Abstract
The question of whether animals possess ‘cultures’ or ‘traditions’ continues to generate widespread theoretical and empirical interest. Studies of wild chimpanzees have featured prominently in this discussion, as the dominant approach used to identify culture in wild animals was first applied to them. This procedure, the ‘method of exclusion,’ begins by documenting behavioural differences between groups and then infers the existence of culture by eliminating ecological explanations for their occurrence. The validity of this approach has been questioned because genetic differences between groups have not explicitly been ruled out as a factor contributing to between-group differences in behaviour. Here we investigate this issue directly by analysing genetic and behavioural data from nine groups of wild chimpanzees. We find that the overall levels of genetic and behavioural dissimilarity between groups are highly and statistically significantly correlated. Additional analyses show that only a very small number of behaviours vary between genetically similar groups, and that there is no obvious pattern as to which classes of behaviours (e.g. tool-use versus communicative) have a distribution that matches patterns of between-group genetic dissimilarity. These results indicate that genetic dissimilarity cannot be eliminated as playing a major role in generating group differences in chimpanzee behaviour.
Keywords: culture, social learning, genetics, chimpanzees, Pan troglodytes
1. Introduction
The importance of group-specific, socially transmitted behaviour to the lives of non-human animals, and its relevance to the evolution of human culture, is a matter of considerable controversy [1–4]. As our closest living relatives and the most intensively studied non-human primate in the wild, chimpanzees have played a particularly important role in the study of culture in non-human animals [5–8]. A landmark survey of geographical variation in chimpanzee behaviour found 39 behaviours, including various forms of tool use, grooming, and courtship behaviour, that were common in at least one chimpanzee group but absent in at least one other, and for which an ecological explanation for this absence was judged to be unlikely, e.g. a lack of ‘termite fishing’ even though the appropriate termite species was present [9,10]. This so-called ‘method of exclusion’, which infers the existence of culture by eliminating ecological explanations for the patterning of between-group behavioural variation, has since been applied to several other primate and non-primate species, and has until recently been the dominant approach used to identify animal culture in the wild [11–14].
In a pattern reminiscent of between-group variation in human behaviour, each chimpanzee group had its own unique combination of putative cultural variants. This complex, mosaic pattern of between-group variation in behaviour was different than that observed in prior studies of animal culture, where in most cases only one type of behaviour varied among groups (although this may be a result of the fact that usually only one type of behaviour was being investigated, e.g. bird song dialects; [15]). Although other species have now been discovered to show patterns of behavioural diversity that approach levels displayed by chimpanzees [14], for many, chimpanzees remain the prime exemplar of animal culture [1,16,17].
Support for the hypothesis that some of the geographical variation in the behaviour of chimpanzees and other animals may represent socially learned cultural variation comes from three main areas. First, research conducted in captivity suggests that chimpanzees and other animals have the requisite social-learning abilities to produce stable differences in group-specific behaviour [18–25]. In one representative experiment [18], two chimpanzees were isolated from their respective groups and trained in different tool using techniques to gain access to food in an experimental apparatus. These two ‘demonstrators’ were then re-introduced into their original groups. The behaviour of individuals in each group was then compared with each other and with the behaviour of chimpanzees in a no-demonstrator control group. The majority of chimpanzees in the two experimental groups employed the technique of their demonstrator, while chimpanzees in the control group did not typically obtain access to the food. Second, longitudinal naturalistic observations of wild chimpanzees [26] and capuchin monkeys [27,28] show that individuals which spend more time together develop similar techniques for particular extractive foraging tasks, suggesting that social learning plays a role in skill development. And third, as would be predicted if more exposure to models results in more opportunities for social learning, between-site comparisons in wild chimpanzees and orangutans indicate that the number of behavioural variants found at a site is positively correlated with the amount of time individuals spend associating with conspecifics [14,29].
While results such as these indicate that the social learning abilities of chimpanzees and other primates may be sufficient to produce differences in the behaviour of individuals within and between groups, they do not necessarily indicate that all or even most of the behavioural variants observed in the wild arise as a result of social learning, and thus represent true cultural variants [3,7,17,30,31]. In addition to the difficulty of determining whether ecological explanations can ever be definitively excluded as a source of behavioural variation, some critics argue that insufficient attention has been paid to the possibility that genetic differences are responsible for behavioural variation between groups of chimpanzees and other animals [2,4,30,32,33]. Recent research shows that individuals raised with no opportunity to acquire a suspected socially learnt behaviour exhibit sophisticated abilities to use tools (finches and crows; [34,35]) and process foods (gorillas; [36]). Similarly, two naive captive-born chimpanzees presented with rough bristly leaves spontaneously folded and swallowed them in the same way that sick chimpanzees do in the wild (probably in order to expel intestinal parasites; [37]). Together, these results suggest that complex behaviours can be at least partly under genetic control and that group differences in underlying genetic predispositions and abilities could plausibly contribute to group differences in behaviour. Indeed, almost one-third of the putative cultural variants in the original report [9] of geographical variation in chimpanzee behaviour are found only in Pan troglodytes verus, the most genetically divergent of the four chimpanzee subspecies and considered by some to represent a different species [38,39]. Genetic dissimilarity within subspecies may even play a role in generating between-group variation in chimpanzee behaviour. The east African subspecies Pan troglodytes schweinfurthii, for example, shows as much geographical structuring of genetic variation as does the entire human species [40], and genetically influenced variation in body size has been hypothesized to contribute to differences between the calls produced by chimpanzees in two groups belonging to the same east African subspecies [41].
