Abstract
Intra-locus sexual conflict results when sex-specific selection pressures for a given trait act against the intra-sexual genetic correlation for that trait. It has been found in a wide variety of taxa in both laboratory and natural populations, but the importance of intra-locus sexual conflict and sexually antagonistic genetic variation in hermaphroditic organisms has rarely been considered. This is not so surprising given the conceptual and theoretical association of intra-locus sexual conflict with sexual dimorphism, but there is no a priori reason why intra-locus sexual conflict cannot occur in hermaphroditic organisms as well. Here, I discuss the potential for intra-locus sexual conflict in hermaphroditic animals and review the available evidence for such conflict, and for the existence of sexually antagonistic genetic variation in hermaphrodites. I argue that mutations with asymmetric effects are particularly likely to be important in mediating sexual antagonism in hermaphroditic organisms. Moreover, sexually antagonistic genetic variation is likely to play an important role in inter-individual variation in sex allocation and in transitions to and from gonochorism (separate sexes) in simultaneous hermaphrodites. I also describe how sequential hermaphrodites may experience a unique form of intra-locus sexual conflict via antagonistic pleiotropy. Finally, I conclude with some suggestions for further research.
Keywords: intra-locus sexual conflict, hermaphrodite, sexual antagonism, evolutionary transitions, gonochorist, antagonistic pleiotropy
1. Introduction
There is ample evidence that the reproductive interests of males and females do not always coincide. This has traditionally been considered to be a consequence of anisogamy [1], although recent theoretical work suggests that sex-specific mortality rates and adult sex ratios may be a more parsimonious explanation [2]. Different reproductive interests can lead to various types of conflict between the sexes, such as conflict over parental investment or mating rates. In general, there are two main types of sexual conflict: inter-locus and intra-locus. In inter-locus sexual conflict, the sexes experience opposing selection pressures on one or more traits, but the genes affecting the expression of the trait probably differ between the sexes. Much previous research on inter-locus sexual conflict has focused on traits that involve an interaction between the sexes, such as fertilization efficiency, remating behaviour or female reproductive rate [3]. In contrast, in intra-locus sexual conflict, it is the same genes that are subject to conflicting selection pressures between the sexes [3,4]. In the standard definition, intra-locus sexual conflict occurs when (i) males and females have different phenotypic optima for the same trait, resulting in sexually antagonistic selection pressures, and (ii) there is a positive inter-sexual genetic correlation for the trait, resulting in correlated phenotypic expression between the sexes [5]. In practice, inter- and intra-locus sexual conflict probably occupy opposite endpoints of a continuum rather than existing as sharply defined separate phenomena. For example, although mating rate is determined via the interaction between males and females (i.e. inter-locus sexual conflict), one can easily imagine that behavioural differences in propensity to remate (which will be an important contributor to mating rate) may be correlated and antagonistically selected between the sexes (i.e. intra-locus sexual conflict [3,6]). Both phenomena have been extensively studied in recent years [3,7].
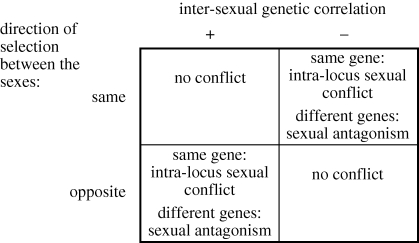
Both inter-locus sexual conflict and intra-locus sexual conflict result from sexual antagonism (i.e. opposing selection pressures between the sexes), and only differ in the genetic basis of the trait(s) in question, which may be either assumed or known. This means that genes involved in both phenomena can be described as sexually antagonistic genetic variation [8]. All that is required for sexual antagonism to exist is that selection acts ‘to change the means of two characters against the sign of their genetic correlation’ [9], so it is also worth noting that sexual antagonism could equally result from similar selection pressures acting on a trait that is negatively correlated between the sexes (in gonochorists, i.e. species with separate sexes) or between sex functions (in hermaphrodites; figure 1). Sexually antagonistic genetic variation plays a central role in both inter- and intra-locus sexual conflict, and is therefore potentially easier to investigate and of wider importance than intra-locus sexual conflict per se. Although it is not part of the formal definition, inter-locus sexual conflict traditionally focuses on reproductive interactions [3], so here I will use the term ‘sexually antagonistic genetic variation’ to describe those traits that are subject to sexually antagonistic selection pressures and have a different genetic basis, but are not related to reproductive interactions between individuals.
Figure 1.
How inter-sexual genetic correlations and the direction of selection combine to produce intra-locus sexual conflict and sexual antagonism. If selection acts against the inter-sexual genetic correlation, then there is conflict (i.e. if the sexes are selected in the same direction and there is a negative inter-sexual genetic correlation, or if the sexes are selected in opposite directions and there is a positive inter-sexual genetic correlation; upper right and bottom left quadrants). In gonochorists (organisms with separate sexes), intra-locus sexual conflict in the lower left quadrant has typically been investigated (traits with positive inter-sexual genetic correlation, but opposite direction of selection between the sexes). Hermaphrodites could be more likely than gonochorists to experience conflict from the upper right quadrant—for example, if increased gonad size is favoured in both sex roles, but there is a trade-off between testis size and ovary size.
Intra-locus sexual conflict has been found in a wide variety of taxa, from plants [10] to mammals [11], and has been found in both natural [12] and laboratory populations [13]. Two comprehensive reviews of this subject [7,14], as well as a meta-analysis of sexually antagonistic selection pressures [15], have recently been published. However, none of these papers examined the potential importance of intra-locus sexual conflict and sexually antagonistic genetic variation in hermaphroditic organisms. Similarly, a recent paper by Bedhomme et al. [16] discussed the relevance of inter- and intra-locus sexual conflict in hermaphrodites, but although they raised a number of interesting points, the shortness of their paper did not permit a thorough development. It is not so surprising that intra-locus sexual conflict and sexually antagonistic genetic variation in hermaphroditic animals have been more or less overlooked to date, since the evolution of sexual dimorphism is predicted to be an indicator of sexually antagonistic selection pressures (past or present [17]). Simultaneously, hermaphroditic organisms are monomorphic almost by definition (but see [18], referenced in [19]), so their potential for sexually antagonistic selection is not intuitively obvious. Yet as much as 5 to 6 per cent of all animal species are hermaphroditic (or 30 per cent if insects are excluded [20]), including 2 per cent of fish species [21]. Here, I hope to fill in the gaps by presenting a more comprehensive discussion of the potential importance of intra-locus sexual conflict and sexually antagonistic genetic variation in hermaphroditic animals. Some of the phenomena I will discuss have already been studied as interesting research topics in themselves (e.g. sex allocation theory in hermaphrodites) but have not been considered in this context.
