Abstract
After preliminary training to open a sliding door using their head and their paw, dogs were given a discrimination task in which they were rewarded with food for opening the door using the same method (head or paw) as demonstrated by their owner (compatible group), or for opening the door using the alternative method (incompatible group). The incompatible group, which had to counterimitate to receive food reward, required more trials to reach a fixed criterion of discrimination performance (85% correct) than the compatible group. This suggests that, like humans, dogs are subject to ‘automatic imitation’; they cannot inhibit online the tendency to imitate head use and/or paw use. In a subsequent transfer test, where all dogs were required to imitate their owners' head and paw use for food reward, the incompatible group made a greater proportion of incorrect, counterimitative responses than the compatible group. These results are consistent with the associative sequence learning model, which suggests that the development of imitation depends on sensorimotor experience and phylogenetically general mechanisms of associative learning. More specifically, they suggest that the imitative behaviour of dogs is shaped more by their developmental interactions with humans than by their evolutionary history of domestication.
Keywords: associative sequence learning, mirror neuron, stimulus–response compatibility, automatic imitation, dogs
1. Introduction
Humans are subject to ‘automatic imitation’; the sight of another person's body movement tends to elicit the same body movement from the observer, even when this imitative tendency interferes with efficient performance of an ongoing task [1–5]. For example, if people are instructed to open their mouths as soon as they see the letters ‘OM’ appear on a screen, responses are slower when the letters are accompanied by an image of an opening hand than when they are accompanied by an image of an opening mouth [6].
Automatic imitation is of interest for at least three reasons. First, studies in naturalistic settings have shown that automatic imitation is pervasive in everyday human life, where it promotes affiliation and cooperation among social partners [7]. Second, it has been suggested that automatic imitation is necessary for imitation learning [8], a capacity that is thought to be crucial for the cultural inheritance of behaviour [9]. According to the associative sequence learning model (ASL; [10]), imitation learning requires not only encoding the order of elements in a novel sequence of body movements (sequence learning), but also that observation of each element automatically activates a corresponding motor programme (automatic imitation). Third, evidence is emerging that automatic imitation is mediated by the ‘mirror neuron system’ [11], areas of the premotor and parietal cortex that are active during passive observation of actions and during execution of the same actions without visual feedback (e.g. [12,13]). For example, Catmur et al. [11] showed that, in humans, disruption of the mirror neuron system, using repetitive transcranial magnetic stimulation (TMS), impairs automatic imitation.
Research with non-human animals could provide insight into the mechanisms, functions and origins of automatic imitation, but to date automatic imitation has been reported in only one non-human species—the budgerigar [14]. The present study sought evidence of automatic imitation in dogs. Domestic dogs are promising candidates because they are known to be highly sensitive to social cues [15–17], and their interactions with humans may provide the kind of sensorimotor experience, which, according to the ASL model, is required for the development of automatic imitation. This experience consists of ‘correlated’ observation and execution of the same body movement, i.e. experience in which observation of a body movement and execution of the same body movement occur close together in time (contiguity), and in which one of these events is predictive of the other (contingency). Experience of this kind is obtained by human infants and children when they watch their own actions, directly or using a mirror; when they are imitated by adults; when the same sound (verbal or non-verbal) is heard during the observation and execution of an action; and when they are explicitly rewarded by an adult for performing actions that match those of the adult [18]. Similarly, dogs are sometimes trained on actions where the body movement of the owner is matched to the body movement of the dog e.g. giving the paw, where the owner usually stretches out the hand with the expectation that the dog stretches out its paw in order to get a reward.
