Abstract
The higher costs of sons compared with daughters extends to a negative effect of brothers on the lifetime reproductive success of their siblings in subsistence and preindustrial societies. In societies with fewer resource constraints, one might expect that these effects would be limited or non-existent. This study investigates the costs of brothers and sisters in a contemporary western society of adult Australians. Girls with elder brothers had a delayed age at menarche. Younger brothers were associated with delayed onset of sexual activity in sisters, but not in brothers. Neither younger nor elder brothers influenced fitness parameters (number of pregnancies, number of children, age at first pregnancy or age at first birth) in siblings of either sex. This study provides evidence that brothers negatively affect their sisters' onset of reproductive maturity and sexual activity; however, this delay is not associated with a fitness cost in contemporary Australia. We suggest this is due to the long period of independence prior to child bearing.
Keywords: brothers, sisters, menarche, onset of sexual activity, costs of brothers, helper-at-the-nest
1. Introduction
In polygynous mammals, there is evidence that male offspring receive higher levels of parental investment than female offspring [1]. Male young in several species of macaques, the red deer (Cervus elaphas), and the elephant seal (Mirounga angustirostris) weigh more at birth than female young [1–3]. Male elephant seal pups are also nursed for longer than are female pups [2]. Male calves of the red deer also have longer gestation periods than female calves [1]. Raising a male calf through one winter means a doe is less likely to rear a calf the following winter than are does producing female offspring [1]. Ovis canadensis ewes are also less likely to wean a lamb in the year following weaning a son compared with weaning a daughter [4]. Furthermore, those lambs whose mother did wean a son in the previous year are less likely to survive to 1 year than those whose mother weaned a daughter [4].
There is ample evidence that sons are more costly to human mothers than are daughters as well. In contemporary human populations, mothers pregnant with a son require 10 per cent more food, indicating that male foetuses have higher energy requirements than female foetuses [5]. Male offspring are carried longer in utero than female offspring [6]. Boys, on average, weigh more at birth than girls [7]. Mothers nurse sons more frequently and sons are weaned at a later age than daughters [6]. In the Gabbra of Kenya, the inter-birth interval after a son is greater than after a daughter; this suggests that even after birth maternal investment is greater in sons than in daughters [8]. In nineteenth century Swedish families, the longest inter-birth interval is between two sons; this suggests a higher physiological cost of carrying a son compared with a daughter [6]. Preindustrial Sami women from northern Scandinavia who had sons rather than daughters had a decreased lifespan, providing evidence that sons are more costly than daughters [9].
This drain on the limited parental resources, owing to the additional cost of sons, also may influence siblings. Studies in the USA and Quebec found that boys born after a brother have reduced birth weights [10,11]; the negative effect of maternal depletion after the birth of a son influences the foetal growth of a subsequent son. In Denmark, controlling for maternal age, time to pregnancy, parity, calendar year, same father and gestational age, both males and females with one or two elder brothers had significantly lower birth weights compared with those with no elder brothers [12].
An increase in the number of brothers, particularly younger brothers, is associated with delayed sexual maturation in their sisters in contemporary Melbourne, Australia [13] and in Great Britain [14], providing evidence that brothers also can negatively affect reproductive maturity of their sisters. In the polygynous Kipsigis of Kenya, men with more brothers have fewer offspring surviving to 5 years of age [15]. In the Gabbra of Kenya, men with more brothers have lower fertility and reproductive success [8,16]. In a historical population of preindustrial Finns, individuals with elder brothers had a later age at first birth, longer inter-birth intervals between their children [17], lower fecundity and lower lifetime reproductive success than those with elder sisters after controlling for socioeconomic status and ecological conditions [18]. In nineteenth century Sweden, males with more brothers had reduced lifetime reproductive success compared with those with fewer brothers [6]. Thus, in numerous societies, brothers negatively influence the reproductive outcomes of their siblings.
