Abstract
In mammals, altricial birds and some invertebrates, parents care for their offspring by providing them with food and protection until independence. Although parental food provisioning is often essential for offspring survival and growth, very little is known about the conditions favouring the evolutionary innovation of this key component of care. Here, we develop a mathematical model for the evolution of parental food provisioning. We find that this evolutionary innovation is favoured when the efficiency of parental food provisioning is high relative to the efficiency of offspring self-feeding and/or parental guarding. We also explore the coevolution between food provisioning and other components of parental care, as well as offspring behaviour. We find that the evolution of food provisioning prompts evolutionary changes in other components of care by allowing parents to choose safer nest sites, and that it promotes the evolution of sibling competition, which in turn further drives the evolution of parental food provisioning. This mutual reinforcement of parental care and sibling competition suggests that evolution of parental food provisioning should show a unidirectional trend from no parental food provisioning to full parental food provisioning.
Keywords: coevolution, environmental conditions, kin selection, nest site selection, parental feeding, parent–offspring interactions
1. Introduction
Parental care includes any behaviour that enables an individual to promote offspring survival, growth and/or development by ameliorating conditions that are harmful to offspring [1]. Parental care is a highly diverse trait that varies with respect to duration, the form and level it takes, and whether it is provided by the male, the female or both parents [1]. The large theoretical literature on the causes and consequences of this diversity has focused on the impact of environmental variation, brood size, mate attractiveness and paternity uncertainty on overall levels of care (e.g. [2–4]), as well as sex differences in care and their consequences for mating systems and sexual selection (e.g. [5]). However, although this is a highly successful branch of theory that has stimulated much empirical work on mammals, birds, fishes and invertebrates (e.g. [6–8]), it has mainly focused on the evolution of overall levels of care, thus overlooking the fact that parental care is often characterized by complex suites of parental and offspring traits [1,9,10].
In mammals, altricial birds and some invertebrates, parental care is associated with multiple parental behaviours such as nest building, protection of eggs or embryos, and guarding and provisioning of food after hatching or birth [1,9,10], as well as with offspring behaviours such as begging and sibling aggression [11,12]. The most conspicuous and well-studied component of care is parental food provisioning, which is often essential for offspring survival and growth [1]. Comparative evidence suggests that parental food provisioning is a derived form of care that evolved from an ancestral state resembling that of contemporary precocial birds, where parents guard their offspring against predators and offspring self-feed independently of the parents [13]. Guarding of offspring after hatching (hereafter termed ‘offspring guarding’), in turn, evolved from egg guarding—the most basal form of care—through the simple extension of guarding beyond hatching [14]. Yet, despite the important role of parental food provisioning in altricial birds and mammals, little is known about the factors driving the evolutionary innovation of this key component of care.
The most important ecological conditions shaping the evolution of parental care are food availability and predation risk [10,15]. These ecological conditions may drive the evolution of parental food provisioning by influencing: (i) the choice of nest site, as parents need to balance predation risk against food availability for offspring after hatching [16,17]; and (ii) the effectiveness of guarding against predators, as any time spent collecting food away from the nest site would conflict with guarding at the nest site [18]. In addition, the evolution of parental food provisioning is associated with changes in offspring development and behaviour, as neonates are often dependent on parents and show high levels of sibling competition [11,14]. Thus, theoretical work needs to take into account how the evolution of parental food provisioning may be shaped by environmental factors, such as predation risk and food availability, and how the subsequent evolution of parental food provisioning may be driven by evolutionary modifications in offspring traits.
Here, we develop a mathematical model examining the conditions favouring the evolutionary innovation of parental food provisioning, and the subsequent coevolution between this and other components of parental care, as well as sibling competition for parentally provisioned food. We first consider the trade-off between safety and food availability at nest sites in the absence of parental food provisioning, and examine the scope for innovation in parental food provisioning to be favoured and to reduce the necessity for food availability at the nest site. We then consider the evolution of sibling competition, and determine its role in the reinforcement of parental nest choice and food-provisioning strategies. Relating this evolutionary progression to ecological factors—in particular, the relative efficiencies of parental guarding and feeding strategies—the model goes some way towards explaining the diversity of parental care behaviours observed in the natural world.
2. Models and analyses
(a). Basic model
We consider the decisions faced by a parent, with respect to the choice of nest site and type of parental care, and by an offspring, with respect to feeding strategy. We develop a simple model of uniparental care that captures the tension, interplay and coevolution of parent and offspring strategies. We assume a trade-off between the intrinsic safety x and food availability 1 − x of a nest site (0 ≤ x ≤ 1). We assume a trade-off between the investment a parent makes into food provisioning y versus guarding 1 − y its offspring (0 ≤ y ≤ 1). And we assume a trade-off between the investment an offspring makes in competition for parentally derived food z versus self-feeding 1 − z (0 ≤ z ≤ 1). For simplicity, we assume linear trade-offs in each case. Model notation is summarized in table 1.
