Abstract
The conflict in Afghanistan has produced injuries similar to those produced from military conflicts for generations. What distinguishes the modern casualty of the conflict in Afghanistan from those of other conflicts is the effectiveness of modern field medical care that has led to individuals surviving with injuries, which would have been immediately fatal even a few years ago. These patients present several challenges to the reconstructive surgeon. These injured individuals present early challenges of massive soft-tissue trauma, unstable physiology, complex bony and soft-tissue defects, unusual infections, limited reconstructive donor sites, peripheral nerve injuries and traumatic amputations. Late challenges to rehabilitation include the development of heterotopic ossification in amputation stumps. This paper outlines the approach taken by the reconstructive team at the Royal Centre for Defence Medicine in managing these most difficult of reconstructive challenges.
Keywords: reconstruction, wounds, amputation
1. Introduction
The modern medical evacuation chain is a highly efficient process, with patients often arriving at the Royal Centre for Defence Medicine within 48 h of wounding. UK service personnel are surviving injuries, which in earlier conflicts would have been fatal, and up until September 2009 there had been 44 unexpected UK survivors, i.e. survivors with an injury severity score (ISS) of greater than 60. This improvement is multi-factorial, reflecting improvements in individual personal protective equipment as well as modern developments in forward medical care. The concentration of experience and maintenance of a corporate clinical memory is paramount. The realization that the system is more important than the individual clinician and can deliver marked improvement in population outcomes [1] is at the forefront of how injured UK servicemen and women are managed [2].
Initial stabilization of the injured is performed at the role 3 facility within the theatre of military operations. The principles of war surgery: arrest of haemorrhage; thorough wound debridement; temporary stabilization of fractures; removal of contamination and foreign bodies; copious wound lavage and administration of antibiotics are well recognized. What is uncertain, however, is the ideal reconstructive protocols for these patients. The timing of reconstruction, whether to embark upon complex reconstruction in an acutely sick patient or to obtain wound closure by the simplest method and defer complex reconstruction until the patient is stabilized poses difficult clinical conundrums for the clinicians charged with treating these patients. Much of the evidence regarding management of injury stems from civilian practice; however, military wounds differ from civilian wounds in a number of important ways. These include the mechanism of injury, the degree of contamination, and presence and severity of associated injuries. Our practice at role 4 in Birmingham has been to attempt to apply best civilian practice while being aware that the evidence derived from civilian practice does not necessarily apply to military wounds.
The hallmark injury seen in the current conflict in Afghanistan is a blast injury produced by the improvized explosive device (IED). Blast injuries are heterogeneous complex events and personnel sustain injuries that differ from civilian injuries by virtue of their distribution, the mechanism and the degree of contamination. Blast injuries are characterized as primary (caused by the effect of peak overpressure on tissues), secondary (caused by flying objects or fragments), tertiary (caused by bodily displacement) or quaternary (caused by explