Abstract
Little is known about the factors causing variation in behavioural plasticity and the interplay between personality and plasticity. Habituation to predators is a special case of behavioural plasticity. We investigated the direct and indirect effects of boldness, exploration and sociability traits on the habituation ability of Iberian wall lizards, considering exposure and sex effects. Individual boldness was consistent across several non-habituation contexts, but it did not significantly affect habituation. Exploration had a strong direct effect on habituation, with more exploratory individuals being able to habituate faster than less exploratory ones, probably because of their ability to assess risk better. Individual variation in habituation was also affected by sociability, but this was an indirect effect mediated by exposure to the predator. Less social individuals avoided refuges with conspecific cues, increasing exposure to the predator and eventually habituation. Finally, the direct effects of sex (females habituated faster than males) were opposite to its indirect effects through exposure. We conclude that risk assessment, instead of the proactivity–reactivity gradient usually considered in the literature, can affect behavioural plasticity through complex interactions between direct and indirect effects, including exploratory behaviour, degree of exposure to the predator and sex, which represent novel mechanisms generating inter-individual variation in plasticity.
Keywords: behavioural plasticity, personality, habituation, boldness, exploration, sociability
1. Introduction
Consistent individual differences in major behavioural traits like boldness, exploration, sociability or aggressiveness, also known as personality, have been found in multiple vertebrates [1–4]. There is evidence indicating that this personality variation may be heritable [5–7] and may have fitness consequences [8,9]. Individuals may not only be consistently different among themselves, but also adjust their behaviour to varying environmental conditions [10]. This behavioural plasticity could be either similar among individuals, hence maintaining personality differences across an environmental gradient, or vary among individuals [10]. Furthermore, individuals may differ in plasticity in one behavioural dimension, but show no plasticity differences in other dimensions [11].
Personality and behavioural plasticity have been extensively studied independently; however, their interplay has received relatively little attention [4,12,13], particularly in terms of inter-individual variability in behavioural plasticity [10,14]. Recently, Dingemanse et al. [10] proposed a framework for the joint study of personality and behavioural plasticity, which is crucial for understanding how selection affects each of these processes and how variation in behaviour is maintained within and among individuals [10].
Habituation to predators is a special case of behavioural plasticity [15,16], by which animals reduce their antipredator responses to a potential predatory stimulus through a process in which the stimulus ceases to be regarded as dangerous after repeated non-threatening exposures to it [17,18]. Previous studies have shown differences in habituation ability among individuals within a population ([17,19,20]; but see Martin & Réale [21]), and these differences may have fitness-related consequences [22]. However, the mechanisms underlying the individual variation in habituation are poorly understood [15,17].
The goal of this study was to establish how habituation to a low-risk predator is influenced by boldness, exploration and sociability [3], taking into consideration potential sex effects, and whether the relationships between these factors agree with the predictions of the proactivity–reactivity gradient (see below), or if other mechanisms are implicated. We conducted a manipulative outdoor experiment in semi-natural conditions using the Iberian wall lizard (Podarcis hispanica) as a model species.
According to the proactivity–reactivity gradient [4,23,24], reactive individuals tend to be shy, less aggressive and more adaptable to new situations, and show greater behavioural flexibility than proactive individuals, which tend to be bolder and more aggressive [4,23,24]. Therefore, it is expected that bolder individuals would be less able to habituate than shyer individuals. Alternatively, bold individuals could tolerate closer and longer encounters with a low-risk predator enabling them to better assess the reduced threat posed by that predator, which would actually facilitate habituation. High exploration behaviour is usually included as a characteristic of proactive individuals [4,25], and as such we could expect exploratory individuals to have reduced habituation. Alternatively, exploratory individuals could also obtain more information about their surroundings [26–28], and this information could actually enhance habituation to low-risk predators. Sociability is a relatively less studied behavioural trait in the context of personality research, but recently Cote et al. [29] suggested that sociability could also be part of the behavioural axis including boldness and exploration, with more social individuals being bolder than less social ones. Thus, we hypothesize that more social individuals are more proactive than reactive, and hence less prone to habituation according to the predictions of the proactivity–reactivity gradient. Finally, previous research in birds [17,30] showed that females habituate faster than males. Thus, we also assessed this sex effect in our lizard study system.
We also tested whether the studied behavioural traits could influence habituation through indirect mechanisms, such as individual differences in the time exposed to low-risk predators. Shy individuals could spend more time in refuges than bold individuals [31], resulting in individual differences in exposure owing to boldness. Similarly, sociability may influence whether individuals spend more or less time out of refuges occupied by conspecifics [29,32]. Therefore, boldness and sociability could directly influence the exposure to a predator, which can indirectly influence habituation. We did not predict exploration behaviour to affect lizard activity patterns out of the refuge in a familiar environment [3]. Finally, sex could also influence activity patterns in lizards [33,34], and hence their exposure to the low-risk predator stimuli. Our study allowed us to test a fundamental question in behavioural ecology: whether individual traits can generate inter-individual variation in behavioural plasticity within a population.
2. Methods
Thirty-two adult Iberian wall lizards were captured by noosing in a population in the Guadarrama Mountains (Central Spain), and later transported to El Ventorrillo field station, 3 km away from the capture site. All lizards had intact tails. Lizards were individually housed outdoors in plastic cages (48 × 29 × 24 cm) for two weeks in early June. During this period, we performed behavioural tests of boldness, exploration and sociability. We added a 15 × 15 cm ceramic refuge to each cage, and we provided water ad libitum and mealworms and crickets as daily food.
We acknowledge that the terms boldness, exploration and sociability may not be completely consistent in the literature [3]; however, we describe specifically how we measured each of these parameters in the following sections.