In many species of birds and mammals, there is evidence of older offspring remaining at the nest/den and helping parents raise their younger siblings [19]. Human-focused studies find daughters, more so than sons, to be beneficial to the reproductive success of the natal family. Fourteenth century German families with more elder daughters had higher reproductive rates and larger sibship sizes, and mothers with more elder daughters actually spent less time breastfeeding [20]. In a subsistence population on the Ifaluk atoll, women with two elder daughters had more children than women with two elder sons; having elder daughters allowed women to have higher reproductive success at older ages, presumably owing to early-born daughters' roles as helpers [21]. Girls are more likely to help raise their siblings, and it is lower birth order daughters who will most often fill this role, potentially delaying their own reproduction. In a proto-industrial population, females with more siblings had decreased lifetime reproduction compared with those with fewer siblings [6]. This study investigates the effect of brothers and sisters, both younger and elder, on reproductive parameters in a contemporary population of Australians. We expect that the effects of brothers and sisters will differ for male and female siblings.
2. Material and methods
Our retrospective study used an anonymous written survey technique. Participants were recruited during the second half of 2007 [22]. The 273 participants were predominantly Western Australians between the ages of 18 and 75; 197 (72%) were female. This female bias does not reflect the wider population, but is common in questionnaire-based studies [23,24].
Each respondent was characterized by the presence or absence of brothers and sisters, both younger and elder. This resulted in two categorical independent variables, one that represents the sex composition of younger siblings and, the second that represents the sex composition of elder siblings. The four categories of these variables were: no younger/elder siblings, exclusively younger/elder sisters, exclusively younger/elder brothers and both younger/elder sisters and brothers. The reference category for each independent variable is no younger/elder siblings—a category that includes singletons. Singletons did not differ in terms of age at menarche and first sexual intercourse from all other groups, resulting in comparisons with the reference group being more conservative. Seven female and three male respondents were singletons.
Seven adult reproductive parameters were investigated (age at menarche for females, age at first sexual intercourse, age at first pregnancy for females, age at first birth, numbers of pregnancies for females, number of live-born children and a dichotomous variable for whether an individual had at least one child or not). One-way ANOVAs were used to investigate age at menarche as every female participant had experienced puberty. Levene's test of homogeneity of variances was non-significant, thus Bonferroni post hoc tests were used to indicate which groups differed. For the three ‘age at first event’ variables (sexual intercourse, pregnancy and birth), we used survival analyses (Cox proportional hazard models). Survival analysis models the distribution of the time-to-event, and controls for censored data—that is, where the individual has not yet experienced the event [25,26]. Thus, survival analysis incorporates age. The two count variables (pregnancies and births) followed Poisson rather than normal distributions, thus generalised linear models with log link functions were employed. The binary outcome variable indicating whether individuals had any children was analysed using generalized linear models with a logit link function and a binomial distribution. In all analyses above, except for age at menarche, Wald's χ2 was used to evaluate the significance of the beta coefficients.
Potentially confounding variables identified from the literature included cohort effect, socioeconomic status, sibship size and preceding inter-birth interval. A cohort effect on the age at menarche was examined using a Pearson's correlation between age and age at menarche. Natal family socioeconomic status (FSES) was categorized as poor (reference category), working or lower class, middle class, and upper class. The influences of FSES on age at menarche (one-way ANOVA) and age at first sexual intercourse (Cox proportional hazards models) were investigated. Sibship size was tested against the independent variables with one-way ANOVAs and Bonferroni post hoc tests. Inter-birth interval was regressed on age at menarche and on age at first sexual intercourse. Analyses were stratified by sex where appropriate and all analyses were performed using SPSS v. 18.
3. Results
(a). Sexual maturation
The presence of younger brothers and/or younger sisters did not influence the age at menarche (F3,191 = 0.942, p = 0.421; table 1). However, the sex composition of elder siblings influenced the age of menarche (F3,188 = 4.797, p = 0.003). Girls with exclusively elder brothers were significantly older (by 0.9 years) at menarche than girls with no elder siblings (p = 0.006) or girls with exclusively elder sisters (p = 0.026). To exclude the effect of birth order and to better control for potential confounders, second-born females with an elder brother were used to investigate the effect of preceding inter-birth interval on age at menarche. Age at menarche was not influenced by preceding inter-birth interval (F1,34 = 0.010, p = 0.920).
Table 1.
