Abstract
Multicellular organisms that benefit from division of labour are presumably descended from colonial species that initially derived benefits from larger colony size, before the evolution of specialization. Life in a colony can have costs as well as benefits, but these can be hard to measure. We measured physiological costs to life in a colony using a novel method based on population dynamics, comparing growth rates of unicells and kairomone-induced colonies of a green alga Desmodesmus subspicatus against a reference co-occurring species. Coloniality negatively affected growth during the initial log growth phase, while no adverse effect was detected under nutrient-limited competitive conditions. The results point to costs associated with traits involved in rapid growth rather than those associated with efficient growth under resource scarcity. Some benefits of coloniality (e.g. defence from herbivory) may be different from when this trait evolved, but our approach shows how costs would have depended on conditions.
Keywords: coloniality, size, detergent, competition, trade-off, phenotypic plasticity
1. Introduction
The evolution of multicellularity, one of evolution's major transitions, has occurred independently several times in the history of life [1]. Understanding of the evolutionary forces acting on this transition is highly incomplete because the costs and benefits of life in a multicellular colony versus life as a unicell are still poorly understood.
In certain groups, individuals can exist in either unicellular or multicellular form, so in those cases coloniality is a plastic trait. Phenotypic plasticity, once considered a nuisance to evolutionary studies, is increasingly a focus of investigation and is now acknowledged as an important concept [2]. One example of phenotypic plasticity is the induction of defence mechanisms against herbivores and predators (e.g. [3–5]). Inducible defences may be beneficial if predator attacks are intermittent and are cued by the proximity of the predator, if the defence is beneficial in reducing predator effectiveness, and if the defence is costly in some way [6]. Thus, there must be different costs and benefits associated with different environmental states for inducible defences to be considered adaptive. The cost–benefit landscape for any given flexible trait, however, is often difficult to measure because (i) any given phenotypic change may have multiple impacts on fitness, and (ii) costs and benefits may not be large. A small cost can easily be masked by inherent variance among individuals.
Coloniality in freshwater phytoplankton is an often-studied inducible defence. Chemical cues, or kairomones, from herbivorous zooplankton Daphnia (Cladocera) induce colony formation in Desmodesmus and Scenedesmus [7]. A morphological change from unicells to 2-, 4-, 8-, 16- or sometimes 32-celled colonies can impose difficulty to grazers with size-limited food particle collection [8–10]. Further, once collected, larger algal particles are less likely to be ingested and killed [11]. Similar instances of algal inducible defences have been widely observed in a variety of grazer–algae pairs and in both freshwater and marine systems (e.g. [12–14]). Thus, a well-established benefit to colony formation is reduced mortality to grazing zooplankton.
Any costs of coloniality in these organisms, however, are still mostly speculated upon. Larger particles sink faster than smaller particles, and Lürling & Van Donk [15] indicated that this would be a significant cost of colony formation for Scenedesmus in that faster sinking will increase the rate at which cells move downward out of a euphotic zone in a stratified water column. Other studies, however, suggest that an increased rate of sinking may increase nutrient uptake [16], and the importance of sinking loss rates will depend on habitat structure, such as whether the environment in question is a stratified lake or unstratified pond. Perhaps the principal selective force involved in induced coloniality is from shifting mortality patterns: algae can minimize sinking by remaining small or minimize grazing by being large, but not both. However, other fitness components seem likely. Coloniality changes several major geometric features, such as surface area to volume ratios, and these may have more universal impact on fitness than sinking rates. It has been postulated that colony formation in phytoplankton may involve physiological costs such as reduction in nutrient uptake [11,17] or in other unknown fitness components [11,18,19]. There is still little or no evidence, however, of any such physiological cost to coloniality.
Direct comparison of unicellular and colonial morphs in terms of nutrient uptake, photosynthetic rates or other physiological aspects would be a straightforward approach to measuring costs of coloniality, but these are insensitive measures; small differences between colonial and unicellular morphs could be lost in measurement noise. In this study, we used a novel means of measuring physiological costs that could potentially be applied to many biological systems. Potential fitness changes resulting from coloniality were measured by examining the population dynamics of two morphs of one species relative to a second, reference species, by pairing either predominantly unicellular or colonial Desmodesmus against the same unicellular reference competitor species. The rate of displacement of one species by another may detect subtle differences in growth because these ecological dynamics integrate small differences over time to produce a measurable effect over ecologically relevant time scales.