Dogs have not been tested previously for automatic imitation. Mixed results have been obtained in research seeking evidence of voluntary imitation in dogs, where it is assumed that any imitative behaviour is under the control of intentional mechanisms. For example, testing a large sample of dogs under a wide range of conditions, Tennie et al. [19] found no evidence that they would copy the intransitive movements (sit and lie) of another dog. Single case studies have found ‘functional matching’ of human actions (e.g. reproducing the object-related effects of an observed action), but were ambiguous with respect to body movement imitation [20,21]. Similarly, Miller et al. [22] showed that dogs that have seen a conspecific pushing a screen door to the right or left are more likely than controls to push the door in the same direction as their demonstrator. However, as the authors pointed out, this result is more likely to have been due to learning by observation about properties of the door (emulation) and their relationship with reward (observational conditioning), than to copying of body movements (imitation). Just one study of imitation in dogs has reported clear, positive findings: Range et al. [23] found that dogs that had observed a conspecific using their paw to press a bar for food were more likely than control dogs, which had not observed bar pressing, to use their paw rather than their head to press the bar. This effect was present in the first test trial and in seven subsequent test trials. Given the two-action design of this experiment and the low baseline probability of the observed paw action, this result provides clear evidence for action imitation in dogs.
The study by Range et al. [23] suggests that, under certain conditions, dogs imitate paw actions, i.e. use of the paw rather than the head to manipulate an object. However, it does not tell us whether dogs are subject to automatic imitation of paw action. In the previous study, the observer dogs were rewarded for pressing the bar with their head and for pressing it with their paw. Thus, paw use did not interfere with efficient task performance, and therefore imitating paw action could have been either automatic or intentional, or both.
In the first part of the present study, we investigated whether dogs are subject to automatic imitation using an analogue of the stimulus–response compatibility (SRC) paradigms that are used to investigate automatic imitation in humans (e.g. [2,6]). In SRC paradigms, participants are required to make responses that match (compatible) or do not match (incompatible) their eliciting stimuli on a specified dimension (e.g. spatial location), and are typically found to respond faster and more accurately on compatible trials. In each trial of phase 1 in the present procedure, the dog observed its owner opening a door using their head or their paw (hand). Over trials, the owner used the two methods equally often, and in an unpredictable order. A few seconds after observing door opening, the dog was allowed to open the door itself, and rewarded for using the same part of its body as the owner (compatible group; e.g. rewarded for head use after observing head use), or for using the alternative part of its body (incompatible group; e.g. rewarded for paw use after observing head use). Under these conditions, imitation will facilitate correct (rewarded) responding in the compatible group, and interfere with correct responding in the incompatible group. Therefore, if dogs are subject to automatic imitation, one would expect the incompatible group to take longer than the compatible group to reach a fixed criterion of correct responding.
In the second phase of the experiment, when each dog had reached criterion performance in the initial task, it was given a transfer test with a door in a different location. On each trial in the transfer test, the dogs in both the compatible group and the incompatible group were required to open the door using the same method as their owner. Thus, when they observed head action they were rewarded for using their head, but not for using their paw, and when they observed paw action, they were rewarded for using their paw, but not for using their head. The first phase asked whether the dogs brought with them to the experiment a tendency automatically to imitate head action and/or paw action. In contrast, the second phase asked whether the sensorimotor experience received by the incompatible group in the first phase had established a new tendency to ‘counterimitate’; to use the head after observing paw use, and to use the paw after observing head use. If the dogs in the incompatible group had a tendency automatically to counterimitate, then compared with the compatible group they should perform a greater proportion of counterimitative responses, in spite of the fact that these responses were not rewarded in the transfer test.
2. Material and methods
(a). Subjects
Ten dogs, and their owners, completed the experiment. All of the dogs were older than eight months and well-trained (agility, rescue and obedience training). They were randomly assigned to two groups: compatible (five dogs: three border collies, one Australian shepherd, one mongrel; three males, two females) and incompatible (five dogs: four border collies, one mongrel; two males, three females). The experiments were conducted at the homes of the participants, between May 2006 and January 2008. The research was carried out in accordance with the regulations of Austria where the research was performed, and any institutional guidelines.
