) suspended in 5 µl sterile Grace's insect medium (Sigma-Aldrich) (sensu [44]). Beads were injected directly into the haemocoel. Because haemolymph loss was minimal following injections, the injection wounds were left unsealed. The injected larvae were returned to their respective host plants and allowed to feed for 24 h. After 24 h, body mass was measured (see above), and larvae were frozen at −80°C. The Sephadex beads were extracted post-mortem, and mounted in glycerol (Sigma-Aldrich) on glass slides. During extraction, 10 beads from each individual were randomly selected for analysis, which mitigated potential biases arising from non-uniform encapsulation coupled with bead orientation on the slide. Final sample sizes of individuals from each treatment group of host plant-by-population (comprising 10 Sephadex beads from each individual) were 15, except for the domesticated UNC population that had 30 individuals each for tobacco and devil's claw.
Melanization was assayed as a binomial response variable for each bead: the presence or absence of melanin deposited either directly on the Sephadex bead or on haemocytes involved in the encapsulation of the Sephadex bead. Visualization of melanization was performed using brightfield microscopy.
Encapsulation (degree of haemocyte aggregation) was assayed as a continuous response variable, by subtracting the area of the Sephadex bead from the area enclosed by the outermost edge of the encapsulating haemocytes. This yielded a measurement of the total encapsulation area. The encapsulated Sephadex beads were visualized using a combination of Nomarski differential interference contrast (DIC) and fluorescence microscopy (Zeiss LSM 510 confocal microscope). A fluorescent image of the Sephadex bead (the Congo red dye used to stain the Sephadex beads fluoresces) was overlaid on a DIC image of encapsulation. The fluorescent image of the bead allowed clear delineation of the bead edges, and the DIC image allowed clear visualization of cellular encapsulation (these surrounding haemocytes are largely transparent, requiring the use of DIC). The encapsulation area and bead area were measured using the Zeiss LSM Image Browser software. Encapsulation measurements were highly repeatable (based on 10 randomly sampled beads from each of the six treatment groups; r = 0.98, p < 0.0001), so measurement error was not incorporated into our statistical analyses.
(c). Statistical analyses
We used two types of statistical analysis: linear models to test the effects of host plant quality (tobacco versus devil's claw) and selection history (wild versus domesticated: population differences in exposure to immune challenges) on pre- and post-challenge growth rates and melanization and encapsulation immune defences; and structural equation models to quantify the direct and indirect associations among these variables. All statistical analyses were performed using R (v. 2.10.1 [45]). To explore the consequences of variation in host plant quality and selection history for melanization, we performed a mixed-model analysis of deviance with melanization (presence/absence) as the response, and host plant quality, selection history and their interaction as fixed factors. In most cases, population comparisons were based on linear contrasts between the wild field population and both domesticated laboratory populations (Duke and UNC); we note deviations from these particular contrasts in §3 when they occur. Bead area was included as a covariate to account for variation in bead size (mean bead size in µm ± 1 s.d.: 118 ± 24); importantly, bead area did not differ significantly across host plant quality-by-selection history treatment groups (F5,1194 = 1.58, p = 0.164). Similarly, to explore the consequences of host plant quality and selection history for encapsulation, we performed a mixed-model ANCOVA with encapsulation area as the response, and host plant quality, selection history and their interaction as fixed factors. Bead area was included as a covariate. The effects of host plant quality and selection history on pre- and post-challenge growth rates were also examined using ANOVA, with pre- or post-challenge growth rate as the response and host plant quality, selection environment and their interaction as fixed effects.
Host plant quality and selection history may affect immune defences both directly and indirectly where their effects on immune defences are mediated via pre-challenge growth rate. Similarly, post-challenge growth rate may be affected directly by host plant quality, selection history and pre-challenge growth rate. Trade-offs in resource allocation following an immune challenge may further indirectly alter either immune defences or post-challenge growth rate. In a strict sense, ‘direct’ and ‘indirect’ may represent relative differences in the complexity of the relationships between predictor and response variables: the direct effect, for example, of host plant quality on immune defences may involve additional, unmeasured components. Here, we use direct and indirect to refer to the major structural relationships between the variables measured in our study, rather than hypothesized unmeasured variables.
We used SEM (also, path analysis) to quantify the relative contributions of the direct effects of host plant quality and selection history on immune defences and post-challenge growth rate versus their indirect effects mediated via pre-challenge growth rate (figure 1). Because strong multi-collinearity among host plant quality and pre-challenge growth rate (variance inflation factor greater than 10 in both cases) violated the assumptions of traditional covariance-based SEM, we used component-based SEM (SEM using partial least squares; SEM PLS) [46,47]. In SEM PLS, the predictor (exogenous) and response (endogenous) variables are reduced to principal components, and the predictor components are used to predict the scores on the response components. Host plant quality and selection history were included as exogenous variables. Pre- and post-challenge growth rates and immune defences were included as endogenous variables; pre-challenge growth rate structurally mediated relationships between the exogenous variables and immune defences and post-challenge growth rate. The standardized path coefficients estimated from the model are regression coefficients (beta weights) of standardized variables (mean = 0, s.d. = 1). For indirect effects, individual path coefficients are multiplied along the path to obtain the total path contribution.
3. Results
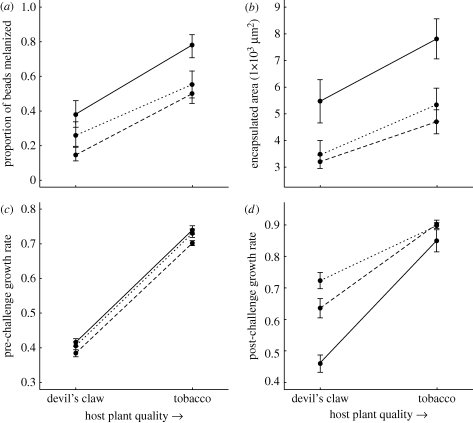
Melanization was greater on the typical host plant relative to the novel host plant, and for the wild field population compared with the domesticated laboratory populations (figure 2a). Analysis of deviance detected significant effects of host plant quality (χ2 = 112, p < 0.0001) and selection history (χ2 = 26.2, p < 0.0001), but not of the interaction between host plant quality and selection history (χ2 = 0.433, p = 0.512), indicating that wild and domesticated populations had qualitatively similar responses to variation in host plant quality. Bead area was non-significant (χ2 < 0.001, p = 1).
Figure 2.
Mean immune responses and growth rates ±1 s.e. (except (a), where 95% binomial confidence intervals are indicated) for the wild field (solid line); UNC domesticated laboratory (dashed line) and Duke domesticated laboratory (dotted line) populations as a function of host plant quality. Specific responses include: (a) proportion of beads melanized, (b) encapsulated area, (c) pre-challenge growth rate, and (d) post-challenge growth rate.
Similarly, encapsulation was greater on the typical host plant relative to the novel host plant, and for the wild field population compared with the domesticated laboratory populations (figure 2b). ANCOVA detected significant effects of host plant quality (F1,116 = 18.3, p < 0.0001) and selection history (F1,116 = 9.76, p < 0.0001), but not of the interaction between host plant quality and selection history (F1,116 = 0.528, p = 0.469), indicating wild and domesticated populations had qualitatively similar responses to variation in host plant quality. Bead area was non-significant (F1,1079 < 0.001, p = 0.994). We also secondarily explored differences among the two domesticated populations. Here, the most striking pattern was the greater mean encapsulation and melanization of the Duke laboratory population relative to the UNC laboratory population (F1,88 = 5.31, p = 0.0214; χ2 = 6.75, p = 0.0342, respectively).
Both pre- and post-challenge growth rates (figure 2c,d) were improved on the typical host plant relative to the novel host plant (F1,116 = 1250, p < 0.0001; F1,116 = 329, p < 0.0001, respectively). Though pre-challenge growth rates were not significantly different between the wild and domesticated populations (F1,116 = 1.64, p = 0.203), post-challenge growth rates were greater for the domesticated populations compared with the wild population (F1,116 = 32.9, p < 0.0001). This reflects the greater growth (and final size) in the last instar in domesticated versus wild populations [41,48]. The interaction between host plant quality and selection history was not significant in the analyses of pre- and post-challenge growth rates (F1,116 = 0.0772, p = 0.782; F1,116 = 0.0473, p = 0.828, respectively).
We used SEM to quantify the direct and indirect associations among host plant quality and selection history, pre- and post-challenge growth rates and immune defences (see above; figure 1 and table 1). The exogenous variables, host plant quality and selection history were included in the same SEM since there were no significant interactions between host plant quality and selection history from the ANCOVAs for encapsulation and melanization. The endogenous variables included encapsulation, melanization and pre- and post-challenge growth rates; pre-challenge growth rate structurally mediated relationships between the exogenous variables and encapsulation, melanization and post-challenge growth rate responses (path diagram is shown in figure 1). The R2 values for endogenous variables were relatively high (R2 = 0.53, 0.83, 0.66 and 0.69 for encapsulation, melanization, pre-challenge and post-challenge growth rates, respectively), indicating the hypothesized SEM adequately fit the data.
Table 1.