2. Material and methods
Desmodesmus subspicatus (R. Chodat) E. Hegewald & A. Schmidt (NIES-802) from the National Institute for Environmental Studies (NIES), Tsukuba, Japan, and Monoraphidium griffithii (Berkeley) Komárková-Legnerová (SAG 202-13) from the culture collection at the University of Göttingen, Germany, had been maintained in our laboratory in a modified NIES-C medium [20] for 9 months and in Algal Stock COMBO medium [21] for 23 months, respectively, at the start of the experiment. Subcultures of these two algae were used as the inocula. Monoraphidium griffithii, a non-motile, non-colony-forming green alga, was chosen as the reference species because it is a cosmopolitan species that often coexists with Desmodesmus in nature, is easily distinguishable from D. subspicatus under the microscope, and has a comparable mean particle volume (approx. 115 × 10−15 l) to D. subspicatus (approx. 70 × 10−15 l for unicells, approx. 191 × 10−15 l for 4-cell colonies). Mostly unicellular Scenedesmus and Monoraphidium were also found to possess comparable qualities as food for a rotifer [22], while Desmodesmus and Scenedesmus are considered a moderately close relative to Monoraphidium within Chlorophyceae (e.g. [23]).
The culture medium was identical to NIES-C [24] except that ZnCl2 replaced ZnSO4 7H2O [20] and that nitrogen (N) and phosphorus (P) contents were adjusted as noted. Estimation of baseline growth rates and the competition experiment described below were conducted with a modified NIES-C medium with molar nitrogen (N) to phosphorus (P) ratio of 5 : 1 (10.5 : 2.1 mM). All cultures were grown at 20°C, 90 µmol quanta m−2 s−1 PAR, provided by cool white fluorescent tubes (Phillips F32T8/TL741, Andover, MA, USA) under a 15 L : 9 D cycle. The dilution rate (D) was defined as the fraction of the culture replaced with fresh growth medium per day. Octyl sodium sulphate (OSS; CAS 142-31-4, Fluka 75074, St Louis, MO, USA), one of the most active components of Daphnia kairomone [25], was dissolved in ultrafiltered water to yield a 100 mmol × l−1 stock solution, which was stored at 4°C. This OSS solution was added to ‘OSS’ treatments to induce colony formation in D. subspicatus. Analyses of unialgal cultures (inocula) were performed electronically, using a CASY 1 Model TTC cell counter (Schärfe System, Reutlingen, Germany) with a 150 µm capillary for an equivalent spherical diameter (ESD) range of 1–10 µm. A typical unicell, 4-cell colony and 8-cell colony of D. subspicatus and a typical M. griffithii cell had an ESD of approximately 5.1, 7.1, 8.8 and 6.0 µm, respectively. Samples for microscopic counting were preserved with Lugol's iodine solution, settled overnight in 10 ml chambers, and quantified under an inverted light microscope at 400× magnification.
A one-factor (control versus 40 µmol l−1 OSS) baseline experiment was run in duplicates in order to estimate maximum growth rate (μ) and examine the general pattern of growth for the two algae. Unialgal batch cultures of D. subspicatus and M. griffithii were started using 50 µl each of the subcultures, which had been maintained for 10 days as semi-continuous cultures (dilution rate = D = 0.3 d−1) with or without 40 µmol l−1 OSS. The last medium change took place 5 days prior to the start of this experiment. Initial biovolume and mean colony size, estimated from a single inoculum, for D. subspicatus were 4.8 × 10−8 ml ml−1 and 1.0 cells colony−1 for control and 7.6 × 10−8 ml ml−1 and 2.2 cells colony−1 with OSS. Similarly, estimated initial biovolume and mean particle volume for M. griffithii were 3.8 × 10−8 ml ml−1 and 113 × 10−15 ml for control and 3.7 × 10−8 ml ml−1 and 132 × 10−15 ml with OSS. Octyl sodium sulphate, if applicable, was used in the pre-cultured inocula, while during the experiment it was added only once, on day 0. The batch cultures were maintained for 20 days.