In an indirect investigation of this issue, Lycett et al. [42,43] reasoned that if the patterning of intergroup variation in behaviour is primarily the product of genetic differences between groups, then a tree of the relationships between chimpanzee groups generated from a cladistic analysis of the 39 putative cultural variants should display more phylogenetic structure when two subspecies are considered together than a tree generated from the east African subspecies alone. They failed to find more phylogenetic structure in the two-subspecies than the single-subspecies tree, and thus concluded that the patterning of between-group variation in chimpanzee behaviour fit a cultural explanation better than a genetic one. This study, however, has at least three major limitations. First, as acknowledged by Lycett et al. [43], at present there is no well-accepted methodology for determining whether the difference in the extent of phylogenetic structure in two different trees is statistically significant. Second, a recent simulation study on trees generated with varying levels of horizontal versus vertical transmission has shown that measures of phylogenetic structure are very sensitive to rates of evolution [44]. If the rate of evolution is sufficiently high, then phylogenetic structure measured for a subset of the branches of a tree can be higher than when measured for the entire tree, even when transmission is completely vertical (i.e. as in genetic transmission), calling the central assumption of the analysis by Lycett et al. [42,43] into question. Finally, the method employed by Lycett et al. [42,43] purports to test only whether genetics can be excluded as a cause for overall levels of similarity in the behavioural repertoires of chimpanzee groups; it furnishes no insight into how strongly the distribution of each of the individual behavioural variants follows or fails to follow patterns of between-group genetic dissimilarity. For example, some authors have suggested that differences in non-vocal communicative behaviours are unlikely to result from differences in underlying genetic predispositions, as these will not be influenced as much as other classes of behaviour that vary according to local ecological conditions and are affected by the operation of natural selection, e.g. tool-use in a foraging context [7,45].
Here, we take a more direct approach to the question of whether genetics plays a role in generating intergroup variation in the behaviour of wild chimpanzees by measuring levels of genetic dissimilarity among nine groups of wild chimpanzees and comparing these with patterns of between-group behavioural variation. We examine the relationship between genetic dissimilarity and both patterns of overall behavioural dissimilarity between groups and the distribution of individual behavioural variants.
2. Material and methods
(a). Study groups and genetic analyses
Of the nine chimpanzee groups we studied, three belonged to the west African P. t. verus and six belonged to the east African P. t. schweinfurthii subspecies. We sequenced most of the members of each of the nine groups at the first hypervariable region of the maternally inherited mitochondrial (mt)DNA (354 bases; numbers of adolescent and adult individuals sequenced/number of adolescent and adult group members present at time of sampling in brackets): the Bossou group (12/12) of Guinea, the North (8/11) and South (16/26) groups of Taï National Park, Cote d'Ivoire, the Sonso group (28/28) of Budongo Forest Reserve, Uganda, the Ngogo (89/89) and Kanyawara (20/20) groups of Kibale National Park, Uganda, the M (32/32) and K (3/Unknown) groups of Mahale Mountains National Park, Tanzania and the Kasekela (38/38) group of Gombe Stream National Park, Tanzania. MtDNA sequences for five of the groups were previously published: Bossou [46], Sonso [47,48], Gombe [49], Ngogo [47,48] and Kanyawara [47,48]. We generated new mtDNA sequences for the Taï North and South groups and the Mahale M and K groups, following previously described procedures [47,48]. Although mtDNA is unlikely to directly code for any of the behavioural variants considered here, it is an excellent marker for determining overall levels of genetic similarity among groups, particularly in species such as chimpanzees where females rather than males move between groups. This is supported by the strong correlation between mtDNA genetic distance and geographical distance among the nine chimpanzee groups included in this study (Pearson's r = 0.96, p = 0.00005, n = 36 pairs of groups).
We calculated AMOVA and pairwise FST values between each of the nine groups (i.e. 36 pairwise comparisons) using Arlequin 3.1 [50], with genetic distances between haplotypes measured using the number of nucleotide differences, and statistical significance assessed by comparison with genetic dissimilarity calculated on random datasets where mtDNA haplotypes were permuted among groups. We set the FST values of two pairs of groups which showed negative values (Mahale M and K = −0.18, Taï North and South =− 0.06) to zero, as is common practice when negative values are suspected to result from sampling error rather than indicating more genetic dissimilarity among individuals of the same group than of individuals between groups [50]. We found only minor quantitative changes in our results depending on whether we set FST as 0 or used the original negative values for these two pairs.
(b). Behavioural analyses
We converted the behavioural data from the original study of geographical variation in chimpanzee behaviour [9], as well as similar data from the two chimpanzee groups newly coded for this study (i.e. the South group at Taï and the Ngogo group at Kibale), into numerical codes in order to assess how frequently the 39 behaviours occurred in each chimpanzee group. For six of the chimpanzee groups in this study (Bossou, Sonso, Kanyawara, Mahale M and K, Kasekela), we used the behavioural codes as described in the original paper on geographical variation in chimpanzee behaviour [9,10]. For Taï North, we changed three codes from the original paper, as one of us (C.B., the director of this field site) decided that these new codes were more accurate: ‘hand-clasp’ was changed from habitual to present, ‘knuckle-knock’ was changed from customary to habitual and ‘branch-slap’ was changed from customary to absent. We repeated all analyses using the original Taï North codes and found only minor quantitative changes in the results. The Taï South group, newly coded for this study by C.B., had the same codes as Taï North, with the following two exceptions: ‘bee-probe’ was coded as present and ‘marrow-pick’ as habitual. Ngogo, newly coded by J.M. and K.L. for this study, had the same codes as Kanyawara, with the following two exceptions: ‘seat-vegetation’ was coded as habitual, and ‘leaf-clip, mouth’ was coded as customary. The behavioural variant ‘branch-din’ was excluded from all analyses because it was absent in all nine of the chimpanzee groups included in this study. Thus, our study examined only 38 of the 39 putative cultural variants described in the original study on geographical variation in chimpanzee behaviour [9,10]. We made no attempt to include behaviours now known or suspected to vary between chimpanzee groups that were not included in this original study, e.g. [51].
We converted the behavioural codes into numbers as follows: (3) ‘customary’, the behaviour occurs in all or most able-bodied members of at least one age–sex class (such as adult males); (2) ‘habitual’, the behaviour is not customary but has occurred repeatedly in several individuals, consistent with some degree of social transmission; (1) ‘present’, the behaviour is neither customary nor habitual but is clearly identified; (0) ‘absent’, the behaviour has not been recorded and no ecological explanation is apparent; (?) ‘ecological explanation’, absence is explicable because of a local ecological feature; and (?) ‘unknown’, the behaviour has not been recorded, but this may be owing to inadequacy of relevant observational opportunities. Both of the (?) codes were treated as missing values in our quantitative analyses. Our numerical coding system differs from that used in the study of Lycett et al. [42,43], in that these authors did not distinguish between behavioural variants that were absent and those that were absent owing to an ecological reason.