Mean value ± s.d. (sample size) for each outcome variable for females having different configurations of younger and elder siblings. The significance (p-value) refers to comparisons with the reference category (none) in each independent variable (younger and elder siblings) for each reproductive factor. Where p-values are greater than or equal to 0.1, they are not provided in this table.
| sex configuration of siblings | age at menarche | age at first sexual intercourse | age at first pregnancy | number of pregnancies | age at first birth | number of children | |
|---|---|---|---|---|---|---|---|
| younger siblings | none | 13.2 ± 1.41 (63) | 18.1 ± 2.15 (63) | 26.5 ± 6.43 (62) | 1.4 ± 1.66 (62) | 28.4 ± 7.56 (62) | 0.9 ± 1.23 (63) |
| exclusively sisters | 13.2 ± 1.21 (38) | 18.3 ± 2.42 (38) | 24.8 ± 5.60 (38) | 1.1 ± 1.64 (38) | 26.0 ± 5.59 (37) | 0.8 ± 1.73 (38) | |
| exclusively brothers | 12.8 ± 1.48 (45) | 19.3 ± 2.94 (41) p = 0.012 | 25.4 ± 6.66 (42) | 1.6 ± 1.99 (42) | 27.1 ± 7.86 (45) | 1.0 ± 1.43 (45) | |
| both brothers and sisters | 12.9 ± 1.56 (39) | 20.0 ± 6.51 (38) p = 0.016 | 24.7 ± 7.03 (38) | 1.6 ± 1.99 (39) | 26.4 ± 7.67 (38) | 1.3 ± 1.70 (38) | |
| elder siblings | none | 12.7 ± 1.38 (71) | 19.6 ± 5.41 (70) | 25.2 ± 6.88 (68) | 1.2 ± 1.75 (69) | 26.7 ± 7.75 (70) | 0.8 ± 1.27 (71) |
| exclusively sisters | 12.7 ± 1.42 (34) | 17.9 ± 2.50 (33) | 26.3 ± 5.94 (33) | 1.4 ± 1.87 (33) | 27.3 ± 5.71 (33) | 0.9 ± 1.37 (33) | |
| exclusively brothers | 13.6 ± 1.35 (42) p = 0.006 | 18.9 ± 2.51 (39) | 23.7 ± 4.41 (40) | 1.4 ± 1.69 (40) | 25.4 ± 7.21 (41) | 1.1 ± 1.39 (42) | |
| both brothers and sisters | 13.3 ± 1.39 (35) | 18.8 ± 2.29 (35) | 27.7 ± 7.50 (36) | 1.9 ± 1.97 (36) | 30.2 ± 7.43 (36) | 1.4 ± 1.66 (36) |
(b). Onset of sexual activity
Age of first sexual intercourse of males was not influenced by the sex composition of either younger (χ2(3) = 3.004, p = 0.391) or elder siblings (χ2(3) = 2.075, p = 0.557; table 2).
Table 2.
Mean value ± s.d. (sample size) for each outcome variable for males having different configurations of younger and elder siblings. No significance values are provided as none of the comparisons to the reference category (none) in either independent variable differs for any of the reproductive factors.