The experiment with a co-occurring reference species was executed with five replicates per treatment as a one-factor experiment, comparing control versus 40 µmol l−1 OSS. The two inocula came from semi-continuous unialgal cultures (D = 0.3 d−1), which had been maintained for 10 days with or without 40 µmol l−1 OSS. On day 0 each of the five control flasks were inoculated with the control inocula at approximately 3.3 × 10−10 ml ml−1 for each species, and the five OSS flasks were inoculated at the same concentration with the OSS inocula. The experimental cultures were then placed on an orbital shaker at 90 r.p.m. and maintained daily for 27 days as semi-continuous cultures at D = 0.3 d−1. The cultures had a constant volume of 150 ml and were grown in 250 ml glass Erlenmeyer flasks covered with inverted 50 ml glass beakers. They were maintained and sampled daily in a laminar-flow clean hood. The following values were determined by microscopic counting: total cell count, total particle count, counts for each size class (unicells up to 8-cell colonies), mean colony size (= total cell count/total particle count) and growth rate, μ (= apparent growth, r + mortality by dilution, D). Net growth rate, r (= ln(Nt/N0)/Δt), was calculated for each day over the last 2 days, except for day 1, where it was calculated over a 1 day period. The natural log of the ratio of abundances at time t, Y(t) = ln(NDesmodesmus,t/NMonoraphidium,t), was calculated and regressed against day. Statistical analyses were performed with statistical software packages SAS 9.1 (SAS Institute, Cary, NC, USA) and Statistica 7.1 (StatSoft, Tulsa, OK, USA). In SAS, proc GLM was used for general linear models and proc MIXED for mixed model repeated-measures ANOVA, where replicates within treatments were specified as the random effect term.
3. Results
Octyl sodium sulphate treatment had no statistically significant effect on the maximum growth rates (μmax) of D. subspicatus or M. griffithii monocultures (contrast between treatments, proc GLM, SAS: p = 0.4, F1,16 = 0.8 for D. subspicatus; p = 0.7, F1,16 = 0.2 for M. griffithii; table 1). Although the estimated means for μmax differed slightly more between control and OSS for D. subspicatus, analysis of its growth rate over the entire 20-day period also indicated no significant treatment effect (proc MIXED, SAS: p = 0.2, power = 0.37 with α = 0.05). Even if the number of replicates was increased to five per treatment, as in our competition experiment, the power to detect a significant, direct growth rate response would still have been 0.61 for D. subspicatus (and even smaller for M. griffithii). Combined with the fact that neither alga entered stationary phase in the baseline experiment until day 18, the absence of statistically significant OSS effect on algal growth further justified the need for more sensitive measures to detect small differences in fitness. Thus, instead of attempting to measure physiological differences associated with coloniality directly through a species-specific response, we turned to measures using comparative population dynamics.
Table 1.
Maximum growth rates (μmax ± 1 s.e. d−1) of the two species when growing alone without (control) and with colony-inducing OSS.