2. Intra-locus sexual conflict in hermaphrodites
There is no a priori reason to assume that sexually antagonistic variation and intra-locus sexual conflict cannot occur in hermaphrodites, as was pointed out by Bedhomme et al. [16]. Just as for gonochorists, we should be able to distinguish between inter-locus and intra-locus sexual conflict. Conflicts between hermaphroditic individuals in mating interactions are typically considered to be inter-locus sexual conflict. For instance, traumatic insemination and conflict over fertilization is a classic example of sexual conflict in simultaneously hermaphroditic organisms [22–24]. However the very nature of the difference between gonochorism and hermaphroditism means that intra-locus sexual conflict and sexually antagonistic genetic variation will manifest somewhat differently in hermaphrodites.
First, sexually antagonistic selection will operate on fitness components (male and female fitness function) in hermaphroditic organisms [25], rather than on total individual fitness, as in gonochorists [5]. This means that sexually antagonistic genetic variation in hermaphrodites must be defined in terms of its effect on fitness components rather than sex-specific individual fitnesses, and sexually antagonistic mutations will only spread in a population of hermaphrodites if the net fitness effect is positive, all else being equal [25]. Second, genes subject to sexually antagonistic selection will experience conflicting selection pressures on a much shorter time scale in hermaphroditic organisms than in gonochorists (i.e. within the lifetime of the individual [25], rather than across generations [5]). Simultaneous hermaphrodites will naturally experience conflicting sexual selection on a shorter time scale than sequential hermaphrodites. Third, there is the question of the relevance of the concept of inter-sexual genetic correlations. Inter-sexual genetic correlations should be applicable to sequential hermaphrodites, since each sex is expressed separately. However, it is less certain whether they can be applied to simultaneous hermaphrodites since the trait will be expressed simultaneously in the same individual. It would obviously be meaningless to calculate an inter-sexual genetic correlation in a simultaneous hermaphrodite for a trait such as overall body size, since it would have to be exactly +1. However, this does not exclude the possibility of sexually antagonistic selection on male and female fitness for body size, for example, if there is a positive relationship between fecundity and body size, but a negative relationship between success in sperm competition and body size. It may also be possible for simultaneous hermaphrodites to have different expression of the same trait as a male or as a female if the trait is not expressed simultaneously (for example, propensity to remate in a given sex role in species that do not engage in reciprocal mating) or if there are epistatic effects (for example, if expression in the testes, but not the ovaries, is dependent on the genotype at another locus). A consensus will have to be reached in future as to whether these differences make intra-locus sexual conflict in hermaphrodites a fundamentally different phenomenon than in gonochorists. Some may argue that differential selection of a given trait via male and female fitness components is more similar to classic intra-individual optimization processes (e.g. sex allocation) than to sexual conflict. However, the term ‘sexual conflict’ has occasionally been used by both plant [26] and animal [24] biologists in such situations, so a case could be made either way. Because conflicting sex-specific selection within the lifetime of the individual is unique to hermaphroditic organisms, I suggest ‘intra-individual sexual antagonism’ as an appropriate alternative term for use in hermaphrodites. I will use it here to collectively denote both intra-locus sexual conflict and sexually antagonistic genetic variation in hermaphrodites. Note that it does not include inter-locus sexual conflict over reproductive interactions, since these are inter-individual conflicts, not intra-individual conflicts.
Data from studies of plants demonstrate that it is indeed possible for the same trait to be subject to antagonistic selection pressures via male and female fitness functions in hermaphroditic organisms. For example, Morgan & Schoen [27] found opposing selection pressures via male and female sex function on the same trait for four traits (corolla pigment, hood width, horn length and slit length) in the hermaphroditic common milkweed plant, Asclepias syriaca. Although concordant selection pressures on floral traits via each sex function seem to be common in plants [28], other examples of antagonistic selection pressures via male and female fitness function have also been found [29,30]. These studies at least confirm that sexually antagonistic selection on the same trait is possible in hermaphrodites, but, to my knowledge, no equivalent study has yet been carried out in a hermaphroditic animal.
According to theory, intra-locus sexual conflict maintains sexually antagonistic genetic variation within populations, and it is therefore thought to be an important contributor to the standing genetic variation for many traits in gonochorists, particularly sexually dimorphic ones [17]. This effect is probably less pronounced in hermaphrodites since sexually antagonistic alleles will be exposed to selection in both sexes within the lifetime of the individual, leading to a greater efficiency of selection [16]. On the other hand, one way intra-locus sexual conflict can be at least partially resolved is via the evolution of sexual dimorphism [5,17]. Simultaneous hermaphrodites cannot evolve sexual dimorphism, so intra-individual sexual antagonism may therefore represent more of a cost or a constraint in hermaphrodites than intra-locus sexual conflict does in gonochorists [16]. Despite a greater efficiency of selection in hermaphrodites and a higher cost of intra-individual sexual antagonism, we should still expect to see some sexually antagonistic variation in hermaphroditic organisms for a number of reasons. One is simply because of mutation–selection balance. A second possibility is if sex-specific optima are variable over space and time, since this will also lead to an increased likelihood of the maintenance of sexually antagonistic genetic variation [14,31]. Sex-specific antagonistic selection pressures are expected to be perfectly balanced at equilibrium [32], so if optima are variable, then sexually antagonistic alleles can spread if they favour the sex function that is under stronger selection. Finally, recent simulations have shown that sexually antagonistic genetic variation can be maintained over a much wider range of selection intensities than has previously been thought (e.g. [33]) if there is even very modest assortative mating for fitness (G. Arnqvist 2010, personal communication). Assortative mating for fitness essentially becomes disassortative if most of the differences in fitness are due to sexually antagonistic genetic variation, and although these simulations have been carried out in a gonochorist context, there are certainly situations where one could expect assortative mating for fitness among hermaphrodites as well (for example, in species with broadcast spawning, where the most frequent gamete genotypes are most likely to encounter each other).
There are several ways in which sexually antagonistic variation could affect male and female fitness function in hermaphrodites. This list is not exhaustive, but some possibilities include:
By increasing allocation to morphological structures involved in one sex function at the expense of the other. In simultaneous hermaphrodites, this could manifest as a trade-off in gonad sizes [34].
By increasing investment in the production of one gamete at the expense of the other. This could be mediated via an energetic trade-off in simultaneous hermaphrodites [34], while in sequential hermaphrodites it could correspond to differences in the timing of sex change [35].