(c). Comparing overall behavioural dissimilarity and genetic dissimilarity
We used these 38 numerical codes to calculate overall behavioural dissimilarity among each of the 36 pairs of groups using two different distance measures: (i) normalized Hamming distance, the number of behavioural variants with a different code, divided by the number of behavioural variants for which the pair of groups was compared, and (ii) Manhattan distance, the sum of the absolute values of the distances between the behavioural variants for a pair of groups. When calculating overall behavioural dissimilarity, behavioural variants with missing values were excluded pairwise rather than listwise (e.g. if for behavioural variant 1 group A had a code of ‘customary’, group B ‘habitual’, and group C ‘unknown’, then behavioural variant 1 would be excluded from the calculations of overall behavioural dissimilarity between groups A and B with C, but not from group A with B). We compared overall behavioural dissimilarity with genetic dissimilarity (FST) using Pearson correlation coefficients. To account for the fact that each of the nine groups appeared multiple times among the 36 pairs of groups, which thus do not represent independent data points, we assessed the statistical significance of the Pearson-test statistic through Mantel matrix permutation [52]. To account for the possibility that outliers might have an undue influence on the result, we also performed Spearman rank correlations with Mantel matrix permutation.
Given that the behavioural codes for each chimpanzee group were determined retrospectively based on the opinions of researchers rather than through a prospective, systematic study of how many individuals performed each of the behavioural variants, the reliability and validity of the coding system used in the original chimpanzee culture study is unclear. We thus performed two additional analyses to assess the robustness of our main analysis of the relationship between overall behavioural dissimilarity and genetic dissimilarity. In the first, we systematically introduced varying amounts of error into the behavioural codes and then re-examined the size and statistical significance of the correlation between overall behavioural dissimilarity and genetic dissimilarity. In the second, we recoded the behavioural data binarily such that the measure of overall behavioural dissimilarity measure was insensitive to the specific levels chosen for the behavioural traits, then re-examined the size and statistical significance of the correlation between overall behavioural dissimilarity and genetic dissimilarity. See the electronic supplementary material for further details.
While a strong correlation between genetic and overall behavioural dissimilarity would indicate that genetic dissimilarity cannot be excluded as playing a major role in the patterning of behavioural variation among chimpanzee groups, it would not necessarily exclude social learning. If emigrating females carry the behavioural variants of their natal group to their new group [53,54], then groups that share many migrants are likely to be behaviourally similar. High migration rates between groups could thus result in a positive correlation between genetic and behavioural dissimilarity, even if behavioural variants were entirely socially learned. Thus, it is only when patterns of behavioural and genetic dissimilarity are discordant that inferences can be made about the role of social learning in generating geographical variation in behaviour.
(d). Behavioural variation between pairs of genetically similar groups
One way to approach this issue is to examine behavioural variation between groups that are genetically similar. In other words, to compare groups in which genetic dissimilarity is so low that it is unlikely to generate differences in their behaviour. Given our lack of knowledge of the specific genetic loci that influence the behaviours considered here, it is impossible to determine definitively what this level of genetic dissimilarity would be. Therefore, we conducted two additional analyses to address this issue, using different criteria for classifying pairs of groups as genetically similar. We started with the method of Whiten et al. [9,10] and identified potential cultural variants as behaviours that were common (i.e. ‘customary’ or ‘habitual’) in one community but absent in another. In the first analysis, we limited our comparisons to groups for which we had strong statistical evidence that genetic dissimilarity between them was not significant. Here we classified the following pairs of groups as genetically similar: Mahale K–Mahale M, Ngogo–Kanyawara, Taï North–Taï South, Bossou–Tai North, Bossou–Taï South. Although the permutation tests conducted in Arlequin indicated that the Mahale K group was not significantly dissimilar from several other non-neighbouring groups within East Africa, i.e. Ngogo, Kanyawara and Kasekela, we excluded these three pairs of groups from this analysis given the poor genetic sampling of the Mahale K group and the other results showing that the well-sampled Mahale M group, which is the direct neighbour of the Mahale K group and has the same set of mtDNA haplotypes, was significantly genetically dissimilar from these other three groups. We interpret results of this analysis cautiously because statistical measures of genetic dissimilarity depend not only on the amount of genetic dissimilarity between communities, but also on the number of individuals sampled and the number and type of loci examined.
In the second, more conservative analysis, we took advantage of the behavioural data we compiled from two new chimpanzee communities. Adding this information to published data from the seven communities in the original study [9] allowed us to make comparisons between pairs of groups located in the same block of continuous forest. Behavioural differences that emerge from this analysis are especially informative because the amount of genetic dissimilarity between groups exhibiting extensive contemporary gene flow is almost certainly insufficient to generate differences in behaviour. Here we included the same pairs of groups as above, minus Bossou–Tai North and Bossou–Tai South.
(e). Genetic dissimilarity and the distribution of the individual behavioural variants
In our final analysis we generated a measure of between-group dissimilarity for each of the individual behavioural variants and investigated the relationships between these and the measure of between-group genetic dissimilarity. Here we created a matrix consisting of the absolute value of the difference in the numerical code between each of the communities, such that communities which were similar for the behavioural variant had a score of 0 (i.e. both had the same code), and communities that were very different for the behavioural variant had a score of 3 (i.e. ‘habitual’ in one group and ‘absent’ in the other). Behavioural variants with missing values were excluded pairwise rather than listwise. We then calculated a Spearman rank correlation matrix permutation test between each of these 38 matrices and a matrix of genetic dissimilarity (FST). The combination of a relatively modest sample size (n = 6 – 36 pairs of groups, depending on the number of groups that had missing values for that particular behavioural variant) and the stringent alpha level (e.g. in this case, a Bonferroni correction of 0.05/38 = 0.001) that is required to correct for multiple comparisons means that there is very low power to reject the null hypothesis that the distribution of each behavioural variant is not predicted by genetic dissimilarity. Thus, we use this analysis only to explore whether there are any general patterns as to which types of behavioural variants are more or less strongly predicted by patterns of genetic dissimilarity.