| sex configuration of siblings | age at first sexual intercourse | age at first birth | number of children | |
|---|---|---|---|---|
| younger siblings | none | 19.5 ± 3.05 (23) | 25.9 ± 4.68 (22) | 1.3 ± 1.46 (24) |
| exclusively sisters | 18.9 ± 2.82 (21) | 28.4 ± 8.29 (22) | 0.7 ± 1.08 (22) | |
| exclusively brothers | 18.2 ± 2.60 (14) | 25.2 ± 5.47 (14) | 0.6 ± 1.01 (14) | |
| both brothers and sisters | 20.6 ± 4.32 (11) | 34.5 ± 7.81 (10) | 0.9 ± 1.14 (11) | |
| elder siblings | none | 19.7 ± 3.20 (26) | 28.7 ± 7.52 (26) | 0.8 ± 1.12 (27) |
| exclusively sisters | 18.5 ± 3.02 (21) | 25.7 ± 4.78 (21) | 1.1 ± 1.49 (21) | |
| exclusively brothers | 19.1 ± 2.78 (12) | 28.8 ± 8.71 (11) | 1.0 ± 1.13 (12) | |
| both brothers and sisters | 18.4 ± 2.96 (7) | 28.9 ± 10.05 (8) | 0.7 ± 1.17 (8) |
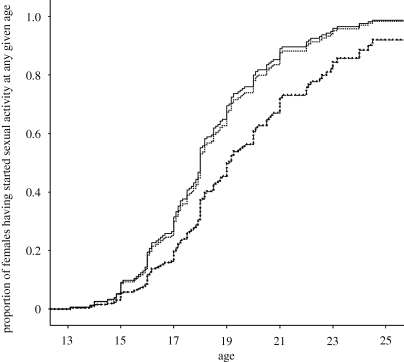
Neither elder brothers nor sisters influenced a female's age at first sexual intercourse (χ2(3) = 5.572, p = 0.134; table 1). However, the sex composition of younger siblings did influence the age at first sexual intercourse for females (χ2(3) = 10.281, p = 0.016). Girls with exclusively younger brothers (HR = 0.581, p = 0.012) or both younger brothers and sisters (HR = 0.579, p = 0.016) had a significantly lower probability of having had sexual intercourse for the first time at any given age than girls with no younger siblings (figure 1). That is, girls with only younger brothers were on average 1.2 years older, and girls with both younger brothers and sisters were on average 1.9 years older at first sexual intercourse than girls with no younger siblings (table 1). These results are independent of age at menarche and subsequent inter-birth interval. There was no correlation between age at menarche and age at first sexual intercourse for females (Pearson's r = 0.009, p = 0.901). To mirror the analysis of preceding inter-birth interval and age at menarche, second last-born females with only one younger brother were used to investigate the relationship between age at first sexual intercourse and subsequent inter-birth interval. No association exists between age at first sexual intercourse and inter-birth interval in years (F1,20 = 0.633, p = 0.436).
Figure 1.
Proportion of females having had sexual intercourse for the first time at any given age by sex composition of their younger siblings. Note: the survival curves for having exclusively younger brothers and both younger brothers and sisters are extremely similar and thus appear superimposed. Having either younger brothers or younger brothers and sisters delays age at first sexual intercourse. Solid line, no younger siblings; dotted line, exclusively younger sisters; line with filled squares, exclusively younger brothers; dashed lines, both younger brothers and sisters.
(c). Onset of reproduction
The age at first birth for males did not significantly differ by the sex composition of younger (χ2(3) = 7.105, p = 0.069) or elder (χ2(3) = 4.949, p = 0.176) siblings (table 2).
Neither having younger siblings (χ2(3) = 1.752, p = 0.625) nor having elder siblings (χ2(3) = 3.803, p = 0.284) significantly influenced the age at which females first became pregnant (table 1). The age at first birth for females also did not differ by the sex composition of younger siblings (χ2(3) = 0.815, p = 0.846) or elder siblings (χ2(3) = 6.130, p = 0.105; table 1).
(d). Reproductive success
Neither sex composition of younger (χ2(3) = 1.430, p = 0.698) nor elder (χ2(3) = 1.287, p = 0.732) siblings was associated with males having fathered any children after controlling for age. In addition, the actual number of children fathered by a male was not significantly influenced by the sex composition of his younger (χ2(3) = 6.052, p = 0.109) or elder siblings (χ2(3) = 3.544, p = 0.315; table 2).
After controlling for age, the number of pregnancies experienced by a female was not significantly influenced by younger siblings (χ2(3) = 0.973, p = 0.808) or by elder siblings (χ2(3) = 3.556, p = 0.314; table 1). Neither sex composition of younger siblings (χ2(3) = 1.015, p = 0.798) nor sex composition of elder siblings (χ2(3) = 1.141, p = 0.767) influenced whether females had given birth to any children (controlling for age). Additionally, the number of children born was not significantly influenced by having younger siblings (χ2(3) = 0.403, p = 0.940) or having elder siblings (χ2(3) = 2.676, p = 0.444; table 1).
(e). Potential confounding variables
There was no correlation between age and age at menarche (Pearson's r = −0.026, p = 0.721); therefore, there was no evidence of a cohort effect on age at menarche.