| species | control | OSS |
|---|---|---|
| Desmodesmus subspicatus | 0.735 ± 0.050 | 0.674 ± 0.056 |
| Monoraphidium griffithii | 0.596 ± 0.046 | 0.612 ± 0.056 |
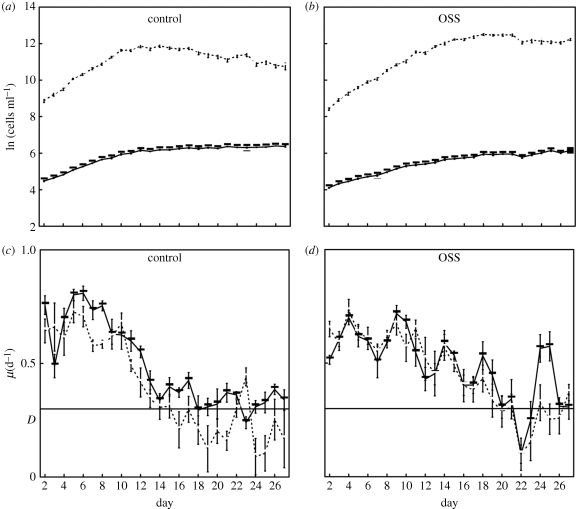
Throughout the competition experiments, D. subspicatus was consistently colonial (predominance of 4 or 8 cells colony−1) in OSS cultures, whereas it was mostly unicellular (<1.5 cells colony−1) in control cultures (electronic supplementary material, figure S1). Overall, 84 per cent of all D. subspicatus particles in control cultures were unicellular, with another 14 per cent as 2- to 4-cell colonies. With OSS, unicells comprised only 2 per cent of the algal particles, while 2- to 4-cell colonies accounted for 76 per cent and 5- to 8-cell colonies 22 per cent. Four-cell colonies were the most dominant colony size on most days; 8-cell colonies became as common as 4-cell colonies around day 11 but rapidly decreased after day 14 (electronic supplementary material, figure S2). In this experiment colonies larger than eight cells were never observed. Figure 1 shows population sizes and growth rates (μ) of the two competing species with or without OSS over the 27-day run. No clear evidence for a physiological cost of coloniality was manifested in the abundance plots (figure 1a,b). In control cultures, the population size of D. subspicatus reached its maximum between day 10 and day 15 and then started to decline, presumably owing to competition with M. griffithii, while with OSS a decline was observed only slightly after day 20. This was manifested as a significant treatment × day effect on D. subspicatus population size (proc MIXED, SAS: p < 0.0001, F27,216 = 22.5) over the 27 day period. The general trend of high growth rates (μ) followed by a period of growth rate approaching the dilution rate (D) corresponds to expectations for semi-continuous cultures beginning at low density then reaching carrying capacity. Desmodesmus subspicatus in control cultures had slightly higher growth rate than M. griffithii on 75 per cent of the days (sign test: p ≈ 0, Z = 5.53), whereas with OSS the number is reduced to 59 per cent (p = 0.044, Z = 2.02; figure 1c,d). Note that at the start of the competition, each culture was inoculated with two species at the same biovolume (approx. 3.3 × 10−10 ml ml−1) based on results of electronic particle analysis. Since particle sizes differed among the four inocula (across species and treatment), the starting population sizes (cells ml−1) were not identical.
Figure 1.
Population sizes (a,b) and growth rates (c,d) of D. subspicatus and M. griffithii plotted against day in competition experiments. Solid lines: D. subspicatus; dashed lines: M. griffithii. Mean values of five replicates were plotted with error bars of ±1 s.e. D = dilution rate = 0.3 d−1.
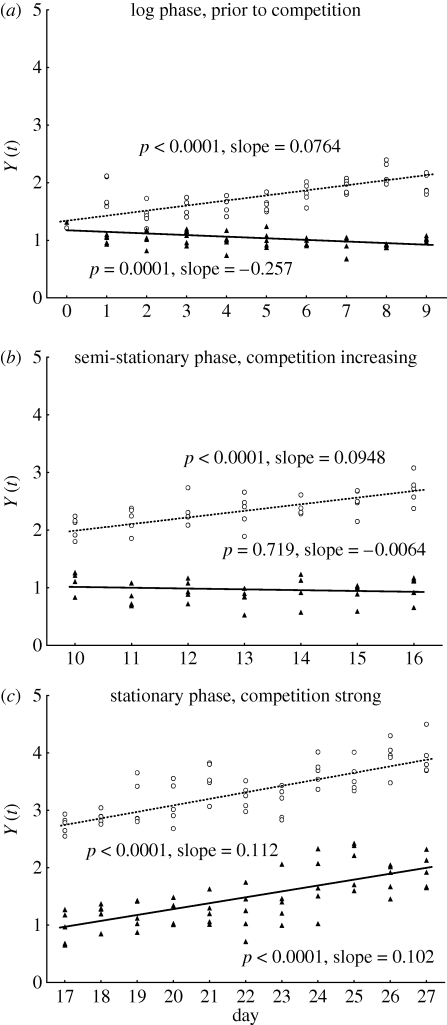
The preceding analyses hint at some differences in population dynamics in unicellular versus colonial morphs. Small physiological differences in these two species may be resolvable by looking more closely at rates of growth of target versus reference species. The natural log of the ratio of the abundances of the two competitors, Y(t) = ln[NDesmodesmus(t)/NMonoraphidium(t)], was therefore plotted against day. The slope of a linear regression of Y(t) versus t has been considered the competitive displacement rate [26,27] and would be appropriate if displacement rate were constant. However, in this study, displacement rate was not constant (electronic supplementary material, figure S2). Thus the dataset was further analysed by splitting it into three time periods: days 0–9, 10–16 and 17–27 (figure 2), which corresponded to different phases of the competition dynamics. The first period was the initial log phase (consistently high μ); the second period was the semi-stationary phase as the species shifted from density-independent growth to a competitive regime (decreasing μ); and the last period corresponds to the stationary phase characterizing competitive dynamics (μ near the dilution rate). Though a smooth polynomial fit is a better description of the data in its entirety, separate line segments are adequate descriptions of these separate periods and provide a convenient basis of interpretation, because displacement rate is approximately constant for short periods of time. In control cultures, the slope of Y(t) versus t was positive throughout, which means that mostly unicellular D. subspicatus consistently displaced M. griffithii through all three phases. Cultures with OSS, on the other hand, initially had a negative slope, indicating that D. subspicatus was performing poorly in comparison with M. griffithii. The slope became undetectable (approx. 