We used 10 000 Mantel permutations in all of our statistical tests.
3. Results
(a). Comparing overall behavioural dissimilarity and genetic dissimilarity
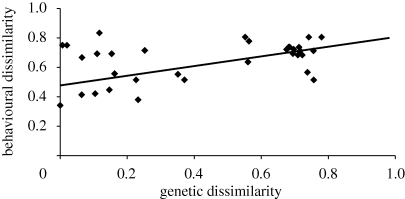
We found that the overall level of genetic dissimilarity among the nine groups was substantial (AMOVA value = 0.49, p = 0.00001), and most pairs of groups were significantly genetically dissimilar from one another (table 1). Levels of genetic dissimilarity and overall behavioural dissimilarity between chimpanzee groups were strongly and significantly correlated, regardless of the method used to calculate overall behavioural dissimilarity (normalized Hamming distance: Pearson's r = 0.52, p = 0.015; Spearman's rs = 0.37, p = 0.031; Manhattan distance: Pearson's r = 0.44, p = 0.018; Spearman's rs = 0.36, p = 0.029; n = 36 for all tests; see figure 1). This relationship approached statistical significance even after excluding the five pairs of groups with strong evidence for no significant genetic dissimilarity (normalized Hamming distance: Pearson's r = 0.48, p = 0.039; Spearman's rs = 0.37, p = 0.116; Manhattan distance: Pearson's r = 0.45, p = 0.091; Spearman's rs = 0.40, p = 0.123; n = 31 for all tests). Additional analyses showed that the relationship between overall behavioural dissimilarity and genetic dissimilarity is robust to error in the behavioural codes (see the electronic supplementary material).
Table 1.
Genetic dissimilarity (below diagonal) and overall behavioural dissimilarity (above diagonal) in 36 pairs of chimpanzee groups. (For genetic dissimilarity, pairs classified as having statistically significant genetic dissimilarity are shown in bold and pairs living in the same block of continuous forest are shown in italics (see main text for details). For overall behavioural dissimilarity, values to the left of the backslash are normalized Hamming distances, and values to the right are Manhattan distances.)
| Bossou | Taï North | Taï South | Kibale Ngogo | Kibale Kanyawara | Budongo Sonso | Gombe Kasekela | Mahale K | Mahale M | |
|---|---|---|---|---|---|---|---|---|---|
| Bossou | 0.75/55 | 0.75/52 | 0.69/36 | 0.69/36 | 0.57/30 | 0.74/58 | 0.64/47 | 0.51/35 | |
| Taï North | 0.02 | 0.05/3 | 0.68/35 | 0.74/37 | 0.71/46 | 0.72/53 | 0.81/51 | 0.81/53 | |
| Taï South | 0.01 | 0 | 0.68/32 | 0.74/34 | 0.71/43 | 0.72/50 | 0.78/48 | 0.81/50 | |
| Kibale Ngogo | 0.71 | 0.72 | 0.71 | 0.05/2 | 0.42/27 | 0.69/34 | 0.41/22 | 0.38/22 | |
| Kibale Kanyawara | 0.69 | 0.71 | 0.68 | 0.02 | 0.45/27 | 0.69/32 | 0.56/22 | 0.55/22 | |
| Budongo Sonso | 0.84 | 0.86 | 0.72 | 0.10 | 0.15 | 0.83/51 | 0.52/34 | 0.52/35 | |
| Gombe Kasekela | 0.68 | 0.70 | 0.68 | 0.11 | 0.15 | 0.12 | 0.67/44 | 0.71/45 | |
| Mahale K | 0.56 | 0.55 | 0.56 | 0.06 | 0.16 | 0.23 | 0.07 | 0.34/20 | |
| Mahale M | 0.76 | 0.78 | 0.74 | 0.23 | 0.35 | 0.37 | 0.25 | 0 |
Figure 1.
The relationship between levels of overall behavioural dissimilarity and levels of genetic dissimilarity in 36 pairs of wild chimpanzee groups. Overall behavioural dissimilarity calculated using normalized Hamming distance.
(b). Behavioural variation between pairs of genetically similar groups
In the first analysis of behavioural variation among genetically similar groups, where we limited comparisons to groups for which we had strong evidence of no statistically significant genetic dissimilarity, we found that genetic differences could be excluded as an explanation for approximately half of the behavioural variants displayed between them (20/38 = 52.6%; see table 2), with most non-excluded behaviours coming from comparisons between the group at Bossou and the Taï North and South groups. In the second, more conservative analysis of behavioural variation among genetically similar groups, where we limited comparisons to groups occupying the same block of continuous forest and thus experiencing high levels of contemporary gene flow, we found that a genetic explanation could be excluded for only five of the 38 behaviours (13.2%; table 2). All five of these were between the K and M groups at Mahale; there were no behaviours that were common in one group and absent in the other in the two comparisons involving groups within Kibale and within Taï.
Table 2.
Genetic dissimilarity and the distributions of the individual behavioural variants. (Shown for each of the individual behavioural variants are their classifications as given in the original study of geographical variation in chimpanzee behaviour [9], the number of groups compared in the correlation between genetic and behavioural dissimilarity, the Spearman's rs and p-values for this correlation, and whether it varies between genetically similar groups: criterion 1: groups classified as having no statistically significant genetic dissimilarity; criterion 2: groups occupying the same block of continuous forest. Behavioural variants are listed in ascending order of strength of positive correlation with genetic dissimilarity. See [10] for more detailed descriptions of the behavioural variants.)