Natal FSES did not significantly influence age at menarche (F3,192 = 1.929, p = 0.126), age at first sexual intercourse for females (χ2(3) = 5.408, p = 0.144), or age at first sexual intercourse for males (χ2(3) = 0.240, p = 0.971).
As would be expected, sibship size was associated with composition of elder siblings (F3,261 = 25.501, p < 0.001) and younger siblings (F3,267 = 25.851, p < 0.001). In both cases, individuals with both elder (or both younger) brothers and sisters came from sibships that had on average two more individuals than girls with no elder (or no younger) siblings.
4. Discussion
(a). Sexual maturation
In this contemporary Australian population, girls with exclusively elder brothers have delayed menarche (table 1). This is not due to sibship size; girls with exclusively elder brothers are not part of larger sibships than girls with no elder brothers. Mechanisms for this delay may include reduced resources, other physiological suppression or psychological, stress-related responses. If sons are more costly, then parental resources may first be directed to elder sons and less available to future or younger children. A negative effect of brothers on sisters' sexual maturation was also documented in an earlier study of Australian families [13]; however, younger brothers had a larger influence on sisters' age at menarche than did elder brothers. Unlike this study, Jones et al. [13] looked at numbers of siblings, not sex composition. Inter-birth interval had no impact on a female's age at menarche, suggesting a prolonged developmental effect rather than resource deprivation as a result of maternal depletion. Elder brothers may reduce nutritional resources; however, there may also be other mechanism(s) that reduce the chance of siblings sexually maturing [27]. If the effect is due to resource deprivation, we would expect that younger brothers would also exhibit delayed sexual maturation; however, we did not have a measure for physiological sexual maturity in boys.
(b). Onset of sexual activity
Having younger brothers delayed the onset of sexual activity of sisters (figure 1), but not of brothers. Girls with both younger brothers and sisters had the oldest age at first sexual reproduction; this may be because they also come from larger sibships. Age at sexual activity, unlike menarche, is not a physiological process, but behaviour largely contingent on social environment. This suggests that the mechanism of a younger brother effect is not resource competition resulting in delayed growth and development but rather behavioural, perhaps involving care-taking roles of elder sisters. Elder brothers delay physiological maturation, while younger brothers delay behavioural maturation.
Reports of daughters helping parents are common. For example, in fourteenth century German families, an elder daughter increased the reproductive success and sibship size of her natal family [20]. Traditional Ifaluk mothers with daughters as first and second born children have, on average, significantly more children than mothers whose first two children were sons [21]. Ifaluk mothers did not differ in numbers of children born within the first 10 years of reproducing; however, mothers with elder daughters had significantly more children during the subsequent 15 years of reproduction, coinciding with the time that eldest children may actually be helpful in caring for younger siblings [21]. Mothers with elder daughters did not differ from mothers with elder sons in mean age at first birth, but the age at last birth was significantly older for those mothers with early-born daughters [21]. Elder daughters may provide more help to their parents in raising their younger brothers than do elder brothers. Associated with this prolonged help is a delay in sexual activity and thus a delay in potential for starting their own family.
(c). Onset of reproduction
Early mortality results in decreased life expectancy and life-history theory then predicts an associated younger age at first birth [28,29]. Data examining 25 genera of mammalian species found that, controlling for body size, the age at first birth was directly influenced by the life expectancy at birth (r = 0.976; [30]). Therefore, if the cost of sons results in a decreased ability of the mother to provide for subsequent children in utero [10,11] and a lower life expectancy for later-born children, then later-born children with elder brothers might be expected to begin reproduction at an earlier age. The relationship between life expectancy at birth and age at first birth across 98 human societies is influenced by the human development index (HDI; [31]). Countries with high HDI ranks, such as New Zealand, Norway and United States, exhibit a strong positive relationship between life expectancy at birth and age at first birth, while no relationship exists for countries with low HDI ranks [31]. Countries with high HDI ranks have life expectancies at birth above 65 years [31]. The approximate life expectancy at birth for a median-aged female in our sample was 78 years (and 71 years for a male; [32]). Australia has a high HDI rank, which is related to an older age at first birth. Given older age at first birth, there is increased time between leaving the natal family home and first birth, so the negative effect of brothers may be reduced during the intervening period of independence prior to family formation. In this Australian sample, sex composition of elder or younger siblings did not influence the onset of reproduction (age at first pregnancy and age at first birth).