0) in the middle phase and then increased in the last phase to a level comparable to that of the control cultures. The displacement rates (slopes) were significantly different between control and OSS treatment for days 0–9 (contrast, proc GLM, SAS: p < 0.0001, F1,96 = 63.5) and 10–16 (p < 0.001, F1,66 = 15.9), but not for days 17–27 (p = 0.6, F1,106 = 0.23).
Figure 2.
Comparison of displacement rates (Y(t)) for D. subspicatus between control (open circles) and OSS (filled triangles) treatments. Separate linear regressions were fitted for the three growth phases: days (a) 0–9, (b) 10–16 and (c) 17–27.
4. Discussion
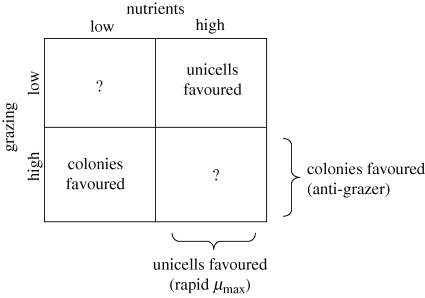
Species dynamics indicated that there was an adverse effect of OSS-induced coloniality on growth of D. subspicatus, but only during the initial log phase of the experiment. No adverse effect was detected during the stationary, competitive phase. Results from this experiment strongly support the hypothesis that there is physiological cost involved in kairomone-induced colony formation in D. subspicatus and further point to traits associated with rapid growth rather than to traits associated with competition at low nutrient levels. Though effects of coloniality on resource uptake and use for growth have often been speculated upon, this work shows, for perhaps the first time, that there is a cost to life in a colony associated with physiological traits related to growth. The growth rate difference between the two algae was not large either with low (control) or high (with OSS) coloniality, and was statistically significant only in reference to growth relative to a reference species. However, phenotypic differences of small effect may have major effects on species success on ecologically relevant time scales, and thus may be important fitness components. In this respect, selection of the reference species may play a critical role in this type of experiment. Using an organism that is phylogenetically very distant from and shares little ecology with the species under question may not reveal interpretable differences in population dynamics under different growth conditions. Coloniality depressed the rate of displacement of M. griffithii by D. subspicatus under high nutrient conditions during the log phase (figure 2a), which could be a critical period of establishment of a population in a previously unoccupied habitat. Coloniality may interfere with rapid resource acquisition necessary for high growth rate, or may have some interfering effect on cell division at high growth rate. Coloniality, however, was not costly under all conditions—the cost of coloniality, visualized in figure 2 as the difference in slopes between control and OSS within each panel, decreased as the cultures entered the semi-stationary phase (figure 2b) and disappeared during the stationary phase (figure 2c). Relying on previous studies documenting anti-grazer benefits of coloniality, our new results indicate the existence of a fundamental underlying trade-off, whereby colonial morphology is favoured under high grazing and low nutrients (figure 3, lower left square) but unicellular morphology is favoured under low grazing and high nutrients (figure 3, upper right square). The high-grazing, low-nutrient condition may, for example, be observed in temperate systems in the middle of the growing season and is expected to be similar to figure 2c, where algae that survive established populations of grazers compete for available nutrients. In contrast, the low-grazing, high-nutrient condition would be similar to figure 2a and may resemble the start of a growing season, where, among a greater variety of algae, those that build up their populations quickly by taking the greatest advantage of the high nutrient availability become dominant species later on. Identifying different algal growth conditions favouring different morphs, as we have done here, provides support for the adaptive nature of herbivore-induced colony formation in phytoplankton.