(b). Apparatus
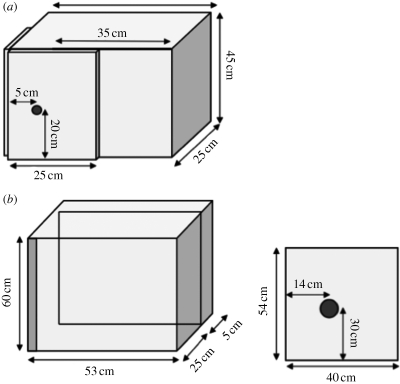
For the first phase, we used a wooden box (45 high × 60 wide × 25 cm deep) with a sliding door at the front (figure 1a). The back of the box was open. On the door was a knob (11 cm long × 2.8 cm diameter) to facilitate opening of the box. In the second phase, we used a new box to test the dogs in both groups. This was to ensure that both groups experienced a change in the test conditions between the first and the second phase, and therefore that, if the incompatible group was slower to reach the criterion in phase 2, this was due to the training they experienced in phase 1 rather than a non-specific effect of alteration in the test conditions. The box used in phase 2 was larger (60 high × 50 wide × 30 cm deep), with a sliding door that was set back from the front of the box by 25 cm (figure 1b). The knob was slightly smaller than in the training box with a length of 6.5 cm and a diameter of 2.5 cm. The door, ceiling and floor of the larger box were made of wood, but the sides were made of Plexiglas to allow the experimenter to see what the dog was doing inside the box. All training and test sessions were videotaped with a digital camera (Sony DCR—TRV 25E).
Figure 1.
Diagrams of (a) the training box, and (b) the test box and its door.
(c). Procedure
(i). Preliminary training
The dogs were first shaped by successive approximation to open the sliding door of the training box using both methods (head and paw). Initially the sliding door was left open, and the dog was allowed to collect a food reward (a small piece of sausage) from the floor inside the box. Over trials, the door was closed gradually, until it was necessary for the dog to move the door to obtain the food. The method (paw or head) chosen by the dog when manipulating the door for the first time was defined as the preferred method. All dogs except two (one from the compatible, one from the incompatible group) preferred the mouth. Once the dog was able to open the door completely, using its head or its paw, the dog received five trials in which it was allowed to use only the method it had chosen. For example, if it had shown a preference for head use, the owner restricted the movement of the dog's paws, so that the dog had to use its head to open the door in each of the five trials. Next, the dog was trained, via a shaping procedure, to use the non-preferred method to open the box. In this procedure, the experimenter kept the door closed until the dog made an attempt to use the non-preferred method after trying unsuccessfully with the preferred method. When the dog made an attempt to use the non-preferred method (e.g. lifting the paw, or manipulating the door with the muzzle), the experimenter immediately opened the door and the dog was rewarded. The owner also praised the dog. This procedure was repeated, with the dog being required to make progressively more complete attempts to open the door using the non-preferred method, until it used the non-preferred method to open the door without the experimenter's assistance, and without first trying the preferred method. Once this was achieved, the dog was given five trials in which it used its non-preferred method to open the door. The owner did not demonstrate door opening at any time during preliminary training.
(ii). Phase 1: training
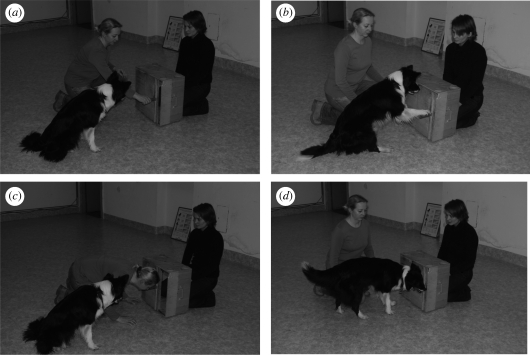
For five of the 10 dogs (two in the compatible group and three in the incompatible group), the training phase began with the following ‘no command’ procedure. Each trial began with the owner opening the door, using their head or paw (hand; figure 2). The dog, positioned alongside its owner, was allowed to observe this demonstration. As soon as the owner had completed her demonstration, the door was closed by the experimenter from inside the box (e.g. invisible to the dog), and the dog was allowed to open it. The interval between observation and testing was 2–3 s. Dogs in the compatible group were rewarded with food if they opened the door using the same method as their owner, i.e. for head use if the owner used their head, and for paw use if the owner used their paw. Dogs in the incompatible group were rewarded with food if they opened the door using the alternative method, i.e. for paw use if the owner used their head, and for head use if the owner used their paw. After 350 trials involving this no command procedure, none of the five dogs had achieved criterion performance; they had not made the correct response in 17 of 20 successive trials (85% correct). Therefore, a new ‘command’ procedure was used for further training of these five dogs, and training of the other five dogs (three in the compatible group, and two in the incompatible group) commenced with this command procedure.