(a). Morphology
Body size can affect personality traits in lizards [31]. Thus, following the methods of López et al. [31], we performed a principal component analysis (PCA) to reduce seven different size variables from each lizard to two independent factors, one representing absolute size and the other representing relative size (body mass and head size relative to snout–vent length). See electronic supplementary material, S1 for details.
(b). Boldness
To obtain a measure of boldness before the habituation protocol (see below), we followed López et al. [31]. We tested the lizards between 16.15 and 18.15 h, when all individuals were active. We placed individual cages separately from one another in an open and sunny location, and the cage walls and the ceramic refuge provided partial shade. We simulated several consecutive attacks to each lizard, recording at the start of each consecutive attack whether the lizard was hiding inside the refuge (body and head inside the refuge), leaning out of the refuge (i.e. individual's body was inside the refuge, but its head was sticking out) or outside the refuge (body and head outside of the refuge; [31]). One observer (I.R.P.) performed the attacks by first crawling slowly on the ground to avoid being seen by the lizards in the other cages. The observer then suddenly appeared over the cage walls simulating a predator attack by tapping the lizard close to its tail with a little stick, which made the animal run and hide into the refuge. If the lizard was already in its refuge, the observer tapped the refuge entrance with the stick. For each lizard, we simulated 12 attacks within a day (one attack every 10 min within a 2 h window). The observer was out of sight from the lizards between attacks. The goal of all these simulated attacks during the 2 h period was to create a context of constant high risk to record the proportion of times lizards were out versus inside the refuge. The 12 attacks were repeated the next day, totalling 24 attacks per individual.
From the observations of the initial position of lizards in relation to the refuge taken at each simulated attack (i.e. every 10 min), we calculated six variables (number of times inside the refuge on day 1, number of times leaning out on day 1, number of times outside the refuge on day 1 and the same variables on day 2) that were included in a PCA to obtain a composite variable indicative of boldness [31]. From this analysis, we used PC1 (hereafter ‘boldness in refuge-use context’) as it represented a gradient from shy to bold individuals (see electronic supplementary material, S2 for details on the PCA and its interpretation).
To test whether individual differences in boldness were consistent across situations, we later compared this boldness in refuge-use context index with other measures likely to represent boldness in different contexts: (i) movement strategy in a novel habitat (see §2c) and (ii) flight initiation distance (FID) at the start of the habituation treatment (see §2e).
(c). Exploration
To measure the exploratory behaviour of lizards, we performed a novel-environment test [5] in an indoor 2.5 × 1.5 m experimental enclosure. Enclosure walls were 60 cm high, and were made of polyethylene sheets that lizards were unable to climb. Nine stacks composed of floor-tiles of similar size and shape were placed at regular intervals from each other inside the enclosure. A 40 × 15 cm area was delimited just beside one of the enclosure short walls to serve as the area where focal lizards were released inside the enclosure at the start of the exploration trials. We ran one trial for each of the 32 lizards, in which the individual lizard was placed in the release area, while an observer directly recorded its behaviour for 3.5 min from a hide. Specifically, we recorded the time it took the lizard to leave the release area and start exploring the enclosure (latency), the number of different stacks visited during the observation period (stacks inspected), the number of movement bouts (i.e. a non-moving lizard starts walking for a given length and then stops moving for more than a second) performed per minute (movements per minute) and the average number of hind legs steps that composed each movement bout (steps per movement). Many lizards tried to climb the enclosure walls unsuccessfully several times, so we also recorded the total number of climbing attempts, and the average time spent at each climb attempt (time per climbing attempt).
We conducted a PCA to reduce these six variables to a smaller number of independent factors (see electronic supplementary material, S2 for details on the PCA and its interpretation). We obtained and used two factors from this analysis. PC1 was interpreted as a gradient from slow to fast explorers, with fast explorers quickly inspecting the enclosure and quickly abandoning each climbing attempt after being unsuccessful. We interpret PC2 as representing another gradient of boldness, with shy individuals taking a long time to initiate the exploration of the novel environment, moving across the enclosure using short movement bouts that allow for pauses to scan the environment [35], while performing few attempts to climb the walls. We speculate that lizards may perceive a climbing attempt as an action entailing high predation risk because of their high exposure while climbing, the noise derived from the forehands slipping repeatedly on the plastic surface and the reduced locomotor ability of this species while climbing relative to horizontal movement [36]. Our interpretation of PC2 as another measure of boldness is reinforced by the significant association between PC2 (boldness in novel habitat) and boldness in refuge-use context (see §2b; Spearman rank correlation, rs = 0.52, n = 31, p = 0.003).
(d). Sociability
Our sociability test followed Cote and co-workers [29,32], who measured how the time spent hidden in a refuge varied as a function of whether the refuge contains olfactory cues from conspecifics or not. Twenty adult lizards, different from the focal individuals, were used as sources of conspecific odour. To obtain the olfactory cues, donor lizards were housed for 10 days in one large terrarium, with a layer of vermiculite as a substrate, which was later used under the refuge of the focal lizard. Focal lizards were tested indoors in a cleaned plastic cage (80 × 50 × 60 cm high) containing a 15 × 15 cm ceramic refuge for hiding and a bulb for heating. All lizards were tested in the afternoon when they were fully active. We had two substrate treatments with varying types of vermiculite under the refuge: (i) new and clean vermiculite and (ii) odorized vermiculite coming from the terrarium with the 20 conspecifics. In both the treatments, vermiculite was moisturized by spraying water. Each lizard was tested separately in a cleaned cage, randomly beginning either with odorized vermiculite or with clean vermiculite. The lizard could choose between staying under the refuge or leaving the refuge and being exposed. Each lizard was introduced in the test cage, and left 4 min to acclimate. Then, the time spent hidden under the refuge was measured during 7 min. These time frames are similar to those used by Cote and co-workers [29,32], and were considered appropriate after preliminary tests on individuals not used in this study. The next day, all lizards were tested again, this time using the opposite substrate type to the one used the first day. Following the interpretation of Cote and co-workers [29,32], we used the odour-dependent time spent hidden (time spent hidden when the refuge substrate was odorized vermiculite minus time spent hidden when the refuge substrate was clean vermiculite) as a sociability index.