Behaviourally mediated effects on fitness. Some hermaphroditic species experience significant conflict over fertilization [22–24]. In such species, it could pay to increase investment in fertilization attempts, even if this results in lowered egg production. Choosing to invest in sexual conflict arms races over fecundity should be widespread among simultaneous hermaphrodites, according to recent theoretical work [36,37]. There is also empirical evidence suggesting that elevated mating rates may have opposite fitness effects via male and female sex functions [38,39].
Mediation of parasite-associated effects. Parasitic castration is common in many trematode–snail systems, and some parasites primarily affect only one sex function [34]. Mutations that restore the affected sex function at some cost to the unaffected one would spread in these cases.
Points (i) and (ii) fall under the umbrella of sex allocation theory. Sex allocation in hermaphrodites is a productive field of research in itself, so I will discuss its relation to intra-individual sexual antagonism in greater detail below. Although most of the processes I have outlined here depend on the existence of a trade-off in investment between sex functions, it is worth noting that such a trade-off is not strictly necessary for intra-individual sexual antagonism to exist, since all that is required is discordance between the direction of selection and the inter-sexual genetic correlation (figure 1). For example, mutations that increase fitness in one sex function but have deleterious pleiotropic effects in the other (perhaps via common signalling pathways) are sexually antagonistic without being dependent on an energetic trade-off in investment.
3. Asymmetric fitness effects
For researchers used to working on organisms with separate sexes, the measurement of fitness in hermaphrodites involves some interesting modifications, an issue I have touched on above. Because hermaphrodites can gain fitness via both sex functions, it is obviously necessary to measure offspring output from both sperm and eggs when calculating fitness. Studies of selection in hermaphroditic plants commonly measure male and female fitness separately in the same individual (e.g. [25–27,40]). Total absolute fitness will be the sum of offspring output from each sex role, and total relative fitness will then be total offspring output relative to the population mean [25]. Whole-organism relative fitness will be the mean of the relative fitnesses from each sex role, assuming each role is played equally often (in simultaneous hermaphrodites [25,41]) or the time spent as male and as female is equal (in sequential hermaphrodites). What is interesting here is that the same relative total fitness can be achieved in different ways. Individuals with relative fitness 1.1, for example, could achieve this either by having average fitness as males and above average fitness as females, or average fitness as females and above average fitness as males.
Because of the two-part nature of fitness in hermaphrodites, mutations with asymmetric fitness effects will be particularly relevant to intra-individual sexual antagonism. A mutation that increases female (male) fitness substantially at a small cost to male (female) fitness will be positively selected. It seems likely that frequency-dependent effects will come into play here. For example, compare the effects of the following two mutations: mutation 1 is a sexually antagonistic asymmetric mutation that increases absolute female fitness by 15 per cent, but decreases male fitness by 5 per cent. In a hermaphroditic organism that plays both sex roles equally often, this will result in a 5 per cent increase in total fitness. Mutation 2 is a symmetric mutation that increases absolute fitness in both sexes by 5 per cent, also resulting in a 5 per cent increase in total fitness. Although both mutations will experience equally strong positive selection, we can expect frequency-dependent effects to play a larger role in the population in which mutation 1 appears. As mutation 1 spreads, the marginal benefit of investing in female fitness will decrease, while the benefit of investing in male fitness will increase, resulting in frequency-dependent selection for alleles that increase male fitness. In comparison, because the effects of mutation 2 are symmetric, as mutation 2 spreads in the population there will be no frequency-dependent selection favouring increased fitness in a particular sex function. The frequency-dependent selection of asymmetric mutations suggested here is qualitatively similar to the frequency-dependent dynamics that have been found in polymorphic systems (e.g. [42,43]).
Mutations with asymmetric fitness effects should also result in selection for increased investment in the sex function that is favoured by the mutation [16]. In gonochorists, offspring sex ratio is predicted to vary according to the level of sexual antagonism. For example, a high-quality male is expected have an excess of sexually antagonistic male-benefit/female-detriment alleles present in his genome, so a female mated to such a male should bias her offspring sex ratio towards sons in order to avoid detrimental fitness effects on daughters. Consistent with such predictions, Calsbeek & Sinervo [44] found that female lizards mated to males of different sizes exhibited cryptic choice of sperm, such that daughters were sired by small males and sons by large males. In a similar process, hermaphroditic animals should increase their allocation to the sex function for which they have an excess of beneficial alleles [16], either by increasing energetic investment in that sex function or by increasing the amount of time spent in that sex role. It is known that hermaphrodites can bias sex allocation according to size and social situation (e.g. [31,45,46]), and individuals whose sex allocation has been experimentally manipulated change their mating behaviour [47], so bias as a result of accommodation to sexually antagonistic genetic variation seems likely.
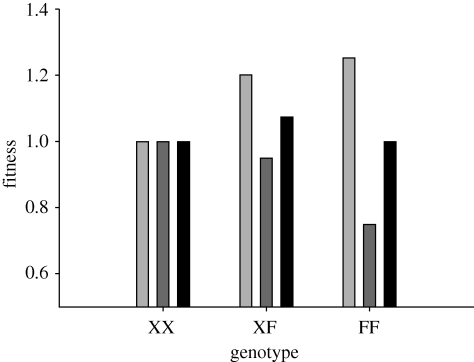
Asymmetric dominance effects and epistasis may also be important in maintaining sexually antagonistic variation in hermaphrodites. It has been predicted in gonochorists that sexually antagonistic sex-linked loci will spread even at considerable cost to one sex, depending whether they are dominant (favouring female-benefit/male-detriment alleles in XY systems) or recessive (favouring female-detriment/male-benefit alleles in XY systems [4]). Such effects should be possible in sequential hermaphrodites possessing sex chromosomes. Simultaneous hermaphrodites generally do not possess sex chromosomes ([16], but see [48]), so in such cases this effect would not apply. However, a recent analysis has demonstrated that the expected contribution of the sex chromosomes to sexually antagonistic genetic variation may have been overestimated and that sexually antagonistic autosomal variation can be maintained in gonochorists via sex-specific asymmetric dominance effects [49]. Sexually antagonistic alleles with asymmetric dominance effects could therefore also be maintained within simultaneously hermaphroditic populations via overdominant selection (heterozygote advantage). For example, a female-benefit/male-detriment allele that produces a large increase in female fitness both when heterozygous and when homozygous, but which produces a small cost to male fitness when heterozygous and a large cost when homozygous, will result in highest mean fitness for heterozygous individuals (figure 2). By modifying the definition of each parameter where appropriate in Fry's eqn (3) [49], we should be able to apply this model to simultaneous hermaphrodites and show that polymorphism will be maintained whenever
where hm (hf) is the dominance of an allele which has deleterious effects on the male (female) component of fitness, and sm (sf) is the selection coefficient against the less fit homozygote for the male (female) component of fitness. This allows maintenance of sexually antagonistic genetic variation over a much broader range of selection intensities than for additive traits and does not require perfectly balanced opposing selection pressures on each sex function.