(d). Reproductive success
Life-history theory also predicts the numbers of children; if there is a delay in age at first birth, then there is less time to have as many children as individuals who start reproducing at a younger age. Australia, however, has low average fertility; a delay in reproductive onset may not limit the ability to have the average number of children. The sex composition of elder or younger siblings was not related to reproductive success (numbers of pregnancies or numbers of children). It was expected that costly sons would lead to decreased resource availability for other siblings and ultimately result in decreased reproductive success of siblings with many brothers. In numerous previous studies, brothers negatively influence the lifetime reproductive success and fitness of their siblings [15,16,18]. However, these studies involved subsistence economies and historical, preindustrial populations with high mortality rates, early age at first birth and higher fertility. High mortality rates select for earlier and more frequent reproduction; because an individual has a higher probability of dying in any given year, delaying reproduction is more likely to reduce reproductive success (and fitness) than is a delay in a low-mortality population [29]. Consequently, most individuals from subsistence economies reproduce at a younger age, and the time between leaving the natal family home and starting a family is often negligible. The time between leaving home and starting a family is effectively the amount of time an individual or couple has to obtain resources through their own means to provide for their future family. In contrast, reduced time for amassing individual resources means any resources provided by a couple's parents are a greater proportion of the total resources available to the couple for their own family. Families from subsistence economies with elder sons have fewer resources to provide for later born children [16]. Therefore, these later born individuals would not be as successful in raising as many offspring as those with no elder brothers (as is seen in studies of subsistence economies). Women may choose to delay reproduction to study and/or work in order to acquire resources (resource-acquisition strategy) if the cost per child is high, as is the case in developed, highly competitive societies [31]. This allows a woman to increase her resource base to provide for her family when she does eventually start to reproduce [31].
In this Australian sample, the mean age at menarche (13.1, s.d. = 1.4) and mean age at first sexual intercourse (18.6, s.d. = 2.7) are below the mean age at which people move out of home (19.4, s.d. = 3.2), while the mean ages at first pregnancy (25.7, s.d. = 5.0; females only) and birth (29.1, s.d. = 5.2) are substantially greater than the mean age at leaving home. The older mean age at birth results in increased time for collecting resources and a decreased reliance on resources provided by parents. Thus, we argue that the effect of brothers is only strong enough to affect life-history events in the natal home, and does not impact reproductive parameters that are temporally removed from natal family life in this western, industrialized society. Additionally, the use of contraception and family planning facilitates time for gathering individual resources, decreases the relationship between age and reproductive success, decreases the average number of children and reduces the effect of brothers on their siblings after they leave the natal home. With differing ecological contexts wherein natal family resources are not essential for adult reproductive success, it is unlikely that the cost of brothers will influence reproductive success of their siblings in developed populations such as contemporary Australia.
In conclusion, we find brothers impose an early-life cost on their sisters' sexual maturation. Having elder brothers delays physiological maturation, while (presumably care-taking of) younger brothers delay behavioural maturation. Both of these occur in the context of the natal family. However, in the ecological context of a contemporary low-fertility, low-mortality population, early delays are not translated into actual fitness costs: the age at first birth and parity are not significantly influenced by factors that result in delays earlier in life. The difference we see between effects in western industrialized populations and subsistence or agricultural populations is related to the strength of the impact of natal family conditions on adult conditions, and to the reduced variation in reproductive success in industrialized societies. The demographic fact that disconnects mechanisms in natal family life and adult reproductive outcomes is delayed reproductive onset. We predict that sibling costs will occur in most societies, but that these early costs will only translate into actual fitness effects when the conditions of the natal family strongly influence the acquisition of mates and thus determine reproductive onset (age at first birth).
Acknowledgements
The University of Western Australia Human Research Ethics Committee approved this project.
The School of Anatomy and Human Biology at The University of Western Australia provided funding. We thank the AnHB ecology discussion group for comments. We also thank all participants; without their candid responses, this study could not have taken place. This manuscript benefitted from comments from Prof. Jim Chisholm, Prof. Charles Oxnard, Dr Katherine Sanders and Prof. Linc Schmitt and reviewers for this journal.