| behavioural variant | classification | n | Spearman's rs | p | varies among non-genetically differentiated groups? |
|
|---|---|---|---|---|---|---|
| criterion 1 | criterion 2 | |||||
| termite-fish using leaf midrib | fishing actions | 6 | −0.62 | 0.26 | yes | yes |
| leaf-squash (squash ecto-parasite on leaf) | exploitation of leaf properties/grooming | 28 | −0.21 | 0.26 | no | no |
| termite-fish using non-leaf materials | fishing actions | 6 | −0.21 | 0.77 | yes | yes |
| self-tickle (tickle self using objects) | misc. exploitation of vegetation properties | 36 | −0.14 | 0.56 | no | no |
| leaf-clip, fingers (rip single leaf with fingers) | exploitation of leaf properties | 36 | −0.11 | 0.49 | yes | no |
| pestle-pound (mash palm crown with petiole) | pounding actions | 6 | −0.10 | 1.00 | yes | no |
| leaf-clip, mouth (rip parts off leaf, with mouth) | exploitation of leaf properties | 36 | −0.09 | 0.61 | no | no |
| ant-dip-wipe (manually wipe ants off wand) | fishing actions | 36 | −0.07 | 0.64 | no | no |
| leaf-inspect (inspect ecto-parasite on hand) | exploitation of leaf properties/grooming | 28 | −0.03 | 0.89 | no | no |
| leaf-dab (leaf dabbed on wound, examined) | exploitation of leaf properties | 36 | −0.03 | 0.82 | no | no |
| leaf-strip (rip leaves off stem, as threat) | exploitation of leaf properties | 36 | −0.02 | 0.89 | no | no |
| fly-whisk (leafy stick used to fan flies) | comfort behaviour | 21 | 0 | 0.98 | yes | no |
| food-pound onto other (e.g. stone) | pounding actions | 21 | 0 | 0.99 | yes | no |
| stem pull-through (pull stems noisily) | attention-getting | 36 | 0 | 0.97 | yes | yes |
| expel/stir stick expels or stirs insects) | forcing actions | 21 | 0.02 | 0.93 | yes | no |
| ant-fish (probe used to extract ants) | fishing actions | 36 | 0.03 | 0.78 | no | no |
| branch-slap (slap branch, for attention) | attention-getting | 36 | 0.03 | 0.82 | yes | no |
| aimed-throw (throw object directionally) | misc. exploitation of vegetation properties | 36 | 0.06 | 0.58 | yes | yes |
| shrub-bend (squash stems underfoot) | attention-getting | 36 | 0.07 | 0.62 | yes | yes |
| lever open (stick used to enlarge entrance) | forcing actions | 36 | 0.10 | 0.59 | yes | no |
| fluid-dip (use of probe to extract fluids) | probing actions | 36 | 0.14 | 0.31 | yes | no |
| bee-probe (disable bees, flick with probe) | probing actions | 36 | 0.15 | 0.36 | yes | no |
| knuckle-knock (knock to attract attention) | attention-getting | 36 | 0.16 | 0.28 | no | no |
| hand-clasp (clasp arms overhead, groom) | grooming | 36 | 0.18 | 0.20 | no | no |
| nut-hammer, wood hammer on wood anvil | pounding actions | 6 | 0.21 | 0.77 | yes | no |
| club (strike forcefully with stick) | pounding actions | 36 | 0.25 | 0.10 | no | no |
| leaf-napkin (leaves used to clean body) | exploitation of leaf properties | 36 | 0.25 | 0.10 | no | no |
| nut-hammer, other (e.g. on ground) | pounding actions | 15 | 0.28 | 0.21 | yes | no |
| nut-hammer, wood hammer on stone anvil | pounding actions | 15 | 0.28 | 0.21 | yes | no |
| food-pound onto wood (smash food) | pounding actions | 21 | 0.35 | 0.07 | no | no |
| index-hit (squash ecto-parasite on arm) | grooming | 36 | 0.35 | 0.05 | yes | no |
| marrow-pick (pick bone marrow out) | probing actions | 36 | 0.38 | 0.04 | yes | no |
| rain dance (slow display at start of rain) | no code | 36 | 0.39 | 0.03 | yes | no |
| seat-vegetation (large leaves as set) | comfort behaviour | 36 | 0.49 | 0.02 | no | no |
| nut-hammer, stone hammer on wood anvil | pounding actions | 6 | 0.59 | 0.17 | no | no |
| leaf-groom (intense 'grooming' of leaves) | exploitation of leaf properties | 36 | 0.72 | 0.01 | no | no |
| ant-dip-single (one handed dip stick on ants) | fishing actions | 36 | 0.84 | 0 | no | no |
| nut-hammer, stone hammer on stone anvil | pounding actions | 15 | 0.85 | 0 | no | no |
(c). Genetic dissimilarity and the distribution of the individual behavioural variants
Our Spearman rank correlation matrix permutation tests between genetic dissimilarity and behavioural dissimilarity for each of the behavioural variants showed that the individual behaviours showed considerable variation in how strongly their distributions were predicted by patterns of genetic dissimilarity (table 2). However, there was no obvious pattern regarding which types of behaviours had a distribution that was more or less strongly predicted by genetic dissimilarity. For example, ‘ant-dip-single’ and ‘ant-dip-wipe’, both described in the original study of chimpanzee cultural variation [9] as ‘fishing actions’, were among the behaviours whose distributions are the most and least strongly predicted, respectively, by genetic dissimilarity.
4. Discussion
We found that levels of overall behavioural dissimilarity and genetic dissimilarity between nine chimpanzee groups were strongly correlated, that few behaviours varied between groups classified as genetically similar according to our most conservative criteria, and that there was no obvious pattern as to which types of behaviours had a distribution that was more or less strongly predicted by patterns of between-group genetic dissimilarity. Together, these results indicate that genetic differences cannot be excluded as playing a major role in structuring patterns of behavioural variation among chimpanzee groups. These results do not, however, necessarily indicate that a substantial proportion of the behavioural variation between chimpanzees groups is not cultural in nature, but rather testify to the difficulty inherent in using the method of exclusion to identify the processes responsible for between-group behavioural variation. As others have noted, a strict application of the method of exclusion may lead to an underestimation of the true number of cultural variants that exist in the wild [2,7,17]. Indeed, if the method of exclusion were applied to humans, the strong correlations between behavioural and both genetic [55] and ecological [56] similarity would indicate that a considerable amount of human between-group diversity is not necessarily cultural in nature.