(e). Habituation protocol
To perform the habituation test, we transferred the lizards to four 6 × 4 m outdoor enclosures placed in an open area surrounded by woodland. Enclosures had a natural herbaceous substrate. We supplied water ad libitum but not food; thus, individuals were forced to search and capture naturally occurring invertebrates within the enclosures. While lizards were not able to climb out of the walls, there was a constant flux of arthropods resulting in diverse and abundant prey availability. We added to each enclosure a standardized array of rocks, tiles and bricks for refuge and thermoregulation. Details on enclosure layout are presented in [22].
Individual lizards were sorted by size (four categories) and sex, and eight lizards were randomly allocated into each of the four enclosures (n = 32 individuals), but keeping a similar proportion of size and sex classes per enclosure. The attained density (3.3 lizards 10 m−2) represented a high-density scenario for this species [37]. Lizards went through an acclimation phase from mid-June to mid-August (post-reproductive period). Three days prior to the habituation experiment, each individual was dorsally marked with three painted dorsal colour circles to allow for visual identification.
Lizards were subjected to a 6 day habituation protocol, with one observer (I.R.P.) entering and longitudinally crossing each enclosure every 20 min during the peak activity period (12.00–14.00 h and 16.45–18.45 h), totalling 12 intrusions per enclosure per day. Lizard activity was very low outside of these peak activity periods because of the shadows projected by the surrounding trees before 12.00 and after 19.00 h, and because of the high temperatures experienced between 14.00 and 16.30 h. Iberian wall lizards are not territorial [38]. Individual lizards did not associate themselves with specific sectors of the enclosures, and frequently changed locations to position themselves in the best spots for thermoregulation as the sun and tree shadows moved across the enclosure.
A measurable effect of habituation is the progressive reduction in magnitude of a behavioural response to the repeated application of a stimulus [39]. Therefore, we used the progressive reduction of FID as our proxy of habituation to repeated human intrusions. This indicator of habituation has been frequently used in the recent literature (e.g. [15,18,20,40–42]).
Flight initiation distance was defined as the distance between the observer and the lizard at the point at which the latter flushed in response to the approaching threat. As the observer crossed the enclosure, he was able to mark on a detailed scaled map of the enclosure his own position and the positions of lizards as they individually flushed. When the observer reached the end of the enclosure, he went back to the points marked on the map and measured FID with a measuring tape without stepping out of the crossing path. We also measured distance to the nearest refuge, but it did not affect FID significantly (r = 0.04, p = 0.524). Thus, we did not use this variable in the analyses.
We estimated the reduction of FID over the course of the 6 day experimental phase by regressing FID over time for each individual. A 6 day period was considered enough to observe habituation effects, based on our previous experience with this species as well as on other studies on habituation [17,43]. Some individuals maintained their FID mostly unaltered over the course of the experiment (slope of the FID/time regression close to 0), while other individuals experienced a strong decrease in FID over time (high negative slopes), and some others slightly increased their FID (low positive slopes). Individual behavioural plasticity can be characterized as the slope of the variation for a given behaviour across an environmental gradient [10]. Thus, we were not interested in whether the slope was significant or not, but in the value of the slope of individual FID/time regressions to characterize the degree to which each individual responded to the habituation protocol. For clarity, we multiplied the slopes (by −1) in order to obtain a ‘habituation index’ with higher scores indicating rapid habituation.
To test the consistency and appropriateness of our boldness measure (see §2b), we also calculated for each individual its mean FID over the first day of the protocol to compare with the boldness in refuge-use context index obtained in the boldness test (Spearman rank correlation, rs = −0.47, p = 0.008; [22]): lizards with a high boldness score in the boldness test had a low FID when approached by the observer two months later in a very different context. This finding further emphasizes the consistency of our estimate of boldness across contexts. Note that boldness in refuge-use context was also consistent with our measure of boldness in a novel habitat (see §2c).
(f). Exposure
When performing the habituation treatment, the observer also recorded all lizards that were out of the refuges just before entering each enclosure. By summing up all observations over the course of the habituation protocol, we obtained an index of exposure that represented the number of times each individual lizard was exposed to the low-risk predator.
(g). Statistical analyses
We removed from the dataset an individual that lost its tail during the acclimation phase. All variables were checked for normality, and sociability was log-transformed.
We used a general linear model (GLM) to assess the effects of behaviour (boldness, exploration, sociability), body size (relative body size, absolute body size) and sex on the habituation index. We used boldness in refuge-use context as our measure of boldness to include in the GLM. Flight initiation distance during the first day of habituation was highly correlated with boldness in the refuge-use context (see §2e), so we did not include it in the GLM. Nevertheless, the results remained the same by including it (available upon request). We included an enclosure identity factor in the model to control statistically for potential confounding effects. All enclosures were structurally similar; however, the different position of surrounding trees affected the amount of direct sunlight received. Moreover, differences in the initial behavioural composition of each enclosure could have affected the habituation process, although we note that there was no significant variation in behavioural composition among enclosures (boldness, F3,27 = 0.54, p = 0.659; sociability, F3,27 = 0.72, p = 0.551; exploration, F3,27 = 0.50, p = 0.687).