References
- 1.Clutton-Brock T., Albon S., Guinness F. 1981. Parental investment in male and female offspring in polygynous mammals. Nature 289, 487–489 10.1038/289487a0 (doi:10.1038/289487a0) [DOI] [Google Scholar]
- 2.Reiter J., Stinson N., Le Boeuf B. 1978. Northern elephant seal development: the transition from weaning to nutritional independence. Behav. Ecol. Sociobiol. 3, 337–367 10.1007/BF00303199 (doi:10.1007/BF00303199) [DOI] [Google Scholar]
- 3.Dittus W. P. 1979. The evolution of behaviours regulating density and age-specific sex ratios in a primate population. Behaviour 69, 265–302 [Google Scholar]
- 4.Berube C. H., Festa-Bianchet M., Jorgenson J. T. 1996. Reproductive costs of sons and daughters in Rocky Mountain bighorn sheep. Behav. Ecol. 7, 60–68 10.1093/beheco/7.1.60 (doi:10.1093/beheco/7.1.60) [DOI] [Google Scholar]
- 5.Tamimi R. M., Lagiou P., Mucci L. A., Hsieh C. C., Adami H. O., Trichopoulos D. 2003. Average energy intake among pregnant women carrying a boy compared with a girl. Br. Med. J. 326, 1245–1246 10.1136/bmj.326.7401.1245 (doi:10.1136/bmj.326.7401.1245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Low B. 1991. Reproductive life in nineteenth century Sweden: an evolutionary perspective on demographic phenomena. Ethol. Sociobiol. 12, 411–448 10.1016/0162-3095(91)90024-K (doi:10.1016/0162-3095(91)90024-K) [DOI] [Google Scholar]
- 7.Marsal K., Persson P. H., Larsen T., Lilja H., Selbing A., Sultan B. 1996. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 85, 843–848 10.1111/j.1651-2227.1996.tb14164.x (doi:10.1111/j.1651-2227.1996.tb14164.x) [DOI] [PubMed] [Google Scholar]
- 8.Mace R., Sear R. 1997. Birth interval and the sex of children in a traditional African population: an evolutionary analysis. J. Biosoc. Sci. 29, 499–507 10.1017/S0021932097004999 (doi:10.1017/S0021932097004999) [DOI] [PubMed] [Google Scholar]
- 9.Helle S., Lummaa V., Jokela J. 2002. Sons reduced maternal longevity in preindustrial humans. Science 296, p. 1085. 10.1126/science.1070106 (doi:10.1126/science.1070106) [DOI] [PubMed] [Google Scholar]
- 10.Blanchard R., Ellis L. 2001. Birth weight, sexual orientation and the sex of preceding siblings. J. Biosoc. Sci. 33, 451–467 10.1017/S0021932001004515 (doi:10.1017/S0021932001004515) [DOI] [PubMed] [Google Scholar]
- 11.Cote K., Blanchard R., Lalumiere M. L. 2003. The influence of birth order on birth weight: does the sex of preceding siblings matter?. J. Biosoc. Sci. 35, 455–462 [DOI] [PubMed] [Google Scholar]
- 12.Nielsen H. S., Mortensen L., Nygaard U., Schnor O., Christiansen O. B., Anderson A. N. 2007. Brothers and reduction of the birth weight of later-born siblings. Am. J. Epidemiol. 167, 480–484 10.1093/aje/kwm330 (doi:10.1093/aje/kwm330) [DOI] [PubMed] [Google Scholar]
- 13.Jones B., Leeton J., McLeod I., Wood C. 1972. Factors influencing the age of menarche in a lower socio-economic group in Melbourne. Med. J. Aust. 2, 533–535 [DOI] [PubMed] [Google Scholar]
- 14.Bogaert A. F. 2008. Menarche and father absence in a national probability sample. J. Biosoc. Sci. 40, 623–636 [DOI] [PubMed] [Google Scholar]
- 15.Borgerhoff-Mulder M. 1998. Brothers and sisters—how sibling interactions affect optimal parental allocations. Hum. Nat. 9, 119–161 [DOI] [PubMed] [Google Scholar]
- 16.Mace R. 1996. Biased parental investment and reproductive success in Gabbra pastoralists. Behav. Ecol. Sociobiol. 38, 75–81 10.1007/s002650050219 (doi:10.1007/s002650050219) [DOI] [PubMed] [Google Scholar]