Standardized path coefficient estimates and 95% CI (obtained from n = 500 bootstrap replications) for the direct effects of host plant quality and selection history on melanization, encapsulation and post-challenge growth rates, and their indirect effects mediated through pre-challenge growth rate. Host plant quality and selection history are dichotomous variables, where 0 corresponds with devil's claw and the domesticated laboratory populations, and 1 corresponds with tobacco and the wild field population, respectively. Confidence intervals that do not contain 0 are significantly different from 0 at the p = 0.05 level.
| path | estimate | 95% CI |
|---|---|---|
| host plant quality → encapsulation | 0.39 | 0.19, 0.60 |
| host plant quality → melanization | 0.72 | 0.59, 0.85 |
| host plant quality → post-challenge growth rate | 1.1 | 1.0, 1.3 |
| host plant quality → pre-challenge growth rate | 0.81 | 0.76, 0.86 |
| pre-challenge growth rate → encapsulation | 0.90 | 0.69, 1.1 |
| pre-challenge growth rate → melanization | 0.083 | −0.043, 0.23 |
| selection history → encapsulation | 0.58 | 0.44, 0.71 |
| selection history → melanization | 0.46 | 0.40, 0.53 |
| selection history → post-challenge growth rate | −0.35 | −0.51, −0.21 |
| selection history → pre-challenge growth rate | −0.19 | −0.29, −0.09 |
| pre-challenge growth rate → post-challenge growth rate | −0.59 | −0.77, −0.43 |
SEM confirmed that melanization and encapsulation were improved with greater host plant quality and for the wild population relative to the domesticated populations, and that pre- and post-challenge growth rates were improved with greater host plant quality. We further used SEM to quantify the relative magnitude of direct effects of host plant quality and selection history on immune defences versus their indirect effects mediated via body condition (pre-challenge growth rate). The magnitude of the indirect effect of host plant quality on encapsulation (host plant quality to body condition path: 0.81 × body condition to encapsulation path: 0.90 = 0.73) was nearly twice the magnitude of the direct effect of host plant quality on encapsulation (0.39; figure 1). In contrast, while the magnitude of the direct effect of host plant quality on melanization was relatively high (0.72), the indirect effect was negligible (0.067). Selection history had moderate direct effects on encapsulation and melanization, but indirect effects of selection history mediated via body condition were quite small.
SEM also allowed us to explore potential allocation trade-offs by examining the relationships between each of the endogenous variables. Pre-challenge growth rate—a proxy for condition—had little effect on melanization, but was strongly positively related to encapsulation, and strongly negatively related to post-challenge growth rate (figure 1). This indicates that individuals in a better condition prior to an immune challenge have greater encapsulation; however, following an immune challenge, those individuals which allocate more resources to encapsulation are able to allocate fewer resources to growth. Interestingly, this allocation trade-off between post-challenge growth rate and encapsulation was largely independent of melanization. The growth rate and encapsulation trade-off is further corroborated by larvae from the wild field population given a sham injection (5 µl sterile Grace's insect medium at the start of the fourth instar; devil's claw reared: n = 8, tobacco reared: n = 6). Prior to injection, growth rates (residual growth rates removing the mean effect of host plant quality) of sham- and Sephadex-injected larvae were comparable (t = 0.701, d.f. = 42, p = 0.487), but following injection, growth rates of sham-injected larvae were greater than those injected with an immune challenge (t = 2.17, d.f. = 42, p = 0.0354).
4. Discussion
These studies explored how selection history, host plant quality and allocation trade-offs alter the immune defences of the tobacco hornworm, M. sexta. Our analyses quantified the direct and indirect paths by which these factors contribute to immune responses in this system.
Several recent lines of evidence suggest selection has played an important role in shaping or maintaining immune defences. For example, immune genes tend to have greater rates of amino acid substitution than random samples of genes [7,8], and signatures of natural selection on genes involved in immune defences are concordant with variation among populations in their exposure to different suites of pathogens [49]. However, comparatively little is known regarding the short-term evolutionary dynamics of immune defences [8]. We used a population comparative approach to ask whether contemporary selection is important for maintaining immune defences. The results of our study are consistent with this hypothesis: both components of immune defences were reduced for each of the two domesticated laboratory populations of M. sexta compared with the wild field population from which they were derived over 35 years (more than 260 laboratory generations) ago. However, we note that further replication, particularly at the level of the wild population, would be required to corroborate this pattern. The laboratory environments, in which M. sexta are protected from natural enemies and bacterial and fungal pathogens, relaxes selection on immune defences. Indeed, observational studies of wild populations of M. sexta have shown that less than 2 per cent of eggs laid survive to maturity, owing to the combined effects of predation, parasitism and pathogen infection [36]. In contrast, survival of domesticated populations from egg to maturity typically exceeds 90 per cent [50]. The fact that there is greatly relaxed selection on immune defences in domesticated M. sexta, coupled with the fact that two distinct domesticated populations of M. sexta (from Duke and UNC) had reduced immune defences compared with their wild population ancestors, is consistent with our interpretation that selection is important for maintaining immune defences. It also implies that caution should be exercised in generalizing immune defence results of domesticated laboratory populations to natural populations.
The results of our study provide strong empirical support for the hypothesis that immune defences are improved with greater resource quality. We demonstrated that both melanization and encapsulation immune defences were improved on the typical, high-quality host plant, tobacco, relative to the novel, low-quality host plant, devil's claw, for all three of our M. sexta populations (figure 2a,b). Accumulating evidence suggests resource quality is an important determinant of immune defences in an organism's current environment [13–16]. Yet it seems likely that the relationship between resource quality and immune defences may alter the probability of an organism invading and persisting in a novel environment. This is particularly valid for phytophagous insects, which tend to be relatively specialized on their host plant resources [51]. In the case of M. sexta, reduced immune defences on the novel host plant, devil's claw, compared with the typical host plant, tobacco, may be one of the factors retarding the adoption of devil's claw as a host plant in the southeastern [37] and perhaps southwestern [36] USA. In contrast, a recent study examining immune defences of the autumnal moth, Epirrita autumnata, found that immune defences were the same or better across typical and alternative host plant species, perhaps facilitating the adoption of alternative host plants in this species [52]. The relationship between host immune defences and resource quality may play a key role in determining the ability of hosts to invade and persist in novel environments.
Clearly, host plant quality and selection history impact immune defences, but to what extent are these relationships driven by the indirect effects of host plant quality and selection history on immune defences mediated via non-immune defence aspects of the host's biology? Recent work by Smilanich et al. [29] has demonstrated the utility of SEM for disentangling the contributions of direct and indirect effects to overall immune defences. In this study, we used SEM to distinguish the relative contributions of the direct effects of host plant quality and selection history on immune defences from their indirect effects mediated via host body condition prior to an immune challenge and allocation trade-offs following an immune challenge.
In general, we found substantial variation in the relative importance of direct and indirect effects on immune defences. We emphasize that while direct and indirect effects refer to the major structural relationships between the variables measured in our study, in reality such direct and indirect effects may represent relative differences in the complexity of the relationships between predictor and response variables, owing to potentially important unmeasured variables. In our study, the most important indirect effect was that of host plant quality on encapsulation mediated via body condition prior to the immune challenge, which explained nearly twice as much of the variation in encapsulation as the direct effect of host plant quality (figure 1). In our study system, this result indicates that resource quality most strongly impacts immune defences indirectly through body condition; however, the generality of this pattern is unclear, as most previous work on immune defences cannot distinguish direct from indirect effects. The extent to which the accumulating evidence for positive effects of resource quality on immune defences (see above) reflects intermediary effects of improved body condition is therefore an important open question.
In contrast, the indirect effect of selection history on encapsulation was quite small (figure 1). This result is not surprising, as wild and domesticated populations of M. sexta have similar growth rates (our proxy for body condition) at the beginning of the fourth larval instar when the pre-challenge growth rate was assessed [39,41]. Yet this result does indicate that the reduced immune defences of the domesticated laboratory populations relative to the wild field population are largely a direct consequence of domestication rather than intermediary effects on body condition.
In addition to examining the indirect effects of body condition prior to an immune challenge, we also used SEM to investigate indirect effects of allocation following an immune challenge. The positive association between pre-challenge growth rate and encapsulation coupled with the negative association between pre- and post-challenge growth rates suggests an allocation trade-off, in which encapsulation is maintained at the cost of slower growth. Much of the positive evidence for trade-offs with immune defences comes from selection experiments ([6]; e.g. [53,54]), with relatively mixed support for such trade-offs based on standing genetic variation (e.g. for positive support, see [55–57]; for negative support, see [58,59]; for mixed support within the same study system, see [60]). Our result showing that encapsulation and post-challenge growth rate are related to pre-challenge body condition in opposite ways is consistent with an allocation trade-off. Particularly for the wild field population M. sexta, this lends further support for the importance of allocation trade-offs with immune defences in natural populations.
Despite encapsulation being costly, the lack of association between pre-challenge growth rate and melanization suggests melanization is maintained without incurring observable costs, either owing to investment in growth or other aspects of immune defence such as encapsulation. A possible explanation for this pattern is that the absolute amount of energy required for encapsulation (a process involving the deposition of large numbers of haemocytes) may be greater than that for melanization (a process involving the enzymatic conversion and subsequent deposition of melanin) [61]. An alternative, but non-mutually exclusive hypothesis is that the energetic costs of encapsulation and melanization may differ through ontogeny. In larval Lepidoptera, including M. sexta, circulating haemocytes—a general classification of various types of blood cells involved in immune defences—originate from the proliferation of embryonically derived haemocytes already in circulation, and the production of haemocytes from haematopoietic organs [62,63]. Particularly in more advanced larval developmental stages, the haematopoietic organs are the main source of plasmatocytes, or haemocytes involved in encapsulation, whereas haemocytes already in circulation are the main source of oenocytoids, or haemocytes containing phenoloxidase, an enzyme necessary for the conversion of melanin precursors to active melanin [64]. The production of new plasmatocytes in response to an immune challenge may therefore be more energetically costly than recruiting oenocytoids already in circulation. Thus, at the time of the immune challenge in our experiments with M. sexta, the energetic costs of encapsulation may have been more prominent because they were more recent compared with the costs involved with melanization. There is a limited amount of empirical evidence demonstrating a pattern of highly divergent responses among different immune components [65–68]. The generality of this pattern and the underlying mechanisms are unclear but deserving of further study. SEM may prove useful in this regard, and more generally in disentangling the relative contributions of physiological, ecological and evolutionary factors to variation in immune defences.