To better asses the direct and indirect effects of individual factors on habituation, we ran a path analysis [44,45] using AMOS 18.0 (SPSS Inc.). We included behavioural variables (boldness, exploration, sociability), body size (relative body size, absolute body size) and sex as exogenous predictors (i.e. variables with no causal arrows pointing to them in the diagram), while exposure was included as an endogenous mediator (i.e. a variable with both incoming and outgoing causal arrows in the diagram), and habituation index as the endogenous dependent variable (i.e. a variable with only incoming causal arrows). We coded sex as male = 1, and female = 2 for inclusion in the path analysis. To control for the potential confounding effects of enclosure, we used the residuals of the relationship between enclosure and habituation, and the residuals of the relationship between enclosure and exposure as the dependent and mediator variables, respectively. Non-significant paths can be dropped from a path analysis, especially those paths for which the biological interpretation is weak [46,47]. Thus, we included in the model only those non-significant paths that a priori predicted to have a biologically relevant effect [45,48]. Then, we employed an Akaike Information Criteria (AIC) model selection procedure to further improve our selection of the effects included in the final model. We selected the model with the lowest AIC score (i.e. best model), and we also calculated the AIC score differences between each competing model and the best model to identify any other model equally probable to the best model [49]. We also assessed whether the indirect effects of the predictor through a mediator variable on the dependent variable were significant using two methods: (i) bootstrap estimation and (ii) Baron and Kenny criteria. According to the Baron and Kenny criteria, an indirect effect is considered significant when the path leading to the mediator variable and the path from the mediator to the dependent variable are both significant [50]. The goodness-of-fit of the path analysis model was measured with the Comparative Fit Index (CFI), which ranges from 0 to 1; a value of >0.9 indicates an acceptable fit to the data [51].
(h). Ethical note
None of the lizards used showed any sign of stress or pain, and all lizards behaved and fed normally at the end of each experimental trial. Lizards were released in the same spots in which they were captured and were in good condition. Procedures also complied with recommended guidelines for the treatment of animals in behavioural research [52] and more specifically with the guidelines for the use of live reptiles in research [53].
3. Results
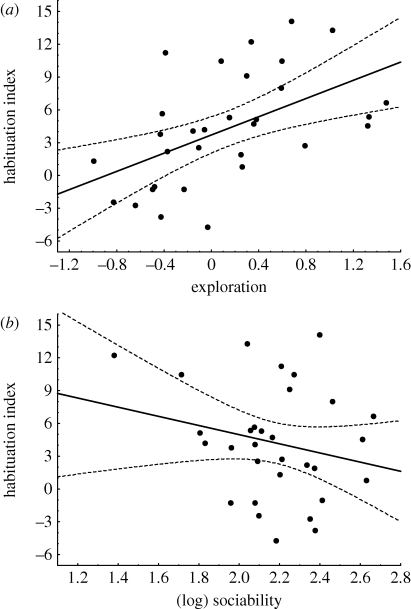
Based on the GLM, habituation index was significantly associated with the exploration behaviour and sociability of individual lizards, while boldness, sex and body size did not significantly affect habituation (table 1, overall model r2 = 0.63, F9,21 = 3.93, p = 0.005). Fast explorers and less social individuals habituated faster than slow explorers and more social individuals (figure 1). Relative body size was highly associated to sex (coefficient of covariation = 0.85), but the results of the GLM did not change when we removed relative body size from the model.
Table 1.
Effects of enclosure, sex, behavioural and size traits on habituation. (Significant (p < 0.05) p-values are shown in bold.)
| variables | F | d.f. | p |
|---|---|---|---|
| intercept | 8.95 | 1,21 | 0.007 |
| boldness | 0.02 | 1,21 | 0.893 |
| exploration | 20.11 | 1,21 | <0.001 |
| sociability | 5.84 | 1,21 | 0.025 |
| relative size | 1.15 | 1,21 | 0.296 |
| absolute size | 2.51 | 1,21 | 0.128 |
| sex | 1.74 | 1,21 | 0.201 |
| enclosure | 3.02 | 3,21 | 0.052 |
Figure 1.
Relationships between habituation index and (a) exploration behaviour, and (b) sociability, in Iberian wall lizards.
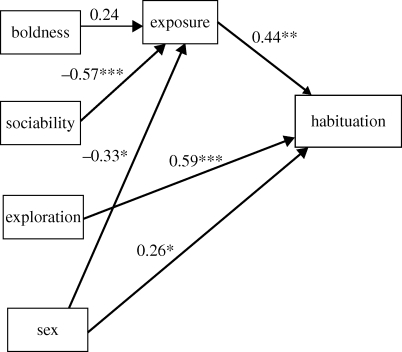
The results of the path analysis confirmed the associations encountered with the GLM, while providing extra insight into the nature of these relationships. Relative and absolute body size had no significant effect on exposure or habituation, and these two factors were dropped from the analysis. Exploration did not have a significant effect on exposure and hence the path exploration-exposure was not included in the final model. Standardized path coefficients are shown in figure 2. Our path analysis model explained 53.4 per cent of the variance in habituation, and presented an acceptable goodness-of-fit (CFI = 0.99).
Figure 2.
Path diagram and path coefficients for predictors of habituation. Correlation lines among the exogenous variables are not shown. Significant paths are in bold. *p < 0.05; **p < 0.01; ***p < 0.001.