Figure 2.
Photographs of (a) a dog owner demonstrating how to open the box with the hand (paw); (b) dog matching the paw action; (c) dog owner demonstrating how to open the box with the head; and (d) dog matching the head action.
The command procedure differed from the no command procedure as follows. While opening the door, and again as the dog approached the door on test, the owner spoke a command appropriate to the action the dog should perform. Thus, dogs in the compatible group heard the command ‘Paw!’ (in German) when the owner demonstrated paw use, and ‘Head!’ when she demonstrated head use. Dogs in the incompatible group heard the command ‘Head!’ when the owner demonstrated paw use, and ‘Paw!’ when she demonstrated head use. Once the dogs reached the criterion (85% correct) with two commands, the second command was omitted. Consequently, the dog now had to pay close attention to the owner's demonstration because the timing of the dog's own action was no longer cued by a command. Thus, the command procedure was used to direct the dog's attention to the demonstration. When each dog reached the criterion with a single command (85% correct), the first command was also omitted. Training then continued, using the no command procedure, until each dog reached criterion performance, and thereby completed the first phase of the experiment.
Over trials, in both the command and no command procedures, the owners demonstrated head use and paw use in an unpredictable sequence, and with approximately equal frequency in each session. If a dog began to perseverate, using the same method to open the door in every trial, the method requiring the alternative response was demonstrated in successive trials until the dog used the alternative method in at least one trial. Sessions were conducted at approximately weekly intervals, and each session ended when the dog became inattentive.
(iii). Phase 2: transfer test
The procedure used in the second phase of the experiment was identical for all dogs. Each trial began with the dog's owner opening the inset door in the larger test box using their head or their paw (hand). The dog was then allowed to open the same door, and rewarded only if it used the same method (head or paw) as its owner.
3. Results
For phase 1, we analysed the number of trials required to reach the learning criterion (correct responding in 17 of 20 successive trials; 85%) with two commands, with one command, and with no commands. In each of these three cases, we used analysis of variance (ANOVA) in which group (compatible, incompatible) was the primary between-subjects variable. Half of the dogs (two in the compatible group and three in the incompatible group) began, as well as ended, the first phase with a no command training procedure. Although none of these dogs reached the criterion in the initial no command training, we checked whether this experience influenced their subsequent performance by including the factor, ‘pilot’ (included, not included), as an additional between-subjects variable in each analysis.
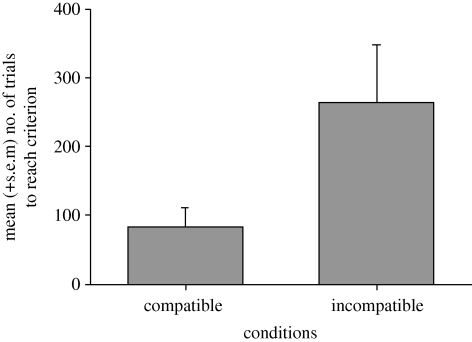
In phase 1, the compatible and incompatible groups did not differ in the number of trials required to reach the criterion with two commands (compatible: mean = 278, s.e.m. = 109; incompatible: mean = 349, s.e.m. = 116) or with one command (compatible: mean = 85, s.e.m. = 30; incompatible: mean = 164, s.e.m. = 61; all F values < 1). However, in the final, no command phase of training, where the dogs were required to use their owners' door opening behaviour as their cue, the compatible group (mean = 83, s.e.m. = 28) took fewer trials to reach the criterion than the incompatible group (mean = 265, s.e.m. = 83; F1,6 = 6.69, p = .041; figure 3). The effect of pilot (F1,6 = 1.79) and the group × pilot interaction (F1,6 = 2.55) was not significant.
Figure 3.
The mean (+ s.e.m.) number of trials to reach criterion for dogs in the compatible and incompatible groups in the training phase when no commands were given (phase 1).