Acknowledgements
We thank C. Sorenson for access to the North Carolina State Research Station for collecting wild M. sexta eggs. C. Angell, A. Kamenel, S. Sheline and M. Smith helped with the experiments. H. F. Nijhout kindly provided M. sexta eggs from the Duke University laboratory colony. T. Perdue and W. Kier provided guidance on the imaging of immune responses. C. Burch, J. Keppel, H. MacLean, R. Martin, C. Mitchell, C. Jones, S. Seiter, M. Servedio, M. Smith and two anonymous reviewers provided helpful comments on an earlier version of this manuscript. The National Science Foundation (IOS-0641179 to J.G.K.) provided funding.
References
- 1.Stock S. P., Vandenburg J., Glazer I., Boemare N. (ed.) 2009. Insect pathogens: molecular approaches and techniques. Wallingford, UK: CAB International [Google Scholar]
- 2.Schmid-Hempel P. 2003. Variation in immune defence as a question of evolutionary ecology. Proc. R. Soc. Lond. B 270, 357–366 10.1098/rspb.2002.2265 (doi:10.1098/rspb.2002.2265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siva-Jothy M. T., Moret Y., Rolff J. 2005. Insect immunity: an evolutionary ecology perspective. Adv. Insect Physiol. 32, 1–48 10.1016/S0065-2806(05)32001-7 (doi:10.1016/S0065-2806(05)32001-7) [DOI] [Google Scholar]
- 4.Schmid-Hempel P. 2005. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 50, 529–551 10.1146/annurev.ento.50.071803.130420 (doi:10.1146/annurev.ento.50.071803.130420) [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann J. A., Kafatos F. C., Janeway C. A., Jr, Ezekowitz R. A. B. 1999. Phylogenetic perspectives in innate immunity. Science 284, 1313–1318 10.1126/science.284.5418.1313 (doi:10.1126/science.284.5418.1313) [DOI] [PubMed] [Google Scholar]
- 6.Lazzaro B. P., Little T. J. 2009. Immunity in a variable world. Phil. Trans. R. Soc. B 364, 15–26 10.1098/rstb.2008.0141 (doi:10.1098/rstb.2008.0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlenke T. A., Begun D. J. 2003. Natural selection drives Drosophila immune system evolution. Genetics 164, 1471–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzaro B. P. 2008. Natural selection on the Drosophila antimicrobial immune system. Curr. Opin. Microbiol. 11, 284–289 10.1016/j.mib.2008.05.001 (doi:10.1016/j.mib.2008.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingvarsson P. K., Ericson L. 1998. Spatial and temporal variation in disease levels of a floral smut (Athracoidea heterospora) on Carex nigra. J. Ecol. 86, 53–61 10.1046/j.1365-2745.1998.00235.x (doi:10.1046/j.1365-2745.1998.00235.x) [DOI] [Google Scholar]
- 10.Bensch S., Åkesson S. 2003. Temporal and spatial variation of hematazoans in Scandinavian willow warblers. J. Parasitol. 89, 388–391 [DOI] [PubMed] [Google Scholar]
- 11.Munster V. J., et al. 2007. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Path. 3, e61. 10.1371/journal.ppat.0030061 (doi:10.1371/journal.ppat.0030061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis R. H. 2004. The age of model organisms. Nat. Rev. Genet. 5, 69–76 10.1038/nrg1250 (doi:10.1038/nrg1250) [DOI] [PubMed] [Google Scholar]
- 13.Feder D., Mello C. B., Garcia E. S., Azambuja P. 1997. Immune response in Rhodinus prolixus: influence of nutrition and ecdysone. J. Insect Physiol. 43, 513–519 10.1016/S0022-1910(97)00010-3 (doi:10.1016/S0022-1910(97)00010-3) [DOI] [PubMed] [Google Scholar]
- 14.Siva-Jothy M. T., Thompson J. J. W. 2002. Short-term nutrient deprivation affects immune function. Physiol. Entomol. 27, 206–212 10.1046/j.1365-3032.2002.00286.x (doi:10.1046/j.1365-3032.2002.00286.x) [DOI] [Google Scholar]
- 15.Ojala K., Julkunen-Tiitto R., Lindström L., Mappes J. 2005. Diet affects the immune defence and life-history traits of an Arctiid moth Prasemia plantaginis. Evol. Ecol. Res. 7, 1153–1170 [Google Scholar]
- 16.Klemola N., Klemola T., Rantala M. J., Ruuhola T. 2007. Natural host-plant quality affects immune defence of an insect herbivore. Entomol. Exp. Appl. 123, 167–176 10.1111/j.1570-7458.2007.00533.x (doi:10.1111/j.1570-7458.2007.00533.x) [DOI] [Google Scholar]