Exploration was again the strongest direct predictor of habituation (figure 2). The direct effects of boldness and sociability on habituation were negligible and they were removed from the model following an AIC model selection procedure. However, sociability affected habituation indirectly through exposure: less social individuals were more frequently out of the refuges than more social individuals, with this higher exposure to the stimulus leading to greater habituation (figure 2). This indirect effect of sociability on habituation was significant according to bootstrap estimation (p = 0.012), and to the Baron and Kenny criteria: the effect of sociability on exposure (p < 0.001) and the effect of exposure on habituation (p = 0.009) were both significant. The indirect effect of sociability on habituation remained significant after removing the two lizards with lowest sociability scores (results available upon request).
Sex had a significant direct effect on habituation with females habituating more than males (figure 2). However, the direct effect of sex is counteracted by the indirect effect of sex on habituation mediated by exposure. Exposure to the predator was significantly greater in males than in females (path coefficient =−0.33, p = 0.022), which coupled with the significant effect of exposure on habituation yielded a significant indirect effect of sex on habituation according to the bootstrap estimation (p = 0.036) and the Baron and Kenny criteria. This indirect effect means that males habituated to a greater degree than females. Hence, the lack of a significant association between sex and habituation in the GLM may be explained by the conflicting direct and indirect effects of sex on habituation (‘inconsistent mediation’ sensu MacKinnon et al. [44]).
The path boldness-exposure was retained in the final model. However, the AIC score difference between the best model and a similar model with this boldness-exposure path dropped was only 0.61, which indicates that this alternative model lacking any boldness effect is equally probable to the selected model [49]. Additionally, the indirect effect of boldness on habituation through exposure was not significant because it did not meet the Baron and Kenny criteria and the bootstrap estimation of the indirect effect was non-significant (p = 0.097).
4. Discussion
Our results show that exploration behaviour, sociability and sex influenced the individual variation in habituation, which is a special case of behavioural plasticity. Additionally, we showed that these relationships were modulated by both direct and indirect effects. While exploration behaviour had a positive and direct effect on habituation, the negative effect of sociability on habituation was mediated by the frequency of exposure to the low-risk stimuli. Less sociable lizards were out of the refuges more frequently than more social ones, being more frequently exposed to the low-risk predator. This probably allowed them to habituate faster than less exposed individuals. The relationship between sex and habituation also proved to be complex. Females showed a direct tendency to habituate faster than males, but males habituated faster following an indirect effect through exposure (e.g. males had more out-of-refuge activity than females).
Under the framework recently proposed by Dingemanse et al. [10] to jointly study personality and behavioural plasticity, our results suggest that elevation (e.g. relative boldness of an individual) and slope (e.g. plasticity) of the boldness reaction norm do not show covariation despite individuals actually differing in both elevation and slope [10]. Variation in behavioural plasticity (e.g. habituation) proved to be more influenced by other behavioural traits than by boldness itself. This suggests that habituation is not related to proactive or reactive behaviours in lizards. By contrast, Ellenberg et al. [17] found a clear negative association between aggressive behaviour against researchers approaching nests (a measure that may be related to boldness) and habituation. Taken together, these results suggest that the constraints imposed by boldness on habituation could be context- or species-specific.
The positive effect of exploration behaviour on habituation was consistent with the hypothesis on the association between exploration behaviour and risk assessment rather than the proactivity–reactivity gradient. More exploratory individuals may assess actual risk more quickly than less exploratory individuals because they can gather more information from novel stimuli [26–28]. Actually, more exploratory individuals may have an advantage in learning or assessing the characteristics of a novel stimulus even if they spend the same time exposed to it than less exploratory individuals (e.g. by focusing their attention more on the stimulus), as has been shown in birds [54]. Furthermore, the fact that more exploratory individuals stopped trying to climb out of the wall sooner than less exploratory individuals also suggests fast assessment abilities (i.e. lizards were not able to successfully climb the walls in our experimental arena).
Réale et al. [3] pointed out that measures of exploration obtained in past studies may not represent true exploration, but a composite measure of several behavioural traits, mainly boldness and exploration. However, our results suggest that we may have been more successful at separating true exploration behaviour from boldness (see §2c and electronic supplementary material, S2). Alternatively, exploration and boldness could actually be interdependent in other taxa (e.g. birds and mammals) but not in lizards.
Sociability influenced habituation only through an indirect mechanism mediated by the time exposed to a low-risk threat. Less social individuals were more frequently out of the refuges than more social ones, as expected in a high-density scenario as ours. Less social individuals may have used direct (refuges with conspecifics in them) or indirect (conspecific scent in recently empty refugees) cues to avoid close conspecific contact within the refuge. Less time in the refuge means more instances of direct interaction with the low-risk predator, probably allowing lizards to better assess their level of risk. This finding suggests that individual differences in sociability may have major implications for the ecology of lacertid lizards, with density-dependent effects of sociability affecting space use, migration and fitness [29,32].
Our analysis of direct and indirect effects uncovered a complex relationship between sex and habituation. Female lizards tended to habituate more than males, as reported previously in birds [17,30]. Ellenberg et al. [17] suggested that the higher habituation of female penguins could be related to their need to conserve energy during incubation; however, our study was conducted in the non-breeding season. More interestingly, sex also affected habituation in an indirect way. Males were more frequently exposed to the predator than females, which probably allowed them to habituate more rapidly. Therefore, the indirect effect counteracted the direct effect, resulting in an overall lack of significant association between sex and habituation. Path analysis, like the one we performed, may be useful in the future to unmask this type of important process in personality and behavioural plasticity studies.
Our results may have implications for the conservation of species living in human-dominated landscapes (e.g. urban and suburban environments) and in protected areas with recreational activities. Habituation ability could provide benefits for individuals in these areas with abundant low-risk predators (e.g. humans). Thus, colonization into human-dominated landscapes could be facilitated if the greater potential of exploratory individuals to habituate is coupled with their greater dispersion ability [26,55]. Recently, Cote et al. [56] have found a positive association between dispersion and low sociability in the invasive mosquitofish (Gambusia affinis). Similarly, a positive association between dispersion and aggression behaviour has been linked to the range expansion of western bluebirds (Sialia mexicana) across the northwestern United States [57]. We speculate that a similar process could result from the associations we found between exploration and habituation, and between sociability and habituation.