To assess performance in phase 2, we calculated a discrimination ratio for each dog by dividing the number of trials in which it used the same method to open the door as its owner by the total number of trials in which it succeeded in opening the door using either the same method or the alternative method. On this measure, values above 0.5 indicate that a dog tended to imitate, to use the same method as its owner, and values below 0.5 indicate that a dog tended to counterimitate, to use the alternative method. These discrimination ratios were analysed using ANOVA in which group (compatible, incompatible) was the between-subjects factor.
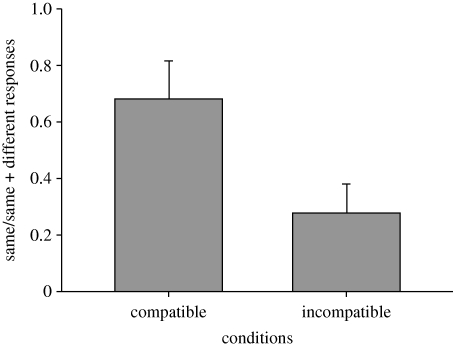
In phase 2, dogs in the compatible group (mean = 0.68, s.e.m. = 0.14) used the same method as their owner to open the door in a greater proportion of trials than did the incompatible group (mean = 0.28, s.e.m. = 0.10; F1,8 = 5.32, p = .05; figure 4). Thus, on average, the dogs in the incompatible group used the alternative method, they counterimitated, in 72 per cent of trials.
Figure 4.
The mean (+s.e.m.) discrimination ratio for dogs in the compatible and incompatible groups in the transfer test (phase 2).
4. Discussion
The results of the present study provide the first evidence of automatic imitation in dogs. In phase 1, the training phase, dogs that were required not to imitate in order to gain food reward, i.e. to use their paw when they saw head use, and to use their head when they saw paw use (incompatible group), took significantly longer to reach a fixed criterion of correct responding than dogs that were required to imitate for food, i.e. to use their head when they saw head use, and to use their paw when they saw paw use. This finding suggests that the dogs brought with them to the experiment a tendency automatically to imitate hand use and/or paw use by their owner; to imitate these actions even when it was costly to do so, when imitation interfered with the efficient performance of an ongoing task. In this respect, the results of phase 1 are analogous to those found in human participants using SRC paradigms (e.g. [1–6]). Experiments using these paradigms have shown that humans are slower to make correct responses when those responses consist of body movements (e.g. mouth opening) that are counterimitative with respect to concurrently presented action stimuli (e.g. hand opening).
In the second, transfer phase of the present experiment, dogs that had previously been rewarded for head use after observing paw use and vice versa (incompatible group), made a greater proportion of incorrect, counterimitative responses than dogs that had previously been rewarded for head use after observing head use and for paw use after observing paw use (compatible group). This group difference occurred in spite of the fact that, in the second phase, all dogs were rewarded only for imitative responses. Therefore, the incompatible group's tendency to make counterimitative responses interfered with efficient performance in the transfer task. Thus, it appears that, as a result of the sensorimotor experience they received in phase 1, in the transfer test the dogs in the incompatible group showed a tendency towards automatic counterimitation.
The results of the transfer test are consistent with those of human studies showing that incompatible sensorimotor experience—performing one action while observing an alternative action—can abolish [4,24] and reverse automatic imitation [25,26]. For example, using TMS, Catmur et al. [25] found that, prior to training, observation of index finger movement yielded larger TMS-induced motor evoked potentials (MEPs) in an index finger muscle than in a little finger muscle, and vice versa for observation of little finger movement. After incompatible training, in which participants were required to make index finger movements whenever they saw little finger movements, and vice versa, this automatic imitation or ‘mirror’ effect was reversed: for example, observation of index finger movement yielded larger MEPs in a little finger muscle than in an index finger muscle.
The occurrence of counterimitation following incompatible sensorimotor training, in dogs and in humans, is consistent with the ASL model. This model suggests that automatic imitation is due to ‘matching vertical associations’; excitatory links between sensory and motor representations of the same action, forged through correlated experience of observing and executing the same action (e.g. [10,18,27]). According to the ASL model, these excitatory links are established through the same mechanisms of associative learning that produce Pavlovian and instrumental conditioning in the laboratory. These mechanisms of associative learning are known to be present in a wide range of species, including dogs. Therefore, the ASL model suggests that, in dogs as well as in humans, incompatible sensorimotor experience—experience in which the observation of one action is correlated with the execution of an alternative action—leads to the development of automatic counterimitation by allowing the mechanisms of associative learning to establish new ‘non-matching vertical associations’, excitatory links between sensory and motor representations of different actions.