Table 1.
A summary of model notation.
| symbol | meaning |
|---|---|
| a | efficiency of parental guarding |
| b | efficiency of parental food provisioning |
| c | efficiency of sibling competition |
| F | offspring feeding (whole brood) |
| F′ | offspring feeding (single individual) |
| p | proportion of offspring food provisioned by the parent |
| r | within-brood genetic relatedness |
| S | offspring safety |
| w | parental fitness |
| w′ | offspring fitness (single individual) |
| x | nest safety strategy |
| y | parental feeding strategy |
| z | sibling competition strategy |
We assume that parental fitness is the product of two quantities: offspring safety (S; i.e. probability of brood survival versus predation) and offspring feeding (F; i.e. expected reproductive success of the brood conditional upon their surviving predation). Offspring safety is a function of intrinsic nest safety (x) and parental guarding (1 − y) strategies:
| 2.1 |
where a denotes the efficiency of guarding (0 ≤ a ≤ 1), i.e. the impact of investment into guarding upon offspring safety, relative to choosing a safer nest. Offspring feeding is a function of the intrinsic food availability at the nest site (1 − x), the investment made by the offspring into self-feeding (1 − z) and the investment made by the parent into provisioning of food for the offspring (y):
| 2.2 |
where b denotes the efficiency of parental food provisioning (0 ≤ b ≤ 1), i.e. the impact of investment into food provisioning upon offspring feeding, relative to choosing a nest with greater food availability. Parental fitness is therefore given by w = S × F, or
| 2.3 |
We assume that offspring safety is the same for all individuals in the brood but that, owing to differential investment in sibling competition, food intake may vary between siblings in the same brood. Thus, offspring fitness is the product of brood safety S (given by equation (2.1)) and own food intake F′, given by
| 2.4 |
where z′ is the investment made by the focal offspring into competition for parentally derived food, z is the average investment made by all offspring within the brood and c is the relative efficiency of competition (0 ≤ c ≤ 1), i.e. the impact of investment into sibling competition upon the individual's share of parentally provisioned food. Thus, the fitness of an offspring is given by w′ = S × F′, or
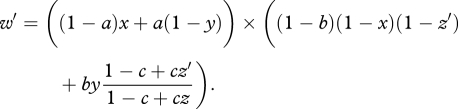 |
2.5 |
Note that the average of w′ over all offspring in the brood (obtained by making the substitution z′ → z in equation (2.5)) is equivalent to parental fitness w (given by equation (2.3)).
Finally, a quantity of key interest is the proportion p of offspring food intake that is derived from parental provisioning versus offspring self-feeding. This is given by
| 2.6 |
This quantity emerges as a function of parental nest choice (x) and food provisioning (y) strategies, and offspring sibling competition (z) strategy. Assuming 0 < b < 1, then all offspring food intake is derived from self-feeding (p = 0) in the absence of parental provisioning (y = 0), and all offspring food intake is derived from parental provisioning (p = 1) if parents choose the safest nests (with no food availability; x = 1) and/or offspring invest fully in sibling competition (z = 1).
(b). Ancestral condition
We consider first the ancestral state of no parental food provisioning (y = 0) and hence no investment in competition for parentally derived food (z = 0). Here, the sole decision faced by the parent is over the choice of nest site, balancing safety (x) against food availability (1 – x). We obtain the following result.
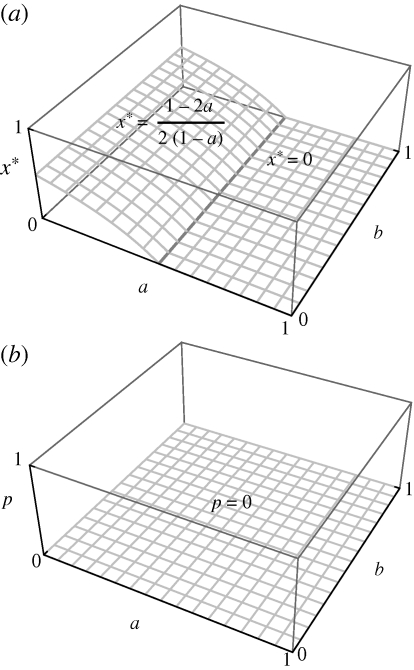
Result 1. If the efficiency of parental guarding is high (a ≥ 1/2), then it is optimal to choose a nest that maximizes food availability (x* = 0), whereas if the efficiency of parental guarding is low (a < 1/2), then a safer nest is optimal (x* = (1 – 2a)/(2(1 − a))). Owing to the lack of parental feeding in this ancestral condition, the safest nest sites with zero food availability are never chosen (x* < 1 for all 0 ≤ a ≤ 1).
Derivation of this analytical result is provided in appendix A. Figure 1 shows graphically how evolutionarily stable choice of nest site (x*; figure 1a) and the proportion of offspring food that is parentally provisioned (p; figure 1b) vary as a function of model parameters (0 < a,b < 1).