Figure 3.
The cost–benefit landscape of coloniality. For a phenotypically plastic trait to be considered adaptive there must be an underlying trade-off that favours one form in one environment but another form in another environment. Previous work on Desmodesmus spp. has shown a benefit to coloniality in offering some protection against herbivores, so coloniality is considered a benefit under high grazing conditions. The costs to coloniality have been more elusive, however, and the present study shows that unicells have higher growth rate under high nutrients. Hence, we can now consider unicells to be favoured over colonies under high nutrient conditions. These costs and benefits resolve into different morphs being favoured under different environmental states. Known costs and benefits do not make unequivocal predictions for two of the environmental states (marked with ‘?’).
This trade-off between grazing resistance and growth at high nutrient availability is in accordance with the argument that evolution of multicellularity resulted from trade-offs between survival and reproduction based on observations in volvocine algae (e.g. Chlamydomonas and Volvox), which vary from unicellular to 50 000 cells per colony [28]. Desmodesmus and Scenedesmus may be considered to represent the very beginning of the unicellular–multicellular transition since their coloniality is plastic: some populations remain almost entirely unicellular while other populations may exhibit varying degrees of colony formation, depending on their environment. Yet some species already show some primitive sign of intra-colonial differentiation and possibility of communication between cells, as in the distinct, long spines that only develop on end cells (e.g. Scenedesmus quadricauda).
Yet another consideration in terms of phenotypic plasticity is the cost of maintaining the flexibility [29]. Van Holthoon [30] showed by repeating colony induction experiments over a 3-year period that the magnitude of colony-formation in Scenedesmus obliquus decreased over time. Verschoor and colleagues [14] attributed this gradual attenuation of the colony-forming response to the ‘hidden’ cost of maintaining the capacity to induce colony formation. This cost is expected to manifest itself when growing in the absence of grazers and underlies the physiological cost of colony formation studied here.
In this study, coloniality was induced using the chemical OSS. Octyl sodium sulphate is not just an active component of Daphnia kairomone [25] but belongs to a class of ubiquitously used detergents called alkyl sulphates (AS). Although AS have a relatively short half-life in surface water (e.g. 1–2 days for linear alkylbenzene sulphonate [31]), because they are used in large quantities worldwide, their influence on phytoplankton populations in nature may be measurable. Typical toxicological studies such as those using Scenedesmus as a representative green alga are based on single-species growth rates. However, competitive dynamics may be a much more sensitive indicator of fitness differential. Effects of compounds such as AS may be much more readily measured when using a competitive trial such as that we used here, and thus may be having impacts at lower concentrations than the existing ‘no observable effect’ concentrations.
Study of costs and benefits of inducible defences will continue to contribute to our understanding of evolution of defence mechanisms in general, and to our effort to model and predict outcomes of ecological interactions including inducible defences. Costs of inducible defence can be difficult to detect; however, population dynamics relative to a reference species can serve as an ecologically relevant and sensitive proxy for relative fitness of the morphologically plastic species. This could be because some of the day-to-day variability in population abundance may be correlated across species, and our method may have effectively cancelled out some of the statistical ‘noise’ that could otherwise have masked subtle differences in fitness between morphs. This new approach can supplement conventional measurements when the expected cost is too small to be measured directly.
Acknowledgements
This research was funded by NSF grant OCE 0344228.