Furthermore, if a high-density population is suddenly exposed to high levels of human disturbance, we could expect less social individuals to habituate more and reduce the non-lethal costs of predation [58]. This could lead to changes in stable population density owing to variations in density-dependent emigration [32] and fitness [29] between social and asocial individuals. Overall, these population processes driven by differential habituation ability could lead to reductions in the genetic diversity of populations in human-disturbed habitats [17].
One of the main implications of our study is that, at least in lizards, behavioural plasticity is not necessarily associated with the proactivity–reactivity gradient found in birds and mammals [4,23,24,59]. Our results actually suggest that differences in information-acquisition ability between individuals play a major role in behavioural plasticity variation in a predator–prey context. Information-acquisition has been previously proposed as one of the maintenance costs of phenotypic plasticity [60], and deemed necessary for individuals to adjust their behaviour to current environmental conditions [61]. This is the first study to our knowledge to find that behavioural traits may be related to the probability of an individual to show behavioural plasticity. In particular, risk assessment can affect behavioural plasticity through a complex interaction of direct and indirect effects, including exploratory behaviour, degree of exposure to the predator, and sex. We show novel mechanisms by which inter-individual variation in behavioural plasticity can be generated and maintained within a population.
Acknowledgements
Lizard capture and experiments were carried out under license (Ref. 10/054082.5/05, resolución 6516/05) from the Madrid Environmental Agency (Consejería de Medio Ambiente de la Comunidad de Madrid). The experimental protocol and procedures were in compliance with the UK Animals Scientific Procedures Act 1986 and the European Communities Council Directive November 24, 1986 (86/609/EEC).
We thank ‘El Ventorrillo’ MNCN Field Station for use of their facilities. Financial funding was provided by the project MCI-CGL 2008-02119. I.R.P. was supported by a postgraduate grant from the Spanish National Research Council (CSIC). We thank Myriam Espelleta for assisting in the collection of lizard specimens, and Alison Bell, Jeff Lucas, Megan Gall, Ken Henry, Mark Nolen, Lauren Brierley, Patrice Baumhardt and Jacquelyn Randolet for useful comments on an earlier version of the draft.
References
- 1.Bell A. M., Hankison S. J., Laskowski K. L. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783 10.1016/j.anbehav.2008.12.022 (doi:10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dall S. R. X., Houston A. I., McNamara J. M. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 10.1111/j.1461-0248.2004.00618.x (doi:10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- 3.Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 4.Sih A., Bell A., Johnson J. C. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 5.Dingemanse N. J., Both C., Drent P. J., Van Oers K., Van Noordwijk A. J. 2002. Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 64, 929–937 10.1006/anbe.2002.2006 (doi:10.1006/anbe.2002.2006) [DOI] [Google Scholar]
- 6.Drent P. J., Van Oers K., Van Noordwijk A. J. 2003. Realized heritability of personalities in the great tit (Parus major). Proc. R. Soc. Lond. B 270, 45–51 10.1098/rspb.2002.2168 (doi:10.1098/rspb.2002.2168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Oers K., de Jong G., Van Noordwijk A. J., Kempenaers B., Drent P. J. 2005. Contribution of genetics to the study of animal personalities: a review of case studies. Behaviour 142, 1185–1206 10.1163/156853905774539364 (doi:10.1163/156853905774539364) [DOI] [Google Scholar]
- 8.Dingemanse N. J., Réale D. 2005. Natural selection and animal personality. Behaviour 142, 1159–1184 10.1163/156853905774539445 (doi:10.1163/156853905774539445) [DOI] [Google Scholar]
- 9.Smith B. R., Blumstein D. T. 2008. Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455 10.1093/beheco/arm144 (doi:10.1093/beheco/arm144) [DOI] [Google Scholar]
- 10.Dingemanse N. J., Kazem A. J. M., Réale D., Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89 10.1016/j.tree.2009.07.013 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 11.Biro P. A., Beckmann C., Stamps J. A. 2010. Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc. R. Soc. B 277, 71–77 10.1098/rspb.2009.1346 (doi:10.1098/rspb.2009.1346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell A. M., Sih A. 2007. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834 10.1111/j.1461-0248.2007.01081.x (doi:10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- 13.Briffa M., Rundle S. D., Fryer A. 2008. Comparing the strength of behavioural plasticity and consistency across situations: animal personalities in the hermit crab Pagurus bernhardus. Proc. R. Soc. B 275, 1305–1311 10.1098/rspb.2008.0025 (doi:10.1098/rspb.2008.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nussey D. H., Wilson A. J., Brommer J. E. 2007. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844 10.1111/j.1420-9101.2007.01300.x (doi:10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- 15.Hemmi J., Merkle T. 2009. High stimulus specificity characterizes anti-predator habituation under natural conditions. Proc. R. Soc. B 276, 4381–4388 10.1098/rspb.2009.1452 (doi:10.1098/rspb.2009.1452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shettleworth S. J. 2010. Cognition, evolution and behavior, 2nd ed. Oxford, UK: Oxford University Press [Google Scholar]