Figure 1.
The ancestral state. (a) In the absence of parental feeding (y = 0), a parent must balance safety (x) and food availability (1 − x) at the nest site. Irrespective of feeding efficiency (b), high guarding efficiency (high a) promotes the choice of an unsafe nest with greater food availability (low x*). (b) In the absence of parental feeding (y = 0), the proportion of offspring food that is parentally provisioned is (obviously) zero.
(c). Evolution of parental food provisioning
Taking the ancestral state (§2b) as an evolutionary starting point, we now consider that parents may provision food for their offspring (y > 0), and allow this trait to coevolve with nest choice (x). We obtain the following results.
Result 2. Parental food provisioning evolves (y* > 0) if it is efficient relative to offspring self-feeding (b > 1/2) and/or efficient relative to parental guarding (b > a).
Result 3. Parental food provisioning favours parental choice of safer nest sites (dx*/dy ≥ 0). The evolution of parental provisioning permits parents to choose the safest nest sites with zero food availability.
Result 4. Parental choice of safer nests favours parental food provisioning (dy*/dx ≥ 0). Taken together with result 3, this reveals that these two traits are mutually reinforcing.
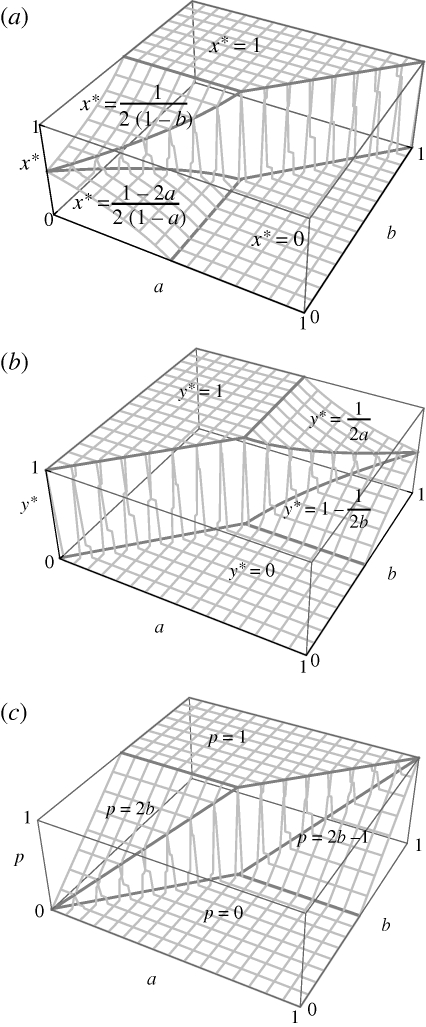
Result 5. More generally: (i) if parental guarding and parental food provisioning are both inefficient (a < 1/2 and b < 1/2), then no parental provisioning evolves when guarding is more efficient than provisioning (x* = (1 – 2a)/2(1 − a) and y* = 0 when a > b), whereas full parental provisioning evolves when provisioning is more efficient than guarding (x* = 1/2(1 − b) and y* = 1 when a < b); (ii) if parental guarding is efficient and parental provisioning is inefficient (a > 1/2 and b < 1/2), then no parental provisioning evolves (x* = 0 and y* = 0); (iii) if parental guarding is inefficient and parental provisioning is efficient (a < 1/2 and b > 1/2), then full parental provisioning evolves (x* = 1 and y* = 1); and (iv) if both parental guarding and parental provisioning are efficient (a > 1/2 and b > 1/2), then an intermediate level of parental provisioning evolves (x* = 0 and y* = (2b − 1)/2b when a > b, and x* = 1 and y* = 1/2a when a < b).
Derivations of these analytical results are provided in the appendix. Figure 2 shows graphically how the choice of nest site (x*; figure 2a), parental food provisioning (y*; figure 2b) and proportion of offspring food that is parentally provisioned (p; figure 2c) vary as a function of model parameters (0 < a,b < 1).
Figure 2.
Optimal nest safety and parental food provisioning in the absence of sibling competition. When parents provision their offspring with food, this allows them to choose safer nest sites with lower food availability and, correspondingly, to invest less in guarding and more in food provisioning. (a) Low guarding efficiency (low a) and high feeding efficiency (high b) promote the choice of a safe nest site with lower food availability (high x*). (b) Low guarding efficiency (low a) and high feeding efficiency (high b) promote investment into parental food provisioning versus guarding (high y*). (c) Low guarding efficiency (low a) and high feeding efficiency (high b) lead to a greater proportion of offspring food being provisioned by the parent versus self-feeding (high p).
(d). Sibling competition
We now consider that offspring may invest in competition with their siblings for parentally derived food (z > 0), and we allow this trait to coevolve with parental choice of nest site (x) and parental food provisioning (y). We obtain the following results.