Figure 2.
The relationship between fitness and genotype for a sexually antagonistic female-benefit/male-detriment allele with asymmetric dominance effects. F is an allele that benefits female sex function at the expense of male sex function, but which is partly dominant for female fitness and partly recessive for male fitness. Heterozygous (XF) individuals have higher total fitness than either homozygote. Light grey bars, female; dark grey bars, male; black bars, mean.
4. Sex allocation and evolutionary transitions in simultaneous hermaphrodites
Much sex allocation theory is based on the assumption that there is a trade-off between allocation to each sex function [34]. Such trade-offs have actually rarely been found, although some examples exist [46,50–52]. Despite the fact that an energetic trade-off is not necessary for intra-individual sexual antagonism to operate, it is one of the simplest ways such antagonism could be mediated. The lack of studies able to demonstrate such a trade-off is therefore perhaps rather surprising, but a possible explanation could be difficulties in measuring allocation [34]. Using a fitness-based perspective (such as in previous studies of intra-locus sexual conflict [11,13,53]) would mitigate these concerns about how to measure allocation and could make it easier to detect variation in allocation patterns due to sexually antagonistic genetic variation.
The most suggestive evidence of the existence of sexually antagonistic genetic variation in a simultaneous hermaphrodite comes from Yund et al. [52]. They used quantitative genetics to demonstrate a negative inter-sexual genetic correlation for gonad size, dependent on investment in asexual growth in a colonial ascidian. There is also evidence that individuals can influence their partners' sex allocation in a sexually antagonistic way via allohormones (hormone-like substances transferred during mating [54]). In Lymnaea stagnalis, peptides transferred in the seminal fluid during mating decrease egg mass production in the inseminated partner [55]. At first glance this seems to benefit neither partner as both the inseminator and the inseminated partner lose fitness if egg mass is reduced. Koene et al. [55] suggest that the benefit to the inseminator may be in increased paternity. Assuming there is some benefit to the inseminator by transferring such peptides, it would be interesting to see whether increased production of male-benefit/female-detriment allohormones also results in decreased female fitness within the same individual.
Much sex allocation theory builds on optimality models ([56,57]; reviewed in [34]), and any sort of departure from model predictions is often presumed to be because of environmental noise or because of the effects of some unmeasured or uncontrolled factors (e.g. [46]). On short (ecological) time scales we may indeed expect some departures from optimality owing to stochastic effects, but over the course of many generations we expect organisms should converge on the optimal sex allocation as predicted from theory. Intra-locus sexual conflict should therefore be most relevant on ecological time scales, for example, by explaining inter-individual variation in departures from optimality. Rather than considering all departures from optimal sex allocation as being rather uninteresting noise, it may be useful to try to determine what portion of the variance in sex allocation within a population is due to genetic effects (i.e. intra-individual sexual antagonism). In fact, Schärer [34] suggests using genetically homogeneous individuals to investigate how environmental factors affect sex allocation, which is an indirect acknowledgement of the confounding effect of genetic variation in allocation patterns. Even in species with plastic sex allocation [47], it seems likely that there can be overall differences in allocation or in the shape/slope of the allocation reaction norm.
The role of intra-individual sexual antagonism in causing departures from optimal sex allocation is not only potentially interesting in itself, however. It may also be important to speciation and evolutionary transitions. Transitions to gonochorism should happen when there is linkage between sexually antagonistic alleles and loci for sex allocation, leading to the evolution of proto-sex chromosomes [16]. The frequency of evolutionary transitions to and from gonochorism varies between taxonomic groups [58–61], and, interestingly, one study even suggests that hermaphroditism is ancestral to gonochorism among metazoans [62]. Various phenomena have recently been suggested as the drivers of such transitions [59,62,63], but, to my knowledge, very little is known about the proximate genetic mechanisms enabling a transition. Better knowledge of standing levels of sexually antagonistic genetic variation should therefore be highly relevant to our understanding of such transitions. Similarly, inter-population variation in departures from optimal sex allocation could be considered the first step towards speciation via an evolutionary transition, and investigation of such inter-population variation would follow in the best traditions of the study of ecological divergence as a precursor to speciation [64]. Conversely, in groups with frequent transitions to and from gonochorism, it would also be useful to look for a depletion of sexually antagonistic variation as a preliminary stage in, or preadaptation to, the evolution of hermaphroditism. An accumulation of female-benefit/male detriment alleles should also be expected in the transition from outcrossing to selfing in hermaphrodites. All of these phenomena make population-level comparisons of sexually antagonistic variation in hermaphroditic species potentially useful.
5. Sexual antagonism in sequential hermaphrodites
Some authors have argued that sequential hermaphrodites have more in common with gonochorists than with simultaneous hermaphrodites (e.g. [58,62]). This is probably also true for intra-individual sexual antagonism. For example, most species of hermaphroditic fish are sequentially hermaphroditic, and many of them are sexually dimorphic as well [21]. Such sexually dimorphic sequential hermaphrodites could experience constraints in the evolution of sexual dimorphism owing to intra-locus sexual conflict, similar to previous results in gonochorists [10,13,65]. Whether such constraints are likely is questionable since the ability to change sex speaks against the existence of strong constraints on morphology, but they are certainly possible. In addition, sequential hermaphrodites should also experience unique forms of intra-individual sexual antagonism. For example, there could be sexually antagonistic effects on the timing of the change in sex. Although in many sequentially hermaphroditic organisms the timing of sex change is plastic [66], there could still be genetic differences in timing between individuals, or in the shape/slope of the sex change reaction norm (similar to the argument for sex allocation above). This does not appear to have been investigated in fish in any detail to date [21]. In Caenorhabditis elegans, there is indeed evidence of genetic variation in the timing of sex change. Caenorhabditis elegans first produces and stores sperm, then changes sex and uses the stored sperm for self-fertilization. Sperm limitation is not uncommon, but a mutation that delays the change to female leads to lower overall population growth despite increasing sperm production [35]. This is because the increase in the number of fertilized eggs is outweighed by the loss incurred by the increase in the time to reproduction. Because C. elegans is selfing, this mutation results in lower total fitness and would be unlikely to spread in natural populations. However, in outcrossing species with high levels of sperm competition, mutations that delay the change from male to female might be favoured.