In the training phase of the present experiment, the dogs in the incompatible group were rewarded for performing counterimitative actions. For example, if the dog used its paw to open the door after observing head use, it was rewarded with a morsel of food. The ASL model suggests that reward is not necessary for the establishment of matching or non-matching vertical associations; that correlated experience of observing and executing the same (matching) or different (non-matching) actions is sufficient. This hypothesis is consistent with the fact that humans show automatic counterimitation after training in which counterimitative responses are not explicitly rewarded. However, further research will be required to determine whether reward is necessary for the establishment of matching and non-matching vertical associations in non-human animals.
From a methodological perspective, it is of interest that, in the first phase of the present experiment, a significant difference between the compatible and incompatible groups did not emerge until the final, no command stage of training. In the preceding one command and two command stages, the owners of dogs in both groups issued commands corresponding to the action the dog should perform. For example, when the correct response was head use, the owner gave the command ‘head’. Therefore, the absence of group differences in the one command and two command stages may indicate that, when verbal commands were available, the dogs paid little or no attention to the door opening behaviour of their owners. However, the five ‘pilot’ dogs failed to reach criterion performance in their initial period of no command training, but succeeded in reaching the criterion after command training. This suggests that the command procedure played some non-specific role in enabling the dogs subsequently to learn to use their owner's behaviour as a discriminative cue. For example, it may have helped the dogs to become accustomed to the turn-taking characteristics of the training procedure, to reduce perseverative responding, or to focus their attention on their owners at the beginning of each trial.
As far as we are aware, this is the first study in which dogs have been tested specifically for automatic imitation. The results extend but do not contradict those of previous studies in which automatic or voluntary imitation would have yielded positive results. For example, Tennie et al. [19] found no evidence that dogs would imitate the postural movements (sit and lie) of another dog. This could be because, in contrast with the present study, the dogs in this experiment received relatively few trials, and were tested for imitation of intransitive actions. However, if the ASL model is correct, it would not be surprising to find that dogs imitate some actions and not others. According to this model, whether a particular action is imitated depends, not on a generalized ‘faculty’ of imitation, but on the nature of the animals' past experience with respect to specific actions. For example, if dogs typically have correlated experience of observing and executing paw action (e.g. giving the paw to humans on command), but rarely receive correlated experience of observing and executing the act of lying down with their owner, then one would expect them to imitate paw action but not lying down.
More generally, the procedure used in the present study had two features that may have facilitated detection of an imitative tendency that has not been detected in previous studies. First, the use of commands in the training phase may have helped to focus the dogs' attention on the models' actions. Second, we used a training procedure, rather than a ‘probe’ procedure, to test for imitation. In probe procedures, animals are typically allowed to observe a target action on several occasions in a single experimental session, their subsequent behaviour is examined for any evidence of similarity with that of the demonstrator, and they are rewarded, or not rewarded, regardless of whether any similarity is observed. In contrast, in our training procedure, the animals received hundreds of observation and test trials, the dimension of similarity under investigation was precisely defined (head versus paw use), and the tendency to imitate was indexed by a difference between animals that were rewarded for imitation (compatible group) and animals that were rewarded for counterimitation (incompatible group). The abundance of trials in our procedure is likely to have both promoted attention to the demonstrators' actions, and enhanced the probability that a tendency to imitate would be detected in spite of between-trial variation in other factors affecting the focal dimension of behaviour (head versus paw use). Similarly, the comparison between compatibly and incompatibly trained groups provides a sensitive test of imitation by allowing it to be measured on a finely differentiated and continuous scale, i.e. number of trials to criterion.
In conclusion, the results of this study provide the first evidence of automatic imitation and of automatic counterimitation in dogs. Dogs are special animals, both in terms of their evolutionary history of domestication, and the range and intensity of their developmental training by humans. Both of these factors may enhance the extent to which dogs attend to human activity, but the results of the present experiment suggest it is the latter—training in the course of development—which plays the more powerful and specific role in shaping their imitative behaviour.