Result 6. Safer nests and increased parental food provisioning promote sibling competition (dz*/dx ≥ 0 and dz*/dy ≥ 0). Both factors reduce the relative importance of self-feeding for an offspring's fitness, and hence reduce the cost of investment in sibling competition. Sibling competition cannot evolve when parental food provisioning is either absent (y = 0) or unhelpful (b = 0), or when siblings are clonally related (r = 1). In all other scenarios, sibling competition may evolve (z* > 0) if the efficiency is sufficiently high (c larger than a threshold value that is always less than 1).
Result 7. Sibling competition promotes parental choice of safer nests and increased parental food provisioning (dx*/dz ≥ 0 and dy*/dz ≥ 0). Increased sibling competition reduces the relative importance of self-feeding, and hence favours safer nests and parental provisioning. Taken together with result 6, this reveals that these traits are mutually reinforcing.
Result 8. Total absence of offspring self-feeding is evolutionarily stable over the whole space of parameters (p = 1 for all 0 < a,b < 1). In particular, the combination of parental choice of the safest nests (x = 1), intermediate or full parental food provisioning (y = min(1/2a,1)) and full sibling competition (z = 1) is always evolutionarily stable. However, this evolutionarily stable state need not be attainable from all starting conditions.
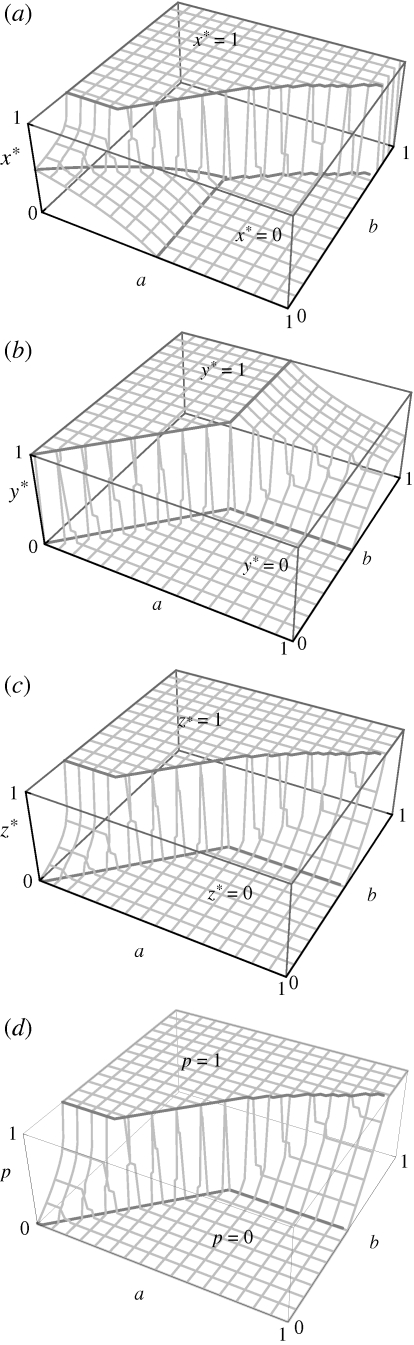
Derivations and more details of these analytical results are provided in the appendix A. Figure 3 presents solutions obtained by numerical simulation, illustrating graphically how the choice of nest site (x*; figure 3a), parental food provisioning (y*; figure 3b), sibling competition (z*; figure 3c) and proportion of offspring food that is parentally provisioned (p; figure 3d) evolve from a condition comprising the absence of sibling competition (§2c), as a function of model parameters (0 < a, b < 1). It is difficult to derive clear predictions of when the total loss of offspring self-feeding will occur, as this will depend upon the relative rate of evolution of each trait in the model, which in turn depends upon such details as standing genetic variance and supply of beneficial mutations.
Figure 3.
Optimal nest safety, parental food provisioning and sibling competition. (a) Sibling competition favours parental choice of safer nests with lower food availability (higher x*, cf. figure 2a). (b). Sibling competition favours parental food provisioning (higher y*, cf. figure 2b). (c) Sibling competition evolves only when parents provision their offspring with food (z* > 0 only when y* > 0). (d) Sibling competition increases the proportion of offspring food that is parentally provisioned (higher p, cf. figure 2c). In all panels, solutions were obtained by numerical simulation, with competition efficiency c = 1 and sibling relatedness r = 1/2, and a range of guarding efficiencies (0 < a < 1) and parental feeding efficiencies (0 < b < 1).
3. Discussion
We have developed a model examining the conditions favouring the evolutionary innovation of parental food provisioning, and the subsequent coevolution between food provisioning, guarding and sibling competition for food provided by the parent. Previous models of parental care have considered this as a unitary trait, and have examined the evolution of overall levels of care rather than specific components of care. Our model shows that: (i) the evolutionary innovation of parental food provisioning is favoured under conditions where a parent is efficient at providing her offspring with food compared with offspring efficiency at self-feeding and/or parental efficiency at guarding the offspring against predators; (ii) the evolution of food provisioning triggers evolutionary changes in parental care by favouring the use of safer nest sites and prompting the evolution of sibling competition for food provided by the parent; and (iii) mutual reinforcement between parental food provisioning, choice of safer nests and sibling competition drives further advances in parental food provisioning. Below, we provide a more detailed discussion of our results and their implications for our understanding of elaborate parental care.