Another form of intra-individual sexual antagonism that should be unique to sequential hermaphrodites is via antagonistic pleiotropy. The idea of antagonistic pleiotropy was originally developed in the context of senescence [67], and states that alleles that increase fitness at early stages but have deleterious effects late in life will tend to accumulate in the population because selection does not operate as efficiently later on in life. Antagonistic pleiotropy has some empirical support in the context of senescence [68,69], and its role in senescence has in fact recently been investigated in a hermaphroditic species [70]. It seems reasonable that sexual antagonism via antagonistic pleiotropy could operate in sequential hermaphrodites, where alleles that increase fitness in the first sex at the expense of the second sex should spread owing to a decreased efficiency of selection at later stages in life. Comparative studies of female-first species versus male-first species could be useful in detecting such effects. Similarly, some species have both sequentially hermaphroditic and gonochoristic morphs with, for example, some individuals that are male throughout their lives (‘primary males’) and others that begin as females and later change sex to become male (‘secondary males’ [21]). Within-species comparisons of sequentially hermaphroditic and gonochoristic morphs could also serve to detect intra-individual sexual antagonism via antagonistic pleiotropy. It is also worth noting that a weaker form of such antagonistic pleiotropy could even occur in simultaneous hermaphrodites that change their allocation patterns over time. Some species of simultaneous hermaphrodite start off with male- (female-) biased sex allocation and then increase their allocation to female (male) fitness with increasing age/body size, making them in effect sequential hermaphrodites with a gradual transition between the sexes (e.g. [45,71–73]). Such species could also experience intra-individual sexual antagonism via antagonistic pleiotropy.
6. Future directions
A common problem when working on intra-locus sexual conflict in gonochorists is being able to demonstrate the existence of sexually antagonistic genetic variation within the study population. Animal model analysis of pedigrees has successfully been used to find evidence of sexually antagonistic genetic variation in organisms with separate sexes (e.g. [11]), so it should be a simple matter to extend this sort of analysis to hermaphroditic organisms. Animal model analysis should in fact be easier in some ways to carry out in hermaphrodites than in organisms with separate sexes, since fitness data from both sexes can be obtained from the same individual. Similarly, a recent paper by Innocenti & Morrow [74] details the calculation of an index of the intensity of sexually antagonistic selection. This index should also be easy to apply to hermaphroditic organisms and will facilitate qualitative comparisons between populations or taxa.
I have suggested that intra-individual sexual antagonism is likely to be important in explaining both standing variation in sex allocation/timing of sex change and evolutionary transitions in hermaphroditic species. To increase our understanding in these areas, some useful lines of research could include:
— Modelling of the role of asymmetric genetic effects, epistasis and assortative mating. The models and simulations I have discussed here of the importance of asymmetric dominance effects and assortative mating in maintaining sexual antagonistic genetic variation were originally developed in the context of gonochorist species. Models that are specific to simultaneous or sequential hermaphrodites would seem to be a logical next step, as would be the development of models dealing with the role of epistasis.
— Molecular and phylogenetic studies. Transitions to and from gonochorism are common in (among others) cnidarians, bivalves, crustaceans, polychaetes, fishes and gastropods [58,60,61]. We might therefore expect that levels of sexually antagonistic genetic variation are elevated in taxa with frequent transitions relative to those with only infrequent transitions. It would be useful to test for a correlation between levels of sexually antagonistic genetic variation and frequency of transitions to and from gonochorism within groups. Linkage between sexually antagonistic alleles and sex allocation loci could also be investigated in groups with sequenced genomes, such as Aplysia (sea hare) and Lottia (owl limpet).
— Population-level studies of sexually antagonistic genetic variation and variance in fitness. Population-level studies of taxa with frequent transitions to and from gonochorism might identify populations that are on the way to evolving gonochorism. This could manifest either as increased levels of sexually antagonistic genetic variation relative to other populations of the same species, or as higher variance (perhaps even bimodality) in sex allocation or sex-specific fitness.
— Experimental evolution and artificial selection. Artificial selection for increased investment in a specific sex function would constitute a direct test of the lability of hermaphroditism and of levels of standing sexually antagonistic genetic variation. Such an approach has also been suggested by Schärer [34], and experimental evolution has been successful in detecting intra-locus sexual conflict in laboratory populations of Drosophila melanogaster [13,75,76]. Colour has occasionally been used as a marker trait in experimental evolution studies (e.g. [8,77]), so this approach could be applied in colour-polymorphic hermaphroditic species. Colour would function as a marker for sex such that only offspring produced via the appropriate gamete type for that colour morph would be allowed to enter the population.
— Intra-individual sexual antagonism in androdioecious and gynodioecious systems. In a transition to or from gonochorism, it is expected that populations will go through an androdioecious (males and hermaphrodites) or gynodioecious (females and hermaphrodites) stage [78,79]. Androdioecy seems to be relatively more common in animals than in plants [80]. Some species also have variable frequencies of hermaphrodites and gonochorists over space and time [60,80]. The potential for intra-individual sexual antagonism could be elevated in androdioecious and gynodioecious systems if selection in gonochorist morphs opposes selection in hermaphroditic morphs.
The evidence for intra-individual sexual antagonism in hermaphroditic animals is sparse and mostly indirect at this time, but this is probably at least partially owing to a lack of previous research specifically intended to detect such effects. There is obviously ample room for further work in this area.
Acknowledgements
Thanks to Ted Morrow for encouraging me to write this paper. Thanks also to Paolo Innocenti, Stéphanie Bedhomme and two anonymous reviewers for helpful comments on the manuscript. This research was funded by a repatriation grant (Återvändarbidrag) from the Swedish Research Council (Vetenskapsrådet).