- 17.Ellenberg U., Mattern T., Seddon P. J. 2009. Habituation potential of yellow-eyed penguins depends on sex, character and previous experience with humans. Anim. Behav. 77, 289–296 10.1016/j.anbehav.2008.09.021 (doi:10.1016/j.anbehav.2008.09.021) [DOI] [Google Scholar]
- 18.Rodriguez-Prieto I., Fernández-Juricic E., Martín J., Regis Y. 2009. Antipredator behavior in blackbirds: habituation complements risk allocation. Behav. Ecol. 20, 371–377 10.1093/beheco/arn151 (doi:10.1093/beheco/arn151) [DOI] [Google Scholar]
- 19.Stolen E. D. 2003. The effects of vehicle passage on foraging behavior of wading birds. Waterbirds 26, 429–436 10.1675/1524-4695(2003)026[0429:TEOVPO]2.0.CO;2 (doi:10.1675/1524-4695(2003)026[0429:TEOVPO]2.0.CO;2) [DOI] [Google Scholar]
- 20.Runyan A. M., Blumstein D. T. 2004. Do individual differences influence flight initiation distance? J. Wildl. Mngmt. 68, 1124–1129 10.2193/0022-541X(2004)068[1124:DIDIFI]2.0.CO;2 (doi:10.2193/0022-541X(2004)068[1124:DIDIFI]2.0.CO;2) [DOI] [Google Scholar]
- 21.Martin J. G. A., Réale D. 2008. Temperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus. Anim. Behav. 75, 309–318 10.1016/j.anbehav.2007.05.026 (doi:10.1016/j.anbehav.2007.05.026) [DOI] [Google Scholar]
- 22.Rodríguez-Prieto I., Martín J., Fernández-Juricic E. In press Habituation to low-risk predators improves body condition in lizards. Behav. Ecol. Sociobiol. [Google Scholar]
- 23.Koolhaas J. M., Korte S. M., De Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. W., Blokhuis H. J. 1999. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. R 23, 925–935 10.1016/S0149-7634(99)00026-3 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- 24.Jensen P. 2002. The ethology of domestic animals: an introductory text. CABI Publishing [Google Scholar]
- 25.Carere C., Drent P. J., Koolhaas J. M., Groothuis T. G. G. 2004. Personalities in great tits, Parus major, stability and consistency. Anim. Behav. 70, 795–805 10.1016/j.anbehav.2005.01.003 (doi:10.1016/j.anbehav.2005.01.003) [DOI] [Google Scholar]
- 26.Crusio W. E. 2001. Genetic dissection of mouse exploratory behaviour. Behav. Brain Res. 125, 127–132 10.1016/S0166-4328(01)00280-7 (doi:10.1016/S0166-4328(01)00280-7) [DOI] [PubMed] [Google Scholar]
- 27.Greenberg R., Mettke-Hoffmann C. 2001. Ecological aspects of neophobia and exploration. Curr. Ornithol. 16, 119–178 [Google Scholar]
- 28.Tebbich S., Fessl B., Blomqvist D. 2009. Exploration and ecology in Darwin's finches. Evol. Ecol. 23, 591–605 10.1007/s10682-008-9257-1 (doi:10.1007/s10682-008-9257-1) [DOI] [Google Scholar]
- 29.Cote J., Dreiss A., Clobert J. 2008. Social personality trait and fitness. Proc. R. Soc. B 275, 2851–2858 10.1098/rspb.2008.0783 (doi:10.1098/rspb.2008.0783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desforges M. F., Wood-Gush D. G. M. 1975. A behavioural comparison of domestic and mallard ducks. Habituation and flight reaction. Anim. Behav. 23, 692–697 10.1016/0003-3472(75)90145-1 (doi:10.1016/0003-3472(75)90145-1) [DOI] [Google Scholar]
- 31.López P., Hawlena D., Polo V., Amo L., Martín J. 2005. Sources of individual shy-bold variations in antipredator behaviour of male Iberian wall lizards. Anim. Behav. 69, 1–9 10.1016/j.anbehav.2004.05.010 (doi:10.1016/j.anbehav.2004.05.010) [DOI] [Google Scholar]
- 32.Cote J., Clobert J. 2007. Social personalities influence natal dispersal in a lizard. Proc. R. Soc. B 274, 383–390 10.1098/rspb.2006.3734 (doi:10.1098/rspb.2006.3734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aragón P., López P., Martín J. 2001. Seasonal changes in activity and spatial and social relationships of the Iberian rock-lizard Lacerta monticola. Can. J. Zool. 79, 1965–1971 10.1139/cjz-79-11-1965 (doi:10.1139/cjz-79-11-1965) [DOI] [Google Scholar]
- 34.Kerr G. D., Bull C. M. 2006. Movement patterns in the monogamous sleepy lizard (Tiliqua rugosa): effects of gender, drought, time of year and time of day. J. Zool. 269, 137–147 [Google Scholar]
- 35.Avery R. A. 1993. Experimental analysis of lizard pause-travel movement: pauses increase probability of prey capture. Amphibia-Reptilia 14, 423–427 10.1163/156853893X00110 (doi:10.1163/156853893X00110) [DOI] [Google Scholar]
- 36.Van Damme R., Aerts P., Vanhooydonck B. 1997. No trade-off between sprinting and climbing in two populations of the lizard Podarcis hispanica (Reptilia: Lacertidae). Biol. J. Linn. Soc. 60, 493–503 10.1006/bijl.1996.0115 (doi:10.1006/bijl.1996.0115) [DOI] [Google Scholar]
- 37.Galán P. 2003. Reproductive characteristics of an insular population of the lizard Podarcis hispanica from Northwest Spain (Cíes Islands, Galicia). Copeia 2003, 657–665 10.1643/CH-02-235R1 (doi:10.1643/CH-02-235R1) [DOI] [Google Scholar]