Previously, little has been known about the conditions favouring the evolution of parental food provisioning from an ancestral state where parents simply guard their offspring. Our model suggests that parental food provisioning is most likely to evolve when (i) the parent is more efficient at providing the offspring with food than the offspring are at self-feeding or (ii) the parent is more efficient at food provisioning than she is at guarding against predators (result 2; figure 2b). The first condition is likely to be met in situations where a guarding parent can enhance offspring fitness by supplementing the offspring's diet with high-quality food that is otherwise unprofitable to offspring owing to costs associated with catching or digesting such food. There is some support for this prediction from species with facultative parental food provisioning in which parents increase offspring fitness by finding food [19] or pre-digesting food for the offspring [20]. The second condition is likely to be met in situations where the parent can enhance offspring fitness more effectively by supplementing their diet than by guarding them against predators. This condition might be met when parental food provisioning shortens the developmental period during which offspring are most vulnerable to predators, or allows the parent to move her offspring to a safer habitat that otherwise provides insufficient food for offspring. Unfortunately, there are currently no data to test this prediction. To this end, we now need data on parental guarding efficiency, and effects of parental food provisioning on offspring development and habitat use in closely related species with and without parental food provisioning.
Our model also explores how parental food provisioning coevolves with other parental behaviours, such as the choice of nest site and guarding. We first consider how selection shapes parental behaviours in the ancestral state directly preceding the evolution of parental food provisioning. Our model shows that, in this ancestral state where the parent simply guards her offspring against predators, parental efficiency at guarding has a strong impact upon the optimal choice of nest site. The parent should choose a safer nest site if she is inefficient at guarding, whereas she should choose a nest site that provides better access to food if she is efficient at guarding (result 1; figure 1a). There is some support for this prediction from studies on precocial birds showing that parents choose nest sites that provide protection from predators and avoid nest sites that offer the best possible feeding opportunities to the offspring [21–23]. However, further evidence for this prediction requires data on the nest site selection of precocial species with different guarding efficiencies, which is currently lacking. Our model shows that, among precocial species, the parent cannot breed in the safest nest sites with zero food availability (result 1), and that this option only becomes available once parental provisioning has evolved, because the parent can then bring food in from the surrounding area where it may be more abundant (results 3 and 5; figure 2a). There is support for this prediction from studies on many altricial birds and mammals, showing that parents select specific nest sites, such as burrows, hollow trees or even isolated oceanic islands, that are safe from predators but devoid of food for the offspring [24]. An important result of our model is that parental choice of safer nests in turn promotes the evolution of parental food provisioning (result 4). This result suggests that the evolution of parental behaviour is driven by mutual reinforcement between parental food provisioning and choice of nest site.
Finally, our model explores coevolution between parental food provisioning and offspring behaviours such as self-feeding and sibling competition (figure 3). Our model shows that the evolution of parental food provisioning promotes the evolution of sibling competition (result 6; figure 3c). In support of this prediction, it is widely recognized that intense sibling competition is common in species where the parent provides food for the offspring [11]. For example, sibling aggression is common in egrets and raptors [11], while begging scrambles are common in passerine birds [25]. An important result of our model is that the evolution of sibling competition in turn promotes the evolution of parental food provisioning and parental choice of safer nests (result 7; figure 3a,b). Thus, our model shows that the evolution of these traits is driven by mutual reinforcement between parental food provisioning and sibling competition. Indeed, this reinforcement leads to full parental provisioning of offspring resources being evolutionarily stable (though not necessarily attainable) over the whole of the model's parameter space (result 8; figure 3d). Based on this result, we predict that the evolution of parental food provisioning should show a unidirectional trend from no provisioning to full provisioning, while there should be very few or no reversals. This prediction could be tested by comparative analysis of taxa characterized by a mixture of species with and without parental food provisioning.
We have developed the simplest model that captures the tension between nest safety and food availability, between parental feeding and guarding, and between self-feeding and sibling competition. This simple model neglects other factors, such as sibling competition over self-feeding. In order to provide a general overview, this model has not been tailored to the biology of any particular species. For conceptual simplicity and ease of analysis, we have considered that the focal traits (termed x, y and z in our model) may evolve while certain model parameters (a, b, c and r) remain fixed over the course of evolution. It may be more realistic to allow some of these parameters to change in response to evolution of parental care. For example, if parents forage in the immediate vicinity of the nest, the efficiency of parental provisioning may decrease (lower b) as parents choose safer, but more remote, nests (higher x). This could tend to counteract the mutual reinforcement between the choice of nest site and parental provisioning. Moreover, newly arisen traits that have not been well-honed by natural selection will tend to be inefficient relative to other, more established strategies, which may present a barrier to any form of evolutionary innovation. Incorporating such realism, including tuning the model to the biology of particular study species, is an important avenue for future development of theory on this topic.