- 17.Zuk M., Stoehr A. M. 2002. Immune defense and host life history. Am. Nat. 160, S9–S22 10.1086/342131 (doi:10.1086/342131) [DOI] [PubMed] [Google Scholar]
- 18.Sheldon B. C., Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321 10.1016/0169-5347(96)10039-2 (doi:10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 19.Lochmiller R. L., Deerenberg C. 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98 10.1034/j.1600-0706.2000.880110.x (doi:10.1034/j.1600-0706.2000.880110.x) [DOI] [Google Scholar]
- 20.Norris K., Evans M. R. 2000. Ecological immunology: life history trade-offs and immune defense in birds. Behav. Ecol. 11, 19–26 10.1093/beheco/11.1.19 (doi:10.1093/beheco/11.1.19) [DOI] [Google Scholar]
- 21.Awmack C. S., Leather S. R. 2002. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47, 817–844 10.1146/annurev.ento.47.091201.145300 (doi:10.1146/annurev.ento.47.091201.145300) [DOI] [PubMed] [Google Scholar]
- 22.König C., Schmid-Hempel P. 1995. Foraging activity and immunocompetence in workers of the bumble bee, Bombus terrestris L. Proc. R. Soc. Lond. B 260, 225–227 10.1098/rspb.1995.0084 (doi:10.1098/rspb.1995.0084) [DOI] [Google Scholar]
- 23.Benrey B., Denno R. F. 1997. The slow-growth–high mortality hypothesis: a test using the cabbage butterfly. Ecology 78, 987–999 10.1890/0012-9658(1997)078[0987:TSGHMH]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[0987:TSGHMH]2.0.CO;2) [DOI] [Google Scholar]
- 24.Siva-Jothy M. T., Tsubaki Y., Hooper R. E. 1998. Decreased immune response as a proximate cost of copulation and oviposition in a damselfly. Physiol. Entomol. 23, 274–277 10.1046/j.1365-3032.1998.233090.x (doi:10.1046/j.1365-3032.1998.233090.x) [DOI] [Google Scholar]
- 25.Rantala M. J., Koskimäki J., Taskinen J., Tynkkynen K., Suhonen J. 2000. Immunocompetence, developmental stability and wingspot size in the damselfly Calopteryx splendens L. Proc. R. Soc. Lond. B 267, 2453–2457 10.1098/rspb.2000.1305 (doi:10.1098/rspb.2000.1305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKean K. A., Nunney L. 2001. Increased activity reduces male immune function in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 98, 7904–7909 10.1073/pnas.131216398 (doi:10.1073/pnas.131216398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolff J., Siva-Jothy M. T. 2002. Copulation corrupts immunity: a mechanism for a cost of mating in insects. Proc. Natl Acad. Sci. USA 99, 9916–9918 10.1073/pnas.152271999 (doi:10.1073/pnas.152271999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fedorka K. M., Zuk M., Mousseau T. A. 2004. Immune suppression and the cost of reproduction in the ground cricket, Allonemobius socius. Evolution 58, 2478–2485 10.1554/04-399 (doi:10.1554/04-399) [DOI] [PubMed] [Google Scholar]
- 29.Smilanich A. M., Dyer L. A., Chambers J. Q., Bowers M. D. 2009. Immunological cost of chemical defence and the evolution of herbivore diet breadth. Ecol. Lett. 12, 612–621 10.1111/j.1461-0248.2009.01309.x (doi:10.1111/j.1461-0248.2009.01309.x) [DOI] [PubMed] [Google Scholar]
- 30.Lavine M. D., Strand M. R. 2002. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 32, 1295–1309 10.1016/S0965-1748(02)00092-9 (doi:10.1016/S0965-1748(02)00092-9) [DOI] [PubMed] [Google Scholar]
- 31.Kanost M. R., Jiang H., Yu X. Q. 2004. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 198, 97–105 10.1111/j.0105-2896.2004.0121.x (doi:10.1111/j.0105-2896.2004.0121.x) [DOI] [PubMed] [Google Scholar]
- 32.Miller J. S., Nguyen T., Stanley-Samuelson D. W. 1994. Eicosanoids mediate insect nodulation responses to bacterial infections. Proc. Natl Acad. Sci. USA 91, 12 418–12 422 10.1073/pnas.91.26.12418 (doi:10.1073/pnas.91.26.12418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eleftherianos I., et al. 2007. An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase inhibition. Proc. Natl Acad. Sci. USA 104, 2419–2424 10.1073/pnas.0610525104 (doi:10.1073/pnas.0610525104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothschild W., Jordan K. 1903. A revision of the lepidopterous family Sphingidae. Novit Zool. 9, 1–972 [Google Scholar]