Acknowledgements
This work has received research funding from the European Community's Sixth Framework Programme under contract number: NEST 012929. We thank two anonymous referees and Wolfram Schultz for helpful comments to improve a former version of this manuscript. Furthermore, we especially thank the dog owners for participating in this long-term study. The Clever Dog Lab thanks a private sponsor and Royal Canin for financial support.
References
- 1.Sturmer B., Aschersleben G., Prinz W. 2000. Effects of correspondence between complex stimulus and response patterns. J. Exp. Psychol. Hum. Percept. Perform. 26, 1746–1759 10.1037/0096-1523.26.6.1746 (doi:10.1037/0096-1523.26.6.1746) [DOI] [PubMed] [Google Scholar]
- 2.Brass M., Bekkering H., Prinz W. 2001. Movement observation affects movement execution in a simple response task. Acta Psychol. 106, 3–22 10.1016/S0001-6918(00)00024-X (doi:10.1016/S0001-6918(00)00024-X) [DOI] [PubMed] [Google Scholar]
- 3.Kilner J. M., Paulignan Y., Blakemore S. J. 2003. An interference effect of observed biological movement on action. Curr. Biol. 13, 522–525 10.1016/S0960-9822(03)00165-9 (doi:10.1016/S0960-9822(03)00165-9) [DOI] [PubMed] [Google Scholar]
- 4.Heyes C. M., Bird G., Johnson H., Haggard P. 2005. Experience modulates automatic imitation. Cogn. Brain Res. 22, 233–240 10.1016/j.cogbrainres.2004.09.009 (doi:10.1016/j.cogbrainres.2004.09.009) [DOI] [PubMed] [Google Scholar]
- 5.Bach P., Tipper S. P. 2007. Implicit action encoding influences personal-trait judgments. Cognition 102, 151–178 10.1016/j.cognition.2005.11.003 (doi:10.1016/j.cognition.2005.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leighton J., Heyes C. M. In press Hand to mouth: automatic imitation across effector systems. J. Exp. Psychol. Hum. Percept. Perform. [DOI] [PubMed] [Google Scholar]
- 7.Chartrand T. L., Van Baaren R. 2009. Human mimicry. Adv. Exp. Soc. Psych. 41, 219–274 10.1016/S0065-2601(08)00405-X (doi:10.1016/S0065-2601(08)00405-X) [DOI] [Google Scholar]
- 8.Heyes C. M., Ray E. D. 2000. What is the significance of imitation in animals? Adv. Study Behav. 29, 215–245 10.1016/S0065-3454(08)60106-0 (doi:10.1016/S0065-3454(08)60106-0) [DOI] [Google Scholar]
- 9.Tomasello M. 1999. The cultural origins of human cognition. Cambridge, MA: Harvard University Press [Google Scholar]
- 10.Heyes C. M. 2001. Causes and consequences of imitation. Trends Cogn. Sci. 5, 253–261 10.1016/S1364-6613(00)01661-2 (doi:10.1016/S1364-6613(00)01661-2) [DOI] [PubMed] [Google Scholar]
- 11.Catmur C., Walsh V., Heyes C. M. 2009. Associative sequence learning: the role of experience in the development of imitation and the mirror system. Phil. Trans. R. Soc. B 364, 2369–2380 10.1098/rstb.2009.0048 (doi:10.1098/rstb.2009.0048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buccino G., et al. 2001. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 13, 400–404 10.1046/j.1460-9568.2001.01385.x (doi:10.1046/j.1460-9568.2001.01385.x) [DOI] [PubMed] [Google Scholar]
- 13.Gangitano M., Mattaghy F. M., Pascual-Leone A. 2004. Modulation of premotor mirror neuron activity during observation of unpredictable grasping movements. Eur. J. Neurosci. 20, 2193–2202 10.1111/j.1460-9568.2004.03655.x (doi:10.1111/j.1460-9568.2004.03655.x) [DOI] [PubMed] [Google Scholar]