Our model is not the first to address the coevolution of parental food provisioning and sibling competition such as offspring begging. Game-theoretic models show that parents should adjust their food-provisioning behaviour to offspring begging behaviour because it provides parents with honest information on the offspring's condition [26,27], while quantitative genetics models show that coadaptation resulting from the combined effects of parental and offspring behaviours on offspring fitness generate a genetic correlation between parental and offspring behaviour [28,29]. Both types of model have been highly successful in stimulating empirical research (e.g. [12,25,30]). Our model extends this existing theoretical framework by incorporating additional parental and offspring traits to those dealt with in previous models, and by exploring the causes and consequences of the evolution of parental food provisioning. It also provides insights into the conditions favouring the evolutionary innovation of parental care, which is lacking from existing modelling approaches. Indeed, we argue that theoretical modelling provides the most promising avenue with which to elucidate this important yet largely ignored issue. This is because it is difficult for empiricists to disentangle the ancestral conditions that favour evolutionary innovations in parental care from the subsequent coevolutionary changes in parental nest site selection and guarding, and offspring development and behaviour.
Acknowledgements
We thank Jonathan Wright and two anonymous referees for valuable comments. A.G. is supported by a Royal Society University Research Fellowship; P.T.S. is funded by a grant from NERC (NE/G004293/1).
APPENDIX A
(a). Ancestral condition
First we consider the ancestral condition, where we assume no parental feeding and hence no sibling competition for parentally derived food (y = z = 0). From equation (2.3) of the main text, the direction of selection acting upon parental choice of nest site (x) is given by the marginal fitness:
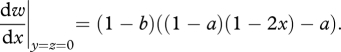 |
A 1 |
Assuming that the evolutionarily stable strategy (ESS; [31]) for the choice of nest site takes an intermediate value (0 < x* < 1), then we can determine this value by setting the right-hand side of equation (A 1) to zero, and solving for x. This obtains x* = (1 – 2a)/2(1 – a), which takes an intermediate value for all a ≤ 1/2. For all a > 1/2, the marginal fitness is negative at x = 0, and hence x* = 0. This recovers result 1 of the main text.
(b). Evolution of parental feeding
Beginning with the ancestral state (previous section), we now allow parental feeding to evolve under the assumption of no sibling competition (z = 0). The direction of selection acting upon the choice of nest site (x) is given by
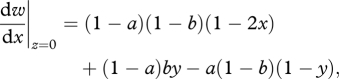 |
A 2 |
and the direction of selection acting upon parental feeding is given by
| A 3 |
For any given value of y, we may calculate the ESS for the choice of nest site as a function of investment into parental feeding in the usual way, by setting the right-hand side of equation (A 2) to zero and solving for x. This obtains
| A 4 |
upon the assumption that it takes an intermediate value. Differentiating the right-hand side of equation (A 4) with respect to y obtains dx*/dy = [(1 − a)b + a(1 − b)]/[2(1 − a)(1 − b)], which is positive for all 0 < a, b < 1, recovering result 3 of the main text.
Similarly, we may set the right-hand side of equation (A 3) to zero and solve for y, obtaining the ESS
| A 5 |
upon the assumption that this takes an intermediate value. Differentiating the right-hand side of equation (A 5) with respect to x obtains dy*/dx = (1 − b)/b, which is positive for all 0 < b < 1, recovering result 4 of the main text.
If we consider the coevolution of nest choice and parental feeding, then there are three possible qualitative outcomes for each of the two traits: it takes value 0, or value 1, or an intermediate value at the ESS. Hence, a priori, there are 32 = 9 qualitatively different joint ESS solutions to consider. Assuming that both traits take intermediate values at the joint ESS, we may employ the usual approach of setting the right-hand side of both equations (A 2) and (A 3) to zero, and simultaneously solving for x and y. This obtains x* = a/(a – b) and y* = (1 − b)(a – b). Note that this solution is never valid, since it implies x* > 1 if a > b, x* < 0 if a < b and x* undefined if a = b. Hence, we rule out the possibility that both traits take intermediate values. For the eight remaining possibilities, we may check the joint ESS condition for each in turn. This reveals that there are six different possible outcomes, each mutually exclusive, and between them covering the entire space of parameter values—as detailed in result 5 (see also result 2) of the main text.