Mathematical methods to identify a statistical ‘signature’ of transmission of a behavioural variant through social learning represent a promising alternative to the method of exclusion for investigating culture in the wild for animals [57–59]. Such methods are especially apt for animals like chimpanzees, for whom definitive translocation experiments [60] are impossible for logistic and ethical reasons. Unfortunately, while we often have a good understanding of the distribution of a behaviour among the individuals within a group, the diffusion processes that led to that distribution are rarely observed [13]. Comparing patterns of behavioural variation with patterns of kinship among individuals within the same social group may be a more tractable way to investigate animal culture in the wild [61], particularly in species like chimpanzees where the relatively weak influence of kinship on patterns of affiliation [47,48,62] means that evidence for social learning (i.e. animals that interact with each other more frequently are more likely to share the behavioural variant) is not confounded with evidence for genetic inheritance (i.e. close relatives are more likely to share the behavioural variant). In summary, diverse methodologies will be essential to resolving the long-standing debate of the relative contributions of genetics, social learning and other factors in generating geographical variation in animal behaviour.
Acknowledgements
This research was approved by the Max Planck Institute for Evolutionary Anthropology.
Thanks to T. Deschner, M. Franz, S. Geidel, C. Rowney, M. Surbeck, C. Tennie and R. Kendal and the anonymous reviewers for discussions and comments on the manuscript, and to R. Mundry for statistical assistance. This research was supported by the Alexander von Humboldt Foundation, the Max Planck Society, the National Science Foundation (USA), the Leakey Foundation and the Wenner-Gren Foundation.
References
- 1.Boesch C. 2003. Is culture a golden barrier between human and chimpanzee? Evol. Anthropol. 12, 82–91 10.1002/evan.10106 (doi:10.1002/evan.10106) [DOI] [Google Scholar]
- 2.Laland K. N., Janik V. M. 2006. The animal cultures debate. Trends Ecol. Evol. 21, 542–547 10.1016/j.tree.2006.06.005 (doi:10.1016/j.tree.2006.06.005) [DOI] [PubMed] [Google Scholar]
- 3.Galef B. G. 2009. Culture in animals? In The question of animal culture (eds Lalande K. N., Galef B. G.), pp. 222–246 Cambridge, MA: Harvard University Press [Google Scholar]
- 4.Heyes C. M., Galef B. G. 1996. Social learning and imitation: the roots of culture. New York, NY: Academic Press [Google Scholar]
- 5.Goodall J. 1973. Cultural elements in a chimpanzee community. In Precultural primate behaviour (ed. Menzel E. W.), pp. 195–249 Basel, Switzerland: Karger [Google Scholar]
- 6.McGrew W. C. 1992. Chimpanzee material culture: implications for human evolution. Cambridge, UK: Cambridge University Press [Google Scholar]
- 7.Boesch C. B. 1996. The emergence of culture among wild chimpanzees. In Evolution of social behaviour patterns in primates and man (eds Runciman W. G., Maynard Smith J., Dunbar R. I. M.), pp. 251–268 Oxford, UK: Oxford University Press [Google Scholar]
- 8.Boesch C., Tomasello M. 1998. Chimpanzee and human cultures. Curr. Anthropol. 39, 591–614 10.1086/204785 (doi:10.1086/204785) [DOI] [Google Scholar]
- 9.Whiten A., Goodall J., McGrew W. C., Nishida T., Reynolds V., Sugiyama Y., Tutin C. E. G., Wrangham R. W., Boesch C. 1999. Cultures in chimpanzees. Nature 399, 682–685 10.1038/21415 (doi:10.1038/21415) [DOI] [PubMed] [Google Scholar]
- 10.Whiten A., Goodall J., McGrew W. C., Nishida T., Reynolds V., Sugiyama Y., Tutin C. E. G., Wrangham R. W., Boesch C. 2001. Charting cultural variation in chimpanzees. Behaviour 138, 1481–1516 10.1163/156853901317367717 (doi:10.1163/156853901317367717) [DOI] [Google Scholar]
- 11.Rendell L., Whitehead H. 2001. Culture in whales and dolphins. Behav. Brain Sci. 24, 309–382 [DOI] [PubMed] [Google Scholar]
- 12.Hohmann G., Fruth B. 2003. Culture in bonobos? Between-species and within-species variation in behavior. Curr. Anthropol. 44, 563–571 10.1086/377649 (doi:10.1086/377649) [DOI] [Google Scholar]
- 13.Perry S., et al. 2003. Social conventions in wild white-faced capuchin monkeys: evidence for traditions in a neotropical primate. Curr. Anthropol. 44, 241–268 10.1086/345825 (doi:10.1086/345825) [DOI] [Google Scholar]
- 14.Van Schaik C. P., Ancrenaz M., Borgen G., Galdikas B. M. F., Knott C. D., Singleton I., Suzuki A., Utami S. S., Merrill M. 2003. Orangutan cultures and the evolution of material culture. Science 299, 102–105 10.1126/science.1078004 (doi:10.1126/science.1078004) [DOI] [PubMed] [Google Scholar]
- 15.Marler P., Tamura M. 1964. Culturally transmitted patterns of vocal behavior in sparrows. Science 146, 1483–1486 10.1126/science.146.3650.1483 (doi:10.1126/science.146.3650.1483) [DOI] [PubMed] [Google Scholar]
- 16.McGrew W. C. 2004. The cultured chimpanzee: reflections on cultural primatology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 17.Laland K. N., Kendal J. R., Kendal R. L. 2009. Animal culture: problems and solutions. In The question of animal culture (eds Lalande K. N., Galef B. G.), pp. 174–197 Cambridge, MA: Harvard University Press [Google Scholar]
- 18.Whiten A., Horner V., de Waal F. B. M. 2005. Conformity to cultural norms of tool use in chimpanzees. Nature 437, 737–740 10.1038/nature04047 (doi:10.1038/nature04047) [DOI] [PubMed] [Google Scholar]