References
- 1.Maynard Smith J., Szathmáry E. 1995. The major transitions in evolution. New York, NY: Oxford University Press [Google Scholar]
- 2.Pigliucci M. 2005. Evolution of phenotypic plasticity: where are we going now? Trends Ecol. Evol. 20, 481–486 10.1016/j.tree.2005.06.001 (doi:10.1016/j.tree.2005.06.001) [DOI] [PubMed] [Google Scholar]
- 3.Tollrian R. 1993. Neckteeth formation in Daphnia pulex as an example of continuous phenotypic plasticity: morphological effects of Chaoborus kairomone concentration and their quantification. J. Plankton Res. 15, 1309–1318 10.1093/plankt/15.11.1309 (doi:10.1093/plankt/15.11.1309) [DOI] [Google Scholar]
- 4.Agrawal A. A. 2000. Benefits and costs of induced plant defense for Lepidium virginicum (Brassicaceae). Ecology 81, 1804–1813 [Google Scholar]
- 5.Van Donk E. 2007. Chemical information transfer in freshwater plankton. Ecol. Inform. 2, 112–120 [Google Scholar]
- 6.Clark C. W., Harvell C. D. 1992. Inducible defenses and the allocation of resources: a minimal model. Am. Nat. 139, 521–539 10.1086/285342 (doi:10.1086/285342) [DOI] [Google Scholar]
- 7.Hessen D. O., Van Donk E. 1993. Morphological changes in Scenedesmus induced by substances released from Daphnia. Arch. Hydrobiol. 127, 129–140 [Google Scholar]
- 8.Geller W., Müller H. 1981. The filtration apparatus of Cladocera: filter mesh sizes and their implications on food selectivity. Oecologia 49, 316–321 10.1007/BF00347591 (doi:10.1007/BF00347591) [DOI] [PubMed] [Google Scholar]
- 9.Jack J. D., Gilbert J. J. 1993. Susceptibilities of different-sized ciliates to direct suppression by small and large cladocerans. Freshwat. Biol. 29, 19–29 10.1111/j.1365-2427.1993.tb00740.x (doi:10.1111/j.1365-2427.1993.tb00740.x) [DOI] [Google Scholar]
- 10.Lürling M., De Lange H. J., Van Donk E. 1997. Changes in food quality of the green alga Scenedesmus induced by Daphnia infochemicals: biochemical composition and morphology. Freshwat. Biol. 38, 619–628 10.1046/j.1365-2427.1997.00225.x (doi:10.1046/j.1365-2427.1997.00225.x) [DOI] [Google Scholar]
- 11.Van Donk E., Lürling M., Lampert W. 1999. Consumer-induced changes in phytoplankton: inducibility, costs, benefits, and the impact on grazers. In The ecology and evolution of inducible defenses (eds Tollrian R., Harvell C. D.), pp. 89–103 Princeton, NJ: Princeton University Press [Google Scholar]
- 12.Jakobsen H. H., Tang K. W. 2002. Effects of protozoan grazing on colony formation in Phaeocystis globosa (Prymnesiophyceae) and the potential costs and benefits. Aquat. Microb. Ecol. 27, 261–273 10.3354/ame027261 (doi:10.3354/ame027261) [DOI] [Google Scholar]
- 13.Lürling M. 2003. The effect of substances from different zooplankton species and fish on the induction of defensive morphology in the green alga Scenedesmus obliquus. J. Plankton Res. 25, 979–989 10.1093/plankt/25.8.979 (doi:10.1093/plankt/25.8.979) [DOI] [Google Scholar]
- 14.Verschoor A. M., van der Stap I., Helmsing N. R., Lürling M., Van Donk E. 2004. Inducible colony formation within the Scenedesmaceae: adaptive responses to infochemicals from two different herbivore taxa. J. Phycol. 40, 808–814 10.1111/j.1529-8817.2004.04007.x (doi:10.1111/j.1529-8817.2004.04007.x) [DOI] [Google Scholar]
- 15.Lürling M., Van Donk E. 2000. Grazer-induced colony formation in Scenedesmus: are there costs to be colonial? Oikos 88, 111–118 10.1034/j.1600-0706.2000.880113.x (doi:10.1034/j.1600-0706.2000.880113.x) [DOI] [Google Scholar]