- 35.Mechaber W. L., Hildebrand J. G. 2000. Novel, non-solanaceous hostplant record for Manduca sexta (Lepidoptera: Sphingidae) in the southwestern United States. Ann. Entomol. Soc. Am. 93, 447–451 10.1603/0013-8746(2000)093[0447:NNSHRF]2.0.CO;2 (doi:10.1603/0013-8746(2000)093[0447:NNSHRF]2.0.CO;2) [DOI] [Google Scholar]
- 36.Mira A., Bernays E. A. 2002. Trade-offs in host use by Manduca sexta: plant characters vs natural enemies. Oikos 97, 387–397 10.1034/j.1600-0706.2002.970309.x (doi:10.1034/j.1600-0706.2002.970309.x) [DOI] [Google Scholar]
- 37.Diamond S. E., Kingsolver J. G. 2010. Fitness consequences of host plant choice: a field experiment. Oikos 119, 542–550 10.1111/j.1600-0706.2009.17242.x (doi:10.1111/j.1600-0706.2009.17242.x) [DOI] [Google Scholar]
- 38.Small J. K. 1903. Flora of the southeastern United States. New York, NY: self published [Google Scholar]
- 39.Diamond S. E., Hawkins S. D., Nijhout H. F., Kingsolver J. G. 2010. Evolutionary divergence of field and laboratory populations of Manduca sexta in response to host plant quality. Ecol. Entomol. 35, 166–174 10.1111/j.1365-2311.2009.01166.x (doi:10.1111/j.1365-2311.2009.01166.x) [DOI] [Google Scholar]
- 40.Nijhout H. F., Davidowitz G., Roff D. A. 2006. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J. Biol. 5, 1–16 10.1186/jbiol43 (doi:10.1186/jbiol43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kingsolver J. G. 2007. Variation in growth and instar number in field and laboratory Manduca sexta. Proc. R. Soc. B 274, 977–981 10.1098/rspb.2006.0036 (doi:10.1098/rspb.2006.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilmos P., Kurucz E. 1998. Insect immunity: evolutionary roots of the mammalian innate immune system. Immunol. Lett. 62, 59–66 10.1016/S0165-2478(98)00023-6 (doi:10.1016/S0165-2478(98)00023-6) [DOI] [PubMed] [Google Scholar]
- 43.Beetz S., Holthusen T. K., Koolman J., Trenczek T. 2008. Correlation of hemocyte counts with different developmental parameters during the last larval instar of the tobacco hornworm, Manduca sexta. Arch. Insect Biochem. Physiol. 67, 63–75 10.1002/arch.20221 (doi:10.1002/arch.20221) [DOI] [PubMed] [Google Scholar]
- 44.Lavine M. D., Beckage N. E. 1996. Temporal pattern of parasitism-induced immunosuppression in Manduca sexta larvae parasitized by Cotesia congregata. J. Insect Physiol. 42, 41–51 10.1016/0022-1910(95)00081-X (doi:10.1016/0022-1910(95)00081-X) [DOI] [Google Scholar]
- 45.R Development Core Team 2009. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; ISBN 3-900051-07-0. See http://www.R-project.org [Google Scholar]
- 46.Chin W. W., Newsted P. R. 1999. Structural equation modeling analysis with small samples using partial least squares. In Statistical strategies for small sample research (ed. Hoyle R.), pp. 307–341 Thousand Oaks, CA: Sage Publications [Google Scholar]
- 47.Monecke A. 2010. semPLS: structural equation modeling using partial least squares. R package version 0.7-3/r3. See http://www.R-Forge.R-project.org/projects/sempls/
- 48.D'Amico L. J., Davidowitz G., Nijhout H. F. 2001. The developmental and physiological basis of body size evolution in an insect. Proc. R. Soc. B 268, 1589–1593 10.1098/rspb.2001.1698 (doi:10.1098/rspb.2001.1698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlenke T. A., Begun D. J. 2005. Linkage disequilibrium and recent selection at three immunity receptor loci in Drosophila simulans. Genetics 169, 2013–2022 10.1534/genetics.104.035337 (doi:10.1534/genetics.104.035337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmad I. M., Waldbauer G. P., Friedman S. 1989. A defined artificial diet for the larvae of Manduca sexta. Entomol. Exp. Appl. 53, 189–191 10.1007/BF00188000 (doi:10.1007/BF00188000) [DOI] [Google Scholar]
- 51.Jaenike J. 1990. Host specialization in phytophagous insects. Annu. Rev. Ecol. Syst. 21, 243–273 10.1146/annurev.es.21.110190.001331 (doi:10.1146/annurev.es.21.110190.001331) [DOI] [Google Scholar]