- 17.Rickard I. J., Lummaa V., Russell A. F. 2009. Elder brothers affect the life history of younger siblings in preindustrial humans: social consequence or biological cost?. Evol. Hum. Behav. 30, 49–57 10.1016/j.evolhumbehav.2008.08.001 (doi:10.1016/j.evolhumbehav.2008.08.001) [DOI] [Google Scholar]
- 18.Rickard I. J., Russell A. F., Lummaa V. 2007. Producing sons reduces lifetime reproductive success of subsequent offspring in pre-industrial Finns. Proc. R. Soc. B 274, 2981–2988 10.1098/rspb.2007.1051 (doi:10.1098/rspb.2007.1051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emlen S. T. 1984. Cooperative breeding in birds and mammals. In Behavioural ecology: an evolutionary approach (eds Krebs J. R., Davies N. B.), pp. 305–339, 2nd edn Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- 20.Kemkes A. 2006. Does the sex of firstborn children influence subsequent fertility behavior? Evidence from family reconstitution. J. Fam. Hist. 31, 144–162 10.1177/0363199005284870 (doi:10.1177/0363199005284870) [DOI] [PubMed] [Google Scholar]
- 21.Turke P. W. 1988. Helpers at the nest: childcare networks on Ifaluk. In Human reproductive behaviour: a Darwinian perspective (eds Betzig L. L., Borgerhoff-Mulder M., Turke P. W.), pp. 173–188 Cambridge, UK: Cambridge University Press [Google Scholar]
- 22.Milne F. H., Judge D. S. 2009. Birth order influences reproductive measures in Australians. Hum. Nat. 20, 294–316 10.1007/s12110-009-9065-5 (doi:10.1007/s12110-009-9065-5) [DOI] [Google Scholar]
- 23.Cottler L., Zipp J., Robins L., Spitznagel E. 1987. Difficult-to-recruit respondents and their effect on prevalence estimates in an epidemiologic survey. Am. J. Epidemiol. 125, 329–339 [DOI] [PubMed] [Google Scholar]
- 24.Dengler R., Roberts H., Rushton L. 1997. Lifestyle surveys—the complete answer? J. Epidemiol. Commun. Health 51, 46–51 10.1136/jech.51.1.46 (doi:10.1136/jech.51.1.46) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox D., Oakes D. 1984. Analysis of survival data. London, UK: Chapman and Hall [Google Scholar]
- 26.Tableman M., Kim J. S. 2004. Survival analysis using S: analysis of time-to-event data. Boca Raton, FL: Chapman and Hall/CRC [Google Scholar]
- 27.Ellis B. J. 2004. Timing of pubertal maturation in girls: an integrated life history approach. Psychol. Bull. 130, 920–958 10.1037/0033-2909.130.6.920 (doi:10.1037/0033-2909.130.6.920) [DOI] [PubMed] [Google Scholar]
- 28.Charlesworth B. 1980. Evolution in age-structured populations. Cambridge, UK: Cambridge University Press [Google Scholar]
- 29.Stearns S. C. 2000. Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87, 476–486 10.1007/s001140050763 (doi:10.1007/s001140050763) [DOI] [PubMed] [Google Scholar]
- 30.Harvey P. H., Zammuto R. M. 1985. Patterns of mortality and age at first reproduction in natural populations of mammals. Nature 315, 319–320 10.1038/315319a0 (doi:10.1038/315319a0) [DOI] [PubMed] [Google Scholar]
- 31.Low B. S., Hazel A., Parker N., Welch K. B. 2008. Influences on women's reproductive lives—unexpected ecological underpinnings. Cross-Cult. Res. 42, 201–219 10.1177/1069397108317669 (doi:10.1177/1069397108317669) [DOI] [Google Scholar]
- 32.AIHW. Australian Institute of Health and Welfare, Australian Government; 2010. Australian trends in life expectancy. See http://www.aihw.gov.au/mortality/life_expectancy/trends.cfm . [Google Scholar]