References
- 1.Trivers R. L. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man 1871–1971 (ed. Campbell B.), pp. 136–207 Chicago, IL: Aldine Publishing Company [Google Scholar]
- 2.Kokko H., Jennions M. D. 2008. Parental investment, sexual selection and sex ratios. J. Evol. Biol. 21, 919–948 10.1111/j.1420-9101.2008.01540.x (doi:10.1111/j.1420-9101.2008.01540.x) [DOI] [PubMed] [Google Scholar]
- 3.Arnqvist G., Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press [Google Scholar]
- 4.Parker G. A., Partridge L. 1998. Sexual conflict and speciation. Phil. Trans. R. Soc. Lond. B 353, 261–274 10.1098/rstb.1998.0208 (doi:10.1098/rstb.1998.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice W. R., Chippindale A. K. 2001. Inter-sexual ontogenetic conflict. J. Evol. Biol. 14, 685–693 10.1046/j.1420-9101.2001.00319.x (doi:10.1046/j.1420-9101.2001.00319.x) [DOI] [Google Scholar]
- 6.Halliday T., Arnold S. J. 1987. Multiple mating by females: a perspective from quantitative genetics. Anim. Behav. 35, 939–941 10.1016/S0003-3472(87)80138-0 (doi:10.1016/S0003-3472(87)80138-0) [DOI] [Google Scholar]
- 7.Bonduriansky R., Chenoweth S. F. 2009. Intra-locus sexual conflict. Trends Ecol. Evol. 24, 280–288 10.1016/j.tree.2008.12.005 (doi:10.1016/j.tree.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 8.Rice W. R. 1992. Sexually antagonistic genes: experimental evidence. Science 256, 1436–1439 10.1126/science.1604317 (doi:10.1126/science.1604317) [DOI] [PubMed] [Google Scholar]
- 9.Lande R. 1979. Quantitative genetic analysis of multivariate evolution, applied to brain : body size allometry. Evolution 33, 402–416 10.2307/2407630 (doi:10.2307/2407630) [DOI] [PubMed] [Google Scholar]
- 10.Delph L. F., Gehring J. L., Frey F. M., Arntz A. M., Levri M. 2004. Genetic constraint on floral evolution in a sexually dimorphic plant revealed by artificial selection. Evolution 58, 1936–1946 [DOI] [PubMed] [Google Scholar]
- 11.Foerster K., Coulson T., Sheldon B. C., Pemberton J. M., Clutton-Brock T. H., Kruuk L. E. B. 2007. Sexually antagonistic genetic variation for fitness in red deer. Nature 447, 1107–1110 10.1038/nature05912 (doi:10.1038/nature05912) [DOI] [PubMed] [Google Scholar]
- 12.Svensson E. I., MacAdam A. G., Sinervo B. 2009. Intra-locus sexual conflict over immune defence and the resolution of gender load in a natural lizard population. Evolution 63, 3124–3135 10.1111/j.1558-5646.2009.00782.x (doi:10.1111/j.1558-5646.2009.00782.x) [DOI] [PubMed] [Google Scholar]
- 13.Prasad N. G., Bedhomme S., Day T., Chippindale A. K. 2007. An evolutionary cost of separate genders revealed by male-limited expression. Am. Nat. 169, 29–37 10.1086/509941 (doi:10.1086/509941) [DOI] [PubMed] [Google Scholar]
- 14.van Doorn G. S. 2009. Intra-locus sexual conflict. Ann. NY Acad. Sci. 1168, 52–71 10.1111/j.1749-6632.2009.04573.x (doi:10.1111/j.1749-6632.2009.04573.x) [DOI] [PubMed] [Google Scholar]
- 15.Cox R. M., Calsbeek R. 2009. Sexually antagonistic selection, sexual dimorphism, and the resolution of intra-locus sexual conflict. Am. Nat. 173, 176–187 10.1086/595841 (doi:10.1086/595841) [DOI] [PubMed] [Google Scholar]
- 16.Bedhomme S., Bernasconi G., Koene J. M., Lankinen Å., Arathi H. M., Michiels N. K., Anthes N. 2009. How does breeding system variation modulate sexual antagonism? Biol. Lett. 5, 717–720 10.1098/rsbl.2009.0401 (doi:10.1098/rsbl.2009.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedhomme S., Chippindale A. K. 2007. Irreconcilable differences: when sexual dimorphism fails to resolve sexual conflict. In Sex, size and gender roles: evolutionary studies of sexual size dimorphism (eds Fairbairn D. J., Blanckenhorn W. U., Székely T.), pp. 185–194 Oxford, UK: Oxford University Press [Google Scholar]
- 18.McLauchlan C. F. 1951. Basic work on the life cycle of some Australian snails. Proc. R. Soc. New South Wales 1949–1950, 26–36 [Google Scholar]
- 19.Leonard J. L. 2006. Sexual selection: lessons from hermaphrodite mating systems. Integr. Comp. Biol. 46, 349–367 10.1093/icb/icj041 (doi:10.1093/icb/icj041) [DOI] [PubMed] [Google Scholar]
- 20.Jarne P., Auld J. R. 2006. Animals mix it up too: the distribution of self-fertilization among hermaphroditic animals. Evolution 60, 1816–1824 [DOI] [PubMed] [Google Scholar]
- 21.Avise J. C., Mank J. E. 2009. Evolutionary perspectives on hermaphroditism in fishes. Sex. Dev. 3, 152–163 10.1159/000223079 (doi:10.1159/000223079) [DOI] [PubMed] [Google Scholar]
- 22.Anthes N., Michiels N. K. 2007. Precopulatory stabbing, hypodermic injections and unilateral copulations in a hermaphroditic sea slug. Biol. Lett. 3, 121–124 10.1098/rsbl.2006.0596 (doi:10.1098/rsbl.2006.0596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koene J. M. 2006. Tales of two snails: sexual selection and sexual conflict in Lymnaea stagnalis and Helix aspersa. Integr. Comp. Biol. 46, 419–429 10.1093/icb/icj040 (doi:10.1093/icb/icj040) [DOI] [PubMed] [Google Scholar]
- 24.Michiels N. K., Newman L. J. 1998. Sex and violence in hermaphrodites. Nature 391, 647. 10.1038/35527 (doi:10.1038/35527) [DOI] [Google Scholar]
- 25.Morgan M. T. 1994. Models of sexual selection in hermaphrodites, especially plants. Am. Nat. 144, S100–S125 10.1086/285655 (doi:10.1086/285655) [DOI] [Google Scholar]
- 26.Lankinen Å., Larsson M. C. 2009. Conflicting selection pressures on reproductive functions and speciation in plants. Evol. Ecol. 23, 147–157 10.1007/s10682-007-9227-z (doi:10.1007/s10682-007-9227-z) [DOI] [Google Scholar]
- 27.Morgan M. T., Schoen D. J. 1997. Selection on reproductive characters: floral morphology in Asclepias syriaca. Heredity 79, 433–441 10.1038/hdy.1997.178 (doi:10.1038/hdy.1997.178) [DOI] [Google Scholar]