(c). Sibling competition
We now consider sibling competition (z > 0), and allow this to coevolve with parental traits. The marginal fitness for nest choice (x), parental feeding (y) and sibling competition (z) strategies are now given by
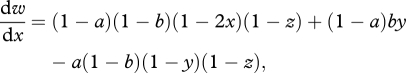 |
A 6 |
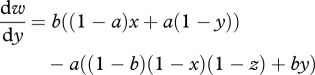 |
A 7 |
and
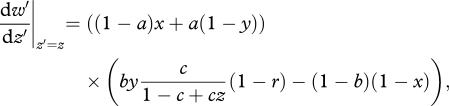 |
A 8 |
where r = dz/dz′ is the ‘whole-group’ kin-selection coefficient of relatedness within sibships [32–34]. Solving for the (intermediate) ESS values as before, we obtain
| A 9 |
| A 10 |
and
| A 11 |
Differentiating the right-hand side of equations (A 9) and (A 10) with respect to z obtains dx*/dz = by/2(1 − b)(1 − z)2 and dy*/dz = (1 − b)(1 − x)/2b, respectively. These are both positive quantities, and hence we recover result 7 of the main text. Differentiating the right-hand side of equation (A 11) with respect to x and y obtains dz*/dx = by(1 − r)/(1 − b)(1 − x)2 and dz*/dy = b(1 − r)/(1 − b)(1 − x), respectively. These are both positive quantities, and hence we recover result 6 of the main text.
The right-hand side of equation (A 11) is positive when
| A 12 |
and this provides the condition for sibling competition to evolve (z* > 0). The right-hand side of inequality (A 12) is always less than unity if by(1 − r) > 0, so fully efficient sibling competition (c = 1) can always evolve. Finally, we can assess the stability of full sibling competition (z* = 1) by examination of the condition dw′/dz′|z′=z=1 > 0, or
| A 13 |
Condition (A 13) is always favoured if x = 1, provided sibling competition is not totally inefficient (c > 0), siblings are not clonally related (r < 1) and parental feeding is both present and useful (b > 0, y > 0). Furthermore, the right-hand side of equation (A 9) tends to infinity as z → 1, provided (1 − a)by > 0, so that if parental food provisioning is present (y > 0), then full sib competition favours parental choice of the safest nests (x* = 1). And evaluating equation (A 10) at x = z = 1 obtains y* = 1/2a, which is always greater than zero, and hence positive parental food provisioning is also stable. Thus, the joint ESS (x*, y*, z*) = (1, min(1/2a,1),1) is evolutionarily stable for all 0 < a, b < 1, recovering result 8 of the main text.
References
- 1.Clutton-Brock T. H. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Winkler D. W. 1987. A general model for parental care. Am. Nat. 130, 526–543 10.1086/284729 (doi:10.1086/284729) [DOI] [Google Scholar]
- 3.Heywood J. S. 1989. Sexual selection by the handicap mechanism. Evolution 43, 1387–1397 10.2307/2409455 (doi:10.2307/2409455) [DOI] [PubMed] [Google Scholar]
- 4.Westneat D. F., Sherman P. W. 1993. Parentage and the evolution of parental behavior. Behav. Ecol. 4, 66–77 10.1093/beheco/4.1.66 (doi:10.1093/beheco/4.1.66) [DOI] [Google Scholar]
- 5.Maynard Smith J. 1977. Parental care: a prospective analysis. Anim. Behav. 25, 1–9 [Google Scholar]
- 6.Wright J. 1998. Paternity and parental care. In Sperm competition and sexual selection (eds Birkhead T. R., Møller A. P.), pp. 117–145 San Diego, CA: Academic Press [Google Scholar]
- 7.Ghalambor C. K., Martin T. E. 2002. Comparative manipulation of predation risk in incubating birds reveals variability in the plasticity of responses. Behav. Ecol. 13, 101–108 10.1093/beheco/13.1.101 (doi:10.1093/beheco/13.1.101) [DOI] [Google Scholar]
- 8.Houston A. I., Székely T., McNamara J. M. 2005. Conflict between parents over care. Trends Ecol. Evol. 20, 33–38 10.1016/j.tree.2004.10.008 (doi:10.1016/j.tree.2004.10.008) [DOI] [PubMed] [Google Scholar]
- 9.Silver R., Andrews H., Ball G. F. 1985. Parental care in an ecological perspective: a quantitative analysis of avian subfamilies. Am. Zool. 25, 823–840 [Google Scholar]
- 10.Tallamy D. W., Wood T. K. 1986. Convergence patterns in subsocial insects. Ann. Rev. Entomol. 31, 369–390 10.1146/annurev.en.31.010186.002101 (doi:10.1146/annurev.en.31.010186.002101) [DOI] [Google Scholar]
- 11.Mock D. W., Parker G. A. 1997. The evolution of sibling rivalry. Oxford, UK: Oxford University Press [Google Scholar]
- 12.Wright J., Leonard M. L. (eds) 2002. The evolution of begging: competition, cooperation and communication. Dordrecht, The Netherlands: Kluwer Academic [Google Scholar]