- 14.Mui R., Hazelgrove M., Pearce J. M., Heyes C. M. 2008. Automatic imitation in budgerigars. Proc. R. Soc. B 275, 2547–2553 10.1098/rspb.2008.0566 (doi:10.1098/rspb.2008.0566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pongrácz P., Miklósi A., Timár-Geng K., Csányi V. 2004. Verbal attention getting as a key factor in social learning between dog and human. J. Comp. Psychol. 118, 375–383 10.1037/0735-7036.118.4.375 (doi:10.1037/0735-7036.118.4.375) [DOI] [PubMed] [Google Scholar]
- 16.Bräuer J., Kaminski J., Riedel J., Call J., Tomasello M. 2006. Making inferences about the location of hidden food: social dog–causal ape. J. Comp. Psychol. 120, 38–47 10.1037/0735-7036.120.1.38 (doi:10.1037/0735-7036.120.1.38) [DOI] [PubMed] [Google Scholar]
- 17.Miklósi A., Soproni K. 2006. A comparative analysis of animals' understanding of the human pointing gesture. Anim. Cogn. 9, 81–93 10.1007/s10071-005-0008-1 (doi:10.1007/s10071-005-0008-1) [DOI] [PubMed] [Google Scholar]
- 18.Ray E. D., Heyes C. M. In press Imitation in infancy: the wealth of the stimulus. Dev. Sci. [DOI] [PubMed] [Google Scholar]
- 19.Tennie C., Glabsch E., Tempelmann S., Bräuer J., Kaminski J., Call J. 2009. Dogs fail to copy intransitive actions in third-party contextual imitation tasks. Anim. Behav. 77, 1491–1499 10.1016/j.anbehav.2009.03.008 (doi:10.1016/j.anbehav.2009.03.008) [DOI] [Google Scholar]
- 20.Topál J., Byrne R. W., Miklósi A., Csányi V. 2006. Reproducing human actions and action sequences: ‘do as I do!’ in a dog. Anim. Cogn. 9, 355–367 10.1007/s10071-006-0051-6 (doi:10.1007/s10071-006-0051-6) [DOI] [PubMed] [Google Scholar]
- 21.Huber L., Range F., Voekl B., Szucsich A., Virányi Z., Miklósi A. 2009. The evolution of imitation: what do the capacities of non-human animals tell us about the mechanisms of imitation. Phil. Trans. R. Soc. B 364, 2299–2309 10.1098/rstb.2009.0060 (doi:10.1098/rstb.2009.0060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller H. C., Rayburn-Reeves R., Zentall T. R. 2009. Imitation and emulation by dogs using a bidirectional control procedure. Behav. Processes 80, 109–114 10.1016/j.beproc.2008.09.011 (doi:10.1016/j.beproc.2008.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Range F., Virányi Z., Huber L. 2007. Selective imitation in domestic dogs. Curr. Biol. 17, 1–5 10.1016/j.cub.2007.04.026 (doi:10.1016/j.cub.2007.04.026) [DOI] [PubMed] [Google Scholar]
- 24.Gillmeister H., Catmur C., Liepelt R., Brass M., Heyes C. 2008. Experience-based priming of body parts: a study of action imitation. Brain Res. 1217, 157–170 10.1016/j.brainres.2007.12.076 (doi:10.1016/j.brainres.2007.12.076) [DOI] [PubMed] [Google Scholar]
- 25.Catmur C., Walsh V., Heyes C. 2007. Sensorimotor learning configures the human mirror system. Curr. Biol. 17, 1527–1531 10.1016/j.cub.2007.08.006 (doi:10.1016/j.cub.2007.08.006) [DOI] [PubMed] [Google Scholar]
- 26.Catmur C., Gillmeister H., Bird G., Liepelt R., Brass M., Heyes C. 2008. Through the looking glass: countermirror activation following incompatible sensorimotor learning. Eur. J. Neurosci. 28, 1208–1215 10.1111/j.1460-9568.2008.06419.x (doi:10.1111/j.1460-9568.2008.06419.x) [DOI] [PubMed] [Google Scholar]
- 27.Heyes C. M. 2005. Imitation by association. In Perspectives on imitation: from cognitive neuroscience to social science (eds Hurley S., Chater N.), pp. 157–176 Cambridge, MA: MIT Press [Google Scholar]