- 19.Horner V., Whiten A., Flynn E., de Waal F. B. M. 2006. Faithful replication of foraging techniques along cultural transmission chains by chimpanzees and children. Proc. Natl Acad. Sci. USA 103, 13 878–13 883 10.1073/pnas.0606015103 (doi:10.1073/pnas.0606015103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnie K. E., de Waal F. B. M. 2007. Copying without rewards: socially influenced foraging decisions among brown capuchin monkeys. Anim. Cogn. 10, 283–292 10.1007/s.10071-006-0069-9 (doi:10.1007/s.10071-006-0069-9) [DOI] [PubMed] [Google Scholar]
- 21.Bonnie K. E., Horner V., Whiten A., de Waal F. B. M. 2007. Spread of arbitrary conventions among chimpanzees: a controlled experiment. Proc. R. Soc. B 274, 367–372 10.1098/rspb.2006.3733 (doi:10.1098/rspb.2006.3733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiten A., Spiteri A., Horner V., Bonnie K. E., Lambeth S. P., Schapiro S. J., de Waal F. B. M. 2007. Transmission of multiple traditions within and between chimpanzee groups. Curr. Biol. 17, 1038–1043 10.1016/j.cub.2007.05.031 (doi:10.1016/j.cub.2007.05.031) [DOI] [PubMed] [Google Scholar]
- 23.Dindo M., Thierry B., Whiten A. 2008. Social diffusion of novel foraging methods in brown capuchin monkeys (Cebus apella). Proc. R. Soc. B 275, 187–193 10.1098/rspb.2007.1318 (doi:10.1098/rspb.2007.1318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredman T., Whiten A. 2008. Observational learning from tool using models by human-reared and mother-reared capuchin monkeys (Cebus apella). Anim. Cogn. 11, 295–309 10.1007/s10071-007-0117-0 (doi:10.1007/s10071-007-0117-0) [DOI] [PubMed] [Google Scholar]
- 25.Price E. E., Lambeth S. P., Schapiro S. J., Whiten A. 2009. A potent effect of observational learning on chimpanzee tool construction. Proc. R. Soc. B 276, 3377–3383 10.1098/rspb.2009.0640 (doi:10.1098/rspb.2009.0640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lonsdorf E. V. 2005. Sex differences in the development of termite-fishing skills in the wild chimpanzees, Pan troglodytes schweinfurthii, of Gombe National Park, Tanzania. Anim. Behav. 70, 673–683 10.1016/j.anbehav.2004.12.014 (doi:10.1016/j.anbehav.2004.12.014) [DOI] [Google Scholar]
- 27.Matthews L. J. 2009. Intragroup behavioral variation in white-fronted capuchin monkeys (Cebus albifrons): mixed evidence for social learning inferred from new and established analytical methods. Behaviour 146, 295–324 10.1163/156853909x410937 (doi:10.1163/156853909x410937) [DOI] [Google Scholar]
- 28.Perry S. 2009. Conformism in the food processing techniques of white-faced capuchin monkeys (Cebus capucinus). Anim. Cogn. 12, 705–716 10.1007/s10071-009-0230-3 (doi:10.1007/s10071-009-0230-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Schaik C. P. 2003. Local traditions in orangutans and chimpanzees: social learning and social tolerance. In The biology of traditions: models and evidence (eds Fragaszy D., Perry S.), pp. 297–328 Cambridge, UK: Cambridge University Press [Google Scholar]
- 30.Galef B. G. 1990. Tradition in animals: field observations and laboratory analyses. In Interpretation and explanation in the study of behavior: comparative perspectives (eds Bekoff M., Jamieson D.), pp. 74–95 Boulder, CO: Westview Press [Google Scholar]
- 31.Tomasello M. 1990. Cultural transmission in the tool use and communicatory signalling of chimpanzees? In ‘Language’ and intelligence in monkeys and apes: comparative developmental perspectives (eds Parker S., Gibson K.), pp. 274–311 Cambridge, UK: Cambridge University Press [Google Scholar]
- 32.Heyes C. M. 1994. Social-learning in animals: categories and mechanisms. Biol. Rev. Camb. Phil. Soc. 69, 207–231 [DOI] [PubMed] [Google Scholar]
- 33.Tennie C., Call J., Tomasello M. 2009. Ratcheting up the ratchet: on the evolution of cumulative culture. Phil. Trans. R. Soc. B 364, 2405–2415 10.1098/rstb.2009.0052 (doi:10.1098/rstb.2009.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tebbich S., Taborsky M., Fessl B., Blomqvist D. 2001. Do woodpecker finches acquire tool-use by social learning? Proc. R. Soc. Lond. B 268, 2189–2193 10.1098/rspb.2001.1738 (doi:10.1098/rspb.2001.1738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenward B., Weir A. A. S., Rutz C., Kacelnik A. 2005. Tool manufacture by naïve juvenile crows. Nature 433, 121–121 10.1038/433121a (doi:10.1038/433121a) [DOI] [PubMed] [Google Scholar]
- 36.Tennie C., Hedwig D., Call J., Tomasello M. 2008. An experimental study of nettle feeding in captive gorillas. Am. J. Primatol. 70, 584–593 10.1002/ajp.20532 (doi:10.1002/ajp.20532) [DOI] [PubMed] [Google Scholar]
- 37.Huffman M. A., Hirata S. 2004. An experimental study of leaf swallowing in captive chimpanzees: insights into the origin of a self-medicative behavior and the role of social learning. Primates 45, 113–118 10.1007/s10329-003-0065-5 (doi:10.1007/s10329-003-0065-5) [DOI] [PubMed] [Google Scholar]
- 38.Morin P. A., Moore J. J., Chakraborty R., Jin L., Goodall J., Woodruff D. S. 1994. Kin selection, social-structure, gene flow, and the evolution of chimpanzees. Science 265, 1193–1201 10.1126/science.7915048 (doi:10.1126/science.7915048) [DOI] [PubMed] [Google Scholar]