- 28.Delph L. F., Ashman L. 2006. Trait selection in flowering plants: how does sexual selection contribute? Integr. Comp. Biol. 46, 465–472 10.1093/icb/icj038 (doi:10.1093/icb/icj038) [DOI] [PubMed] [Google Scholar]
- 29.Campbell D. R. 1989. Measurements of selection in a hermaphroditic plant: variation in male and female pollination success. Evolution 43, 318–334 10.2307/2409210 (doi:10.2307/2409210) [DOI] [PubMed] [Google Scholar]
- 30.Wilson P. 1995. Selection for pollination success and the mechanical fit of Impatiens flowers around bumblebee bodies. Biol. J. Linn. Soc. 55, 355–383 [Google Scholar]
- 31.Anthes N., Putz A., Michiels N. K. 2006. Sex role preferences, gender conflict and sperm trading in simultaneous hermaphrodites: a new framework. Anim. Behav. 72, 1–12 10.1016/j.anbehav.2005.09.017 (doi:10.1016/j.anbehav.2005.09.017) [DOI] [Google Scholar]
- 32.Morgan M. T. 1992. The evolution of traits influencing male and female fertility in outcrossing plants. Am. Nat. 139, 1022–1051 10.1086/285371 (doi:10.1086/285371) [DOI] [Google Scholar]
- 33.Kidwell J. F., Clegg M. T., Stewart F. M., Prout T. 1977. Regions of stable equilibria for models of differential selection in the two sexes under random mating. Genetics 85, 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schärer L. 2009. Tests of sex allocation theory in simultaneously hermaphroditic animals. Evolution 63, 1377–1405 10.1111/j.1558-5646.2009.00669.x (doi:10.1111/j.1558-5646.2009.00669.x) [DOI] [PubMed] [Google Scholar]
- 35.Hodgkin J., Barnes T. M. 1991. More is not better: brood size and population growth in a self-fertilizing nematode. Proc. R. Soc. Lond. B 246, 19–24 10.1098/rspb.1991.0119 (doi:10.1098/rspb.1991.0119) [DOI] [PubMed] [Google Scholar]
- 36.Michiels N. K., Koene J. M. 2006. Sexual selection favors harmful mating in hermaphrodites more than in gonochorists. Integr. Comp. Biol. 46, 473–480 10.1093/icb/icj043 (doi:10.1093/icb/icj043) [DOI] [PubMed] [Google Scholar]
- 37.Preece T., Mao Y., Garrahan J. P., Davison A. 2009. Harmful mating tactics in hermaphrodites. Am. Nat. 173, 632–639 10.1086/597377 (doi:10.1086/597377) [DOI] [PubMed] [Google Scholar]
- 38.Smolensky N., Romero M. R., Krug P. J. 2009. Evidence for costs of mating and self-fertilization in a simultaneous hermaphrodite with hypodermic insemination, the Opisthobranch Alderia willowi. Biol. Bull. 216, 188–199 10.2307/25470740 (doi:10.2307/25470740) [DOI] [PubMed] [Google Scholar]
- 39.Sprenger D., Faber J., Michiels N. K., Anthes N. 2008. Natural female mating rate maximizes hatchling size in a marine invertebrate. J. Anim. Ecol. 77, 696–701 10.1111/j.1365-2656.2008.01376.x (doi:10.1111/j.1365-2656.2008.01376.x) [DOI] [PubMed] [Google Scholar]
- 40.Arista M., Ortiz P. L. 2010. Differential gender selection on floral size: an experimental approach using Cistus salvifolius. J. Ecol. 95, 973–982 10.1111/j.1365-2745.2007.01276.x (doi:10.1111/j.1365-2745.2007.01276.x) [DOI] [Google Scholar]
- 41.Greeff J. M., Michiels N. K. 1999. Sperm digestion and reciprocal sperm transfer can drive hermaphrodite sex allocation to equality. Am. Nat. 153, 421–430 10.1086/303184 (doi:10.1086/303184) [DOI] [PubMed] [Google Scholar]
- 42.Sinervo B., Lively C. M. 1996. The rock–paper–scissors game and the evolution of alternative male strategies. Nature 380, 240–243 10.1038/380240a0 (doi:10.1038/380240a0) [DOI] [Google Scholar]
- 43.Svensson E. I., Abbott J., Härdling R. 2005. Female polymorphism, frequency dependence and rapid evolutionary dynamics in natural populations. Am. Nat. 165, 567–576 10.1086/429278 (doi:10.1086/429278) [DOI] [PubMed] [Google Scholar]
- 44.Calsbeek R., Sinervo B. 2004. Within-clutch variation in offspring sex determined by differences in sire body size: cryptic mate choice in the wild. J. Evol. Biol. 17, 464–470 10.1046/j.1420-9101.2003.00665.x (doi:10.1046/j.1420-9101.2003.00665.x) [DOI] [PubMed] [Google Scholar]
- 45.Schärer L., Karlsson L. M., Christen M., Wedekind C. 2001. Size-dependent sex allocation in a simultaneous hermaphrodite parasite. J. Evol. Biol. 14, 55–67 10.1046/j.1420-9101.2001.00263.x (doi:10.1046/j.1420-9101.2001.00263.x) [DOI] [PubMed] [Google Scholar]
- 46.Schärer L., Sandner P., Michiels N. K. 2005. Trade-off between male and female allocation in the simultaneously hermaphroditic flatworm Macrostomum sp. J. Evol. Biol. 18, 396–404 10.1111/j.1420-9101.2004.00827.x (doi:10.1111/j.1420-9101.2004.00827.x) [DOI] [PubMed] [Google Scholar]
- 47.Janicke T., Schärer L. 2009. Sex allocation predicts mating rate in a simultaneous hermaphrodite. Proc. R. Soc. B 276, 4247–4253 10.1098/rspb.2009.1336 (doi:10.1098/rspb.2009.1336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weeks S. C., Benvenuto C., Sanderson T. F., Duff R. J. 2010. Sex chromosome evolution in the clam shrimp, Eulimnadia texana. J. Evol. Biol. 23, 1100–1106 10.1111/j.1420-9101.2010.01963.x (doi:10.1111/j.1420-9101.2010.01963.x) [DOI] [PubMed] [Google Scholar]
- 49.Fry J. D. 2009. The genomic location of sexually antagonistic variation: some cautionary comments. Evolution 64, 1510–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Visser J. A. G. M., ter Maat A., Zonneveld C. 1994. Energy budgets and reproductive allocation in the simultaneous hermaphrodite pond snail, Lymnaea stagnalis (L.): a trade-off between male and female function. Am. Nat. 144, 861–867 [Google Scholar]
- 51.Koene J. M., ter Maat A. 2004. Energy budgets in the simultaneously hermaphroditic pond snail, Lymnaea stagnalis: a trade-off between growth and reproduction during development. Belgian J. Zool. 134, 41–45 [Google Scholar]
- 52.Yund P. O., Marcum Y., Stewart-Savage J. 1997. Life-history variation in a colonial ascidian: broad-sense heritabilities and tradeoffs in allocation to asexual growth and male and female reproduction. Biol. Bull. 192, 290–299 10.2307/1542722 (doi:10.2307/1542722) [DOI] [PubMed] [Google Scholar]