- 52.Yang S., Ruuhola T., Haviola S., Rantala M. J. 2008. Effects of host-plant shift on immune and other key life-history traits of an eruptive Geometrid, Epirrita autumnata (Borkhausen). Ecol. Entomol. 33, 510–516 10.1111/j.1365-2311.2008.01000.x (doi:10.1111/j.1365-2311.2008.01000.x) [DOI] [Google Scholar]
- 53.Boots M., Begon M. 1993. Trade-offs with resistance to granulosis virus in the Indian meal moth examined by laboratory evolution experiments. Funct. Ecol. 7, 528–534 10.2307/2390128 (doi:10.2307/2390128) [DOI] [Google Scholar]
- 54.Kraaijeveld A. R., Godfray H. J. C. 1997. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature 389, 278–280 10.1038/38483 (doi:10.1038/38483) [DOI] [PubMed] [Google Scholar]
- 55.Hoang A. 2001. Immune response to parasitism reduces resistance of Drosophila melanogaster to dessication and starvation. Evolution 55, 2353–2358 10.1554/0014-3820(2001)055[2353:IRTPRR]2.0.CO;2 (doi:10.1554/0014-3820(2001)055[2353:IRTPRR]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 56.Freitak D., Ots I., Vanatoa A., Hõrak P. 2003. Immune response is energetically costly in white cabbage butterfly pupae. Proc. R. Soc. Lond. B 270, S220–S222 10.1098/rsbl.2003.0069 (doi:10.1098/rsbl.2003.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKean K. A., Yourth C. P., Lazzaro B. P., Clark A. G. 2008. The evolutionary costs of immunological maintenance and deployment. BMC Evol. Biol. 8, 76. 10.1186/1471-2148-8-76 (doi:10.1186/1471-2148-8-76) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Little T. J., Carius H. J., Sakwinska O., Ebert D. 2002. Competitiveness and life-history characteristics of Daphnia with respect to susceptibility to a parasite. J. Evol. Biol. 15, 796–802 10.1046/j.1420-9101.2002.00436.x (doi:10.1046/j.1420-9101.2002.00436.x) [DOI] [Google Scholar]
- 59.Altermatt F., Ebert D. 2007. The genotype specific competitive ability does not correlate with infection in natural Daphnia magna populations. PLoS ONE 2, e1280. 10.1371/journal.pone.0001280 (doi:10.1371/journal.pone.0001280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stoehr A. M. 2010. Responses of disparate phenotypically-plastic, melanin-based traits to common cues: limits to the benefits of adaptive plasticity? Evol. Ecol. 24, 287–298 10.1007/s10682-009-9306-4 (doi:10.1007/s10682-009-9306-4) [DOI] [Google Scholar]
- 61.Cerenius L., Lee B. L., Söderhäll K. 2008. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 29, 263–271 10.1016/j.it.2008.02.009 (doi:10.1016/j.it.2008.02.009) [DOI] [PubMed] [Google Scholar]
- 62.Nardi J. B., Ujhelyi E., Pilas B., Garsha K., Kanost M. R. 2003. Hematopoietic organs of Manduca sexta and hemocyte lineages. Dev. Genes Evol. 213, 477–491 10.1007/s00427-003-0352-6 (doi:10.1007/s00427-003-0352-6) [DOI] [PubMed] [Google Scholar]
- 63.Nardi J. B. 2004. Embryonic origins of the two main classes of hemocytes—granular cells and plasmatocytes in Manduca sexta. Dev. Genes Evol. 214, 19–28 10.1007/s00427-003-0371-3 (doi:10.1007/s00427-003-0371-3) [DOI] [PubMed] [Google Scholar]
- 64.Strand M. R. 2008. The insect cellular immune response. Insect Sci. 15, 1–14 10.1111/j.1744-7917.2008.00183.x (doi:10.1111/j.1744-7917.2008.00183.x) [DOI] [Google Scholar]
- 65.Moret Y., Schmid-Hempel P. 2001. Entomology: immune defence in bumble-bee offspring. Nature 414, 506. 10.1038/35107138 (doi:10.1038/35107138) [DOI] [PubMed] [Google Scholar]
- 66.Cotter S. C., Kruuk L. E., Wilson K. 2004. Costs of resistance: genetic correlations and potential trade-offs in an insect immune system. J. Evol. Biol. 17, 421–429 10.1046/j.1420-9101.2003.00655.x (doi:10.1046/j.1420-9101.2003.00655.x) [DOI] [PubMed] [Google Scholar]
- 67.Freitak D., Wheat C. W., Heckel D. G., Vogel H. 2007. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biol. 5, 56. 10.1186/1741-7007-5-56 (doi:10.1186/1741-7007-5-56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilfert L., Gadau J., Schmid-Hempel P. 2007. The genetic architecture of immune defense and reproduction in male Bombus terrestris bumblebees. Evolution 61, 804–815 10.1111/j.1558-5646.2007.00079.x (doi:10.1111/j.1558-5646.2007.00079.x) [DOI] [PubMed] [Google Scholar]