- 16.Logan B. E., Alldredge A. L. 1989. Potential for increased nutrient uptake by flocculating diatoms. Mar. Biol. 101, 443–450 10.1007/BF00541645 (doi:10.1007/BF00541645) [DOI] [Google Scholar]
- 17.Porter K. G. 1973. Selective grazing and differential digestion of algae by zooplankton. Nature 244, 179–180 10.1038/244179a0 (doi:10.1038/244179a0) [DOI] [Google Scholar]
- 18.Grover J. P. 1995. Competition, herbivory, and enrichment: nutrient-based models for edible and inedible plants. Am. Nat. 145, 746–774 10.1086/285766 (doi:10.1086/285766) [DOI] [Google Scholar]
- 19.Tessier A. J., Bizina E. V., Geedey C. K. 2001. Grazer-resource interactions in the plankton: are all daphniids alike? Limnol. Oceanogr. 46, 1585–1595 10.4319/lo.2001.46.7.1585 (doi:10.4319/lo.2001.46.7.1585) [DOI] [Google Scholar]
- 20.NIES 2004. NIES collection list of strains: microalgae and protozoa, 7th edn. Tsukuba, Japan: National Institute for Environmental Studies [Google Scholar]
- 21.Kilham S. S., Kreeger D. A., Lynn S. G., Goulden C. E., Herrera L. 1998. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377, 1–3 [Google Scholar]
- 22.Rothhaupt K. O. 1990. Population growth rates of two closely related rotifer species: effects of food quantity, particle size, and nutritional quality. Freshwat. Biol. 23, 561–570 10.1111/j.1365-2427.1990.tb00295.x (doi:10.1111/j.1365-2427.1990.tb00295.x) [DOI] [Google Scholar]
- 23.Fawley M. W., Dean M. L., Dimmer S. K., Fawley K. P. 2006. Evaluating the morphospecies concept in the Selenastraceae (Chlorophyceae, Chlorophyta). J. Phycol. 42, 142–154 10.1111/j.1529-8817.2006.00169.x (doi:10.1111/j.1529-8817.2006.00169.x) [DOI] [Google Scholar]
- 24.Watanabe M., Kawachi M., Hiroki M., Kasai F. 2000. NIES collection list of strains: microalgae and protozoa, 6th edn. Tsukuba, Japan: Microbial Culture Collections, National Institute for Environmental Studies [Google Scholar]
- 25.Yasumoto K., Nishigami A., Yasumoto M., Kasai F., Okada Y., Kusumi T., Ooi T. 2005. Aliphatic sulfates released from Daphnia induce morphological defense of phytoplankton: isolation and synthesis of kairomones. Tetrahedron Lett. 46, 4765–4767 10.1016/j.tetlet.2005.05.027 (doi:10.1016/j.tetlet.2005.05.027) [DOI] [Google Scholar]
- 26.Grover J. P. 1991. Dynamics of competition among microalgae in variable environments: experimental tests of alternative models. Oikos 62, 231–243 10.2307/3545269 (doi:10.2307/3545269) [DOI] [Google Scholar]
- 27.Passarge J., Hol S., Escher M., Huisman J. 2006. Competition for nutrients and light: stable coexistence, alternative stable states, or competitive exclusion? Ecol. Monogr. 76, 57–72 10.1890/04-1824 (doi:10.1890/04-1824) [DOI] [Google Scholar]
- 28.Michod R. E., Viossat Y., Solari C. A., Hurand M., Nedelcu A. M. 2006. Life-history evolution and the origin of multicellularity. J. Theor. Biol. 239, 257–272 10.1016/j.jtbi.2005.08.043 (doi:10.1016/j.jtbi.2005.08.043) [DOI] [PubMed] [Google Scholar]
- 29.DeWitt T. J., Sih A., Wilson D. S. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81 10.1016/S0169-5347(97)01274-3 (doi:10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 30.Van Holthoon F. L. 2004. Isolation and identification of kairomone(s) in the Daphnia–Scenedesmus system. Wageningen University, The Netherlands [Google Scholar]
- 31.International Programme on Chemical Safety 1996. Environmental health criteria 169: linear alkylbenzene sulfonates and related compounds. World Health Organization; See http://www.inchem.org/documents/ehc/ehc/ehc169.htm [Google Scholar]