- 53.Fedorka K. M., Mousseau T. A. 2004. Female mating bias results in conflicting sex-specific offspring fitness. Nature 429, 65–67 10.1038/nature02492 (doi:10.1038/nature02492) [DOI] [PubMed] [Google Scholar]
- 54.Koene J. M., ter Maat A. 2001. ‘Allohormones’: a class of bioactive substances favoured by sexual selection. J. Comp. Physiol. A 187, 323–326 10.1007/s003590100214 (doi:10.1007/s003590100214) [DOI] [PubMed] [Google Scholar]
- 55.Koene J. M., Sloot W., Montagne-Wajer K., Cummins S. F., Degnan B. M., Smith J. S., Nagle G. T., ter Maat A. 2010. Male accessory gland protein reduces egg laying in a simultaneous hermaphrodite. PLoS ONE 5, e10117. 10.1371/journal.pone.0010117 (doi:10.1371/journal.pone.0010117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charnov E. L. 1979. Simultaneous hermaphroditism and sexual selection. Proc. Natl Acad. Sci. USA 76, 2480–2484 10.1073/pnas.76.5.2480 (doi:10.1073/pnas.76.5.2480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charnov E. L. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 58.Clark W. C. 1978. Hermaphroditism as a reproductive strategy for metazoans. New Zealand J. Zool. 5, 769–780 [Google Scholar]
- 59.Eppley S. M., Jesson L. K. 2008. Moving to mate: the evolution of separate and combined sexes in multicellular organisms. J. Evol. Biol. 21, 727–736 10.1111/j.1420-9101.2008.01524.x (doi:10.1111/j.1420-9101.2008.01524.x) [DOI] [PubMed] [Google Scholar]
- 60.Ghiselin M. T. 1969. The evolution of hermaphroditism among animals. Q. Rev. Biol. 44, 189–208 10.1086/406066 (doi:10.1086/406066) [DOI] [PubMed] [Google Scholar]
- 61.Heller J. 1993. Hermaphroditism in molluscs. Biol. J. Linn. Soc. 48, 19–42 10.1111/j.1095-8312.1993.tb00874.x (doi:10.1111/j.1095-8312.1993.tb00874.x) [DOI] [Google Scholar]
- 62.Iyer P., Roughgarden J. 2008. Dioecy as a specialization promoting sperm delivery. Evol. Ecol. Res. 10, 867–892 [Google Scholar]
- 63.Michiels N. K., Crowley P. H., Anthes N. 2009. Accessory male investment can undermine the evolutionary stability of simultaneous hermaphroditism. Biol. Lett. 5, 709–712 10.1098/rsbl.2009.0280 (doi:10.1098/rsbl.2009.0280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press [Google Scholar]
- 65.Abbott J. K., Bedhomme S., Chippindale A. K. In press Sexual conflict in wing size and shape in Drosophila melanogaster. J. Evol. Biol. [DOI] [PubMed] [Google Scholar]
- 66.Munday P. L., Buston P. M., Warner R. R. 2006. Diversity and flexibility of sex-change strategies in animals. Trends Ecol. Evol. 21, 89–95 10.1016/j.tree.2005.10.020 (doi:10.1016/j.tree.2005.10.020) [DOI] [PubMed] [Google Scholar]
- 67.Williams G. C. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 10.2307/2406060 (doi:10.2307/2406060) [DOI] [Google Scholar]
- 68.Reed T. E., Kruuk L. E. B., Wanless S., Frederiksen M., Cunningham E. J. A., Harris M. P. 2008. Reproductive senescence in a long-lived seabird: rates of decline in late-life performance are associated with varying costs of early reproduction. Am. Nat. 171, 89–101 [DOI] [PubMed] [Google Scholar]
- 69.Snoke M. S., Promislow D. E. L. 2003. Quantitative genetic tests of recent senescence theory: age-specific mortality and male fertility in Drosophila melanogaster. Heredity 91, 546–556 10.1038/sj.hdy.6800353 (doi:10.1038/sj.hdy.6800353) [DOI] [PubMed] [Google Scholar]
- 70.Escobar J. S., Jarne P., Charmantier A., David P. 2008. Outbreeding alleviates senescence in hermaphroditic snail as expected from the mutation-accumulation theory. Curr. Biol. 18, 906–910 10.1016/j.cub.2008.04.070 (doi:10.1016/j.cub.2008.04.070) [DOI] [PubMed] [Google Scholar]
- 71.Baeza J. A. 2007. Sex allocation in a simultaneously hermaphroditic marine shrimp. Evolution 61, 2360–2373 10.1111/j.1558-5646.2007.00199.x (doi:10.1111/j.1558-5646.2007.00199.x) [DOI] [PubMed] [Google Scholar]
- 72.Petersen C. W., Fischer E. A. 1996. Intra-specific variation in sex allocation in a simultaneous hermaphrodite: the effect of individual size. Evolution 50, 636–645 10.2307/2410837 (doi:10.2307/2410837) [DOI] [PubMed] [Google Scholar]
- 73.Visozo D. B., Schärer L. 2007. Resource-dependent sex-allocation in a simultaneous hermaphrodite. J. Evol. Biol. 20, 1046–1055 [DOI] [PubMed] [Google Scholar]
- 74.Innocenti P., Morrow E. H. In press. A joint index for the intensity of sex-specific selection. Evolution. 10.1111/j.1558-5646.2010.01021.x (doi:10.1111/j.1558-5646.2010.01021.x) [DOI] [PubMed] [Google Scholar]
- 75.Bedhomme S., Prasad N. G., Jiang P.-P., Chippindale A. K. 2008. Reproductive behavior evolves rapidly when intra-locus sexual conflict is removed. PLoS ONE 3, e2187. 10.1371/journal.pone.0002187 (doi:10.1371/journal.pone.0002187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rice W. R. 1996. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381, 232–234 10.1038/381232a0 (doi:10.1038/381232a0) [DOI] [PubMed] [Google Scholar]
- 77.Goodnight C. J. 1990. Experimental studies of community evolution I: the response to selection at the community level. Evolution 44, 1614–1624 10.2307/2409341 (doi:10.2307/2409341) [DOI] [PubMed] [Google Scholar]
- 78.Charlesworth B., Charlesworth D. 1978. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997 10.1086/283342 (doi:10.1086/283342) [DOI] [Google Scholar]
- 79.Charlesworth D., Charlesworth B., Marais G. 2005. Steps in the evolution of heteromorphic sex chromosomes. Heredity 95, 118–128 10.1038/sj.hdy.6800697 (doi:10.1038/sj.hdy.6800697) [DOI] [PubMed] [Google Scholar]
- 80.Weeks S. C., Benvenuto C., Reed S. K. 2006. When males and hermaphrodites coexist: a review of androdioecy in animals. Integr. Comp. Biol. 46, 449–464 10.1093/icb/icj048 (doi:10.1093/icb/icj048) [DOI] [PubMed] [Google Scholar]