- 39.Gagneux P., et al. 1999. Mitochondrial sequences show diverse evolutionary histories of African hominoids. Proc. Natl Acad. Sci. USA 96, 5077–5082 10.1073/pnas.96.9.5077 (doi:10.1073/pnas.96.9.5077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldberg T. L., Ruvolo M. 1997. The geographic apportionment of mitochondrial genetic diversity in East African chimpanzees Pan troglodytes schweinfurthii. Mol. Biol. Evol. 14, 976–984 [DOI] [PubMed] [Google Scholar]
- 41.Mitani J. C., Hunley K. L., Murdoch M. E. 1999. Geographic variation in the calls of wild chimpanzees: a reassessment. Am. J. Primatol. 47, 133–151 (doi:10.1002/(SICI)1098-2345(1999)47:2<133::AID-AJP4>3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- 42.Lycett S. J., Collard M., McGrew W. C. 2007. Phylogenetic analyses of behavior support existence of culture among wild chimpanzees. Proc. Natl Acad. Sci. USA 104, 17 588–17 592 10.1073/pnas.0707930104 (doi:10.1073/pnas.0707930104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lycett S. J., Collard M., McGrew W. C. 2009. Cladistic analyses of behavioural variation in wild Pan troglodytes: exploring the chimpanzee culture hypothesis. J. Hum. Evol. 57, 337–349 10.1016/j.jhevol.2009.05.015 (doi:10.1016/j.jhevol.2009.05.015) [DOI] [PubMed] [Google Scholar]
- 44.Nunn C. L., Arnold C., Matthews L., Borgerhoff Mulder M. In press Simulating trait evolution for cross-cultural comparison. Phil. Trans. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Schaik C. P. 2009. Geographic variation in the behavior of wild great apes: is it really cultural? In The question of animal culture (eds Lalande K. N., Galef B. G.), pp. 70–98 Cambridge, MA: Harvard University Press [Google Scholar]
- 46.Shimada M. K., Hayakawa S., Fujita S., Sugiyama Y., Saitou N. 2009. Skewed matrilineal genetic composition in a small wild chimpanzee community. Folia Primatol. 80, 19–32 10.1159/000181187 (doi:10.1159/000181187) [DOI] [PubMed] [Google Scholar]
- 47.Langergraber K. E., Mitani J. C., Vigilant L. 2007. The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl Acad. Sci. USA 104, 7786–7790 10.1073/pnas.0611449104 (doi:10.1073/pnas.0611449104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langergraber K. E., Siedel H., Mitani J. C., Wrangham R. W., Reynolds V., Hunt K. D., Vigilant L. 2007. The genetic signature of sex-biased migration in patrilocal chimpanzees and humans. PLoS ONE 10, e973. 10.1371/journal.pone.0000973 (doi:10.1371/journal.pone.0000973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W. M., et al. 2008. Molecular ecology and natural history of simian foamy virus infection in wild-living chimpanzees. PLoS Pathog. 4, e1000097. 10.1371/journal.ppat.1000097 (doi:10.1371/journal.ppat.1000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Excoffier L., Laval G., Schneider S. 2005. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol. Bioinform. 1, 47–50 [PMC free article] [PubMed] [Google Scholar]
- 51.Nishida T., Mitani J. C., Watts D. P. 2004. Variable grooming behaviours in wild chimpanzees. Folia Primatol. 75, 31–36 10.1159/000073429 (doi:10.1159/000073429) [DOI] [PubMed] [Google Scholar]
- 52.Sokal R. R., Rohlf F. J. 1995. Biometry. New York, NY: W. H. Freeman and Co [Google Scholar]
- 53.Matsuzawa T. 1994. Field experiments on the use of stone tools in the wild. In Chimpanzee cultures (eds Wrangham R. W., McGrew W. C., de Waal F. B. M.), pp. 351–370 Cambridge, MA: Harvard University Press [Google Scholar]
- 54.Lind J., Lindenfors P. 2010. The number of cultural traits is correlated with female group size but not with male group size in chimpanzee communities. PLoS ONE 5, e9241. 10.1371/journal.pone.0009241 (doi:10.1371/journal.pone.0009241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavalli-Sforza L. L. 2000. Genes, people, and languages. San Francisco, CA: North Point Press [Google Scholar]
- 56.Nettle D. 2009. Ecological influences on human behavioural diversity: a review of recent findings. Trends Ecol. Evol. 24, 618–624 10.1016/j.tree.2009.05.013 (doi:10.1016/j.tree.2009.05.013) [DOI] [PubMed] [Google Scholar]
- 57.Lefebvre L. 1995. The opening of milk bottles by birds: evidence for accelerating learning rates, but against the wave-of-advance model of cultural transmission. Behav. Process. 34, 43–53 10.1016/0376-6357(94)00051-H (doi:10.1016/0376-6357(94)00051-H) [DOI] [PubMed] [Google Scholar]
- 58.Franz M., Nunn C. L. 2009. Network-based diffusion analysis: a new method for detecting social learning. Proc. R. Soc. B 276, 1829–1836 10.1098/rspb.2008.1824 (doi:10.1098/rspb.2008.1824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kendal R. L., Kendal J. R., Hoppitt W., Laland K. N. 2009. Identifying social learning in animal populations: a new ‘option-bias' method. PLoS ONE 4, e6541. 10.1371/journal.pone.0006541 (doi:10.1371/journal.pone.0006541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helfman G. S., Schultz E. T. 1984. Social transmission of behavioral traditions in a coral-reef fish. Anim. Behav. 32, 379–384 10.1016/S0003-3472(84)80272-9 (doi:10.1016/S0003-3472(84)80272-9) [DOI] [Google Scholar]
- 61.Krutzen M., Mann J., Heithaus M. R., Connor R. C., Bejder L., Sherwin W. B. 2005. Cultural transmission of tool use in bottlenose dolphins. Proc. Natl Acad. Sci. USA 102, 8939–8943 10.1073/pnas.0500232102 (doi:10.1073/pnas.0500232102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langergraber K., Mitani J., Vigilant L. 2009. Kinship and social bonds in female chimpanzees (Pan troglodytes). Am. J. Primatol. 71, 840–851 10.1002/ajp.20711 (doi:10.1002/ajp.20711) [DOI] [PubMed] [Google Scholar]