- 13.Ligon J. D. 1999. The evolution of avian breeding systems. Oxford, UK: Oxford University Press [Google Scholar]
- 14.Wesolowski T. 1994. On the origin of parental care and the early evolution of male and female parental roles in birds. Am. Nat. 143, 39–58 10.1086/285595 (doi:10.1086/285595) [DOI] [Google Scholar]
- 15.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Methuen & Co [Google Scholar]
- 16.Martin T. E. 1987. Food as a limit on breeding birds: a life-history perspective. Ann. Rev. Ecol. Syst. 18, 453–487 10.1146/annurev.es.18.110187.002321 (doi:10.1146/annurev.es.18.110187.002321) [DOI] [Google Scholar]
- 17.Martin T. E. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65, 101–127 10.2307/2937160 (doi:10.2307/2937160) [DOI] [Google Scholar]
- 18.Yom-Tov Y. 1974. The effect of food and predation on breeding density and success, clutch size and laying date of the crow (Corvus corone L.). J. Anim. Ecol. 43, 479–498 [Google Scholar]
- 19.Desrochers B. A., Ankney C. D. 1986. Effect of brood size and age on the feeding behavior of adult and juvenile American Coots (Fulica americana). Can. J. Zool. 64, 1400–1406 10.1139/z86-208 (doi:10.1139/z86-208) [DOI] [Google Scholar]
- 20.Smiseth P. T., Darwell C. T., Moore A. J. 2003. Partial begging: an empirical model for the early evolution of offspring signalling. Proc. R. Soc. Lond. B 270, 1773–1777 10.1098/rspb.2003.2444 (doi:10.1098/rspb.2003.2444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters J. R. 1984. The evolution of parental behavior and clutch size in shorebirds. In Shorebirds: breeding behavior and populations (eds Burger J., Olla B. L.), pp. 243–287 New York, NY: Plenum Press [Google Scholar]
- 22.Blomqvist D., Johansson O. C. 1995. Trade-offs in nest site selection in coastal populations of lapwings Vanellus vanellus. Ibis 137, 550–558 10.1111/j.1474-919X.1995.tb03266.x (doi:10.1111/j.1474-919X.1995.tb03266.x) [DOI] [Google Scholar]
- 23.Smith P. A., Gilchrist H. G., Smith J. N. M. 2007. Effects of nest habitat, food, and parental behavior on shorebird nest success. Condor 109, 15–31 [Google Scholar]
- 24.Hansell M. 2000. Bird nests and construction behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- 25.Kilner R., Johnstone R. A. 1997. Begging the question: are offspring solicitation behaviours signals of need? Trends Ecol. Evol. 12, 11–15 10.1016/S0169-5347(96)10061-6 (doi:10.1016/S0169-5347(96)10061-6) [DOI] [PubMed] [Google Scholar]
- 26.Godfray H. C. J. 1991. Signalling of need by offspring to their parents. Nature 352, 328–330 10.1038/352328a0 (doi:10.1038/352328a0) [DOI] [Google Scholar]
- 27.Godfray H. C. J. 1995. Signalling of need between parents and young: parent–offspring conflict and sibling rivalry. Am. Nat. 146, 1–24 10.1086/285784 (doi:10.1086/285784) [DOI] [Google Scholar]
- 28.Wolf J. B., Brodie E. D., III 1998. The coadaptation of parental and offspring characters. Evolution 52, 299–308 10.2307/2411068 (doi:10.2307/2411068) [DOI] [PubMed] [Google Scholar]
- 29.Kölliker M., Brodie E. D., III, Moore A. J. 2005. The coadaptation of parental supply and offspring demand. Am. Nat. 166, 506–516 10.1086/491687 (doi:10.1086/491687) [DOI] [PubMed] [Google Scholar]
- 30.Kölliker M., Brinkhof M. W. G., Heeb P., Fitze P., Richner H. 2000. The quantitative genetic basis of offspring solicitation and parental response in a passerine bird with biparental care. Proc. R. Soc. Lond. B 267, 2127–2132 10.1098/rspb.2000.1259 (doi:10.1098/rspb.2000.1259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maynard Smith J., Price G. R. 1973. The logic of animal conflict. Nature 246, 15–18 10.1038/246015a0 (doi:10.1038/246015a0) [DOI] [Google Scholar]
- 32.Hamilton W. D. 1964. The genetical evolution of altruistic behaviour I & II. J. Theor. Biol. 7, 1–52 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 33.Taylor P. D., Frank S. A. 1996. How to make a kin selection model. J. Theor. Biol. 180, 27–37 10.1006/jtbi.1996.0075 (doi:10.1006/jtbi.1996.0075) [DOI] [PubMed] [Google Scholar]
- 34.Pepper J. W. 2000. Relatedness in trait group models of social evolution. J. Theor. Biol. 206, 355–368 10.1006/jtbi.2000.2132 (doi:10.1006/jtbi.2000.2132) [DOI] [PubMed] [Google Scholar]