- 38.Barbadillo L. J., Lacomba J. I., Pérez-Mellado V., Sancho V., López-Jurado L. F. 1999. Anfibios y reptiles de la Península Ibérica. Barcelona, Spain: Planeta [Google Scholar]
- 39.Rankin C. H., et al. 2009. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 92, 135–138 10.1016/j.nlm.2008.09.012 (doi:10.1016/j.nlm.2008.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemmi J. M. 2005. Predator avoidance in fiddler crabs. 1. Escape decisions in relation to the risk of predation. Anim. Behav. 69, 603–614 10.1016/j.anbehav.2004.06.018 (doi:10.1016/j.anbehav.2004.06.018) [DOI] [Google Scholar]
- 41.Lord A., Waas J. R., Innes J., Whittingham M. J. 2001. Effects of human approaches to nests of northern New Zealand dotterels. Biol. Conserv. 98, 233–240 10.1016/S0006-3207(00)00158-0 (doi:10.1016/S0006-3207(00)00158-0) [DOI] [Google Scholar]
- 42.Magle S., Zhu J., Crooks K. R. 2005. Behavioral responses to repeated human intrusion by black-tailed prairie dogs (Cynomis ludovicianus). J. Mammal. 86, 524–530 10.1644/1545-1542(2005)86[524:BRTRHI]2.0.CO;2 (doi:10.1644/1545-1542(2005)86[524:BRTRHI]2.0.CO;2) [DOI] [Google Scholar]
- 43.Walker B. G., Boersma P. D., Wingfield J. C. 2006. Habituation of adult magellanic penguins to human visitation as expressed trough behavior and corticosterone secretion. Conserv. Biol. 20, 146–154 10.1111/j.1523-1739.2005.00271.x (doi:10.1111/j.1523-1739.2005.00271.x) [DOI] [PubMed] [Google Scholar]
- 44.MacKinnon D. P., Fairchild A. J., Fritz M. S. 2007. Mediation analysis. Ann. Rev. Psychol. 58, 593–614 10.1146/annurev.psych.58.110405.085542 (doi:10.1146/annurev.psych.58.110405.085542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sih A., Lauer M., Krupa J. J. 2002. Path analysis and the relative importance of male–female conflict, female choice and male–male competition in water striders. Anim. Behav. 63, 1079–1089 10.1006/anbe.2002.2002 (doi:10.1006/anbe.2002.2002) [DOI] [Google Scholar]
- 46.Bentler P. M. 1989. EQS structural equations program manual. Los Angeles, CA: BMDP Statistical Software [Google Scholar]
- 47.Iriondo J. M., Albert M. J., Escudero A. 2003. Structural equation modelling: an alternative for assessing causal relationships in threatened plant populations. Biol. Conserv 113, 367–377 10.1016/S0006-3207(03)00129-0 (doi:10.1016/S0006-3207(03)00129-0) [DOI] [Google Scholar]
- 48.Pedhazur E. J. 1982. Multiple regression in behavioural research. Explanation and prediction. New York, NY: Holt, Rinehart & Winston [Google Scholar]
- 49.Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference: a practical information theoretic approach. New York, NY: Springer [Google Scholar]
- 50.Kenny D. A., Kashy D. A., Bolger N. 1998. Data analysis in social psychology. In The handbook of social psychology, vol. 1 (eds Gilbert D., Fiske S., Lindzey G.), pp. 233–265, 4th edn. Boston, MA: McGraw-Hill [Google Scholar]
- 51.Bollen K. A. 1989. Structural equations with latent variables. New York, NY: John Wiley & Sons [Google Scholar]
- 52.ASAB/ABS 2006. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 71, 245–253 10.1016/j.anbehav.2005.10.001 (doi:10.1016/j.anbehav.2005.10.001) [DOI] [PubMed] [Google Scholar]
- 53.ASIH 2004. Guidelines for use of live amphibians and reptiles in field and laboratory research, 2nd edn. Lawrence, KA: Herpetological Animal Care and Use Committee (HACC) of the American Society of Ichthyologists and Herpetologists [Google Scholar]
- 54.Guillette L. M., Reddon A. R., Hurd P. L., Sturdy C. B. 2009. Exploration of a novel space is associated with individual differences in learning speed in black-capped chickadees, Poecile atricapillus. Behav. Process. 82, 265–270 10.1016/j.beproc.2009.07.005 (doi:10.1016/j.beproc.2009.07.005) [DOI] [PubMed] [Google Scholar]
- 55.Dingemanse N. J., Both C., Van Noordwijk A. J., Rutten A. L., Drent P. J. 2003. Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747 10.1098/rspb.2002.2300 (doi:10.1098/rspb.2002.2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cote J., Fogarty S., Weinersmith K., Brodin T., Sih A. 2010. Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc. R. Soc. B 277, 1571–1579 10.1098/rspb.2009.2128 (doi:10.1098/rspb.2009.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duckworth R. A., Badyaev A. V. 2007. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15017–15022 10.1073/pnas.0706174104 (doi:10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Creel S., Christianson D. 2008. Relationships between direct predation and risk effects. Trends Ecol. Evol. 23, 194–201 [DOI] [PubMed] [Google Scholar]
- 59.Verbeek M. E. M., Drent P. J., Wiepkema P. R. 1994. Consistent individual differences in early exploratory behaviour of male great tits. Anim. Behav. 48, 1113–1121 10.1006/anbe.1994.1344 (doi:10.1006/anbe.1994.1344) [DOI] [Google Scholar]
- 60.Auld J. R., Agrawal A. A., Relyea R. A. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511 10.1098/rspb.2009.1355 (doi:10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dall S. R. X., Giraldeau L.-A., Olsson O., McNamara J. M., Stephens D. W. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 10.1016/j.tree.2005.01.010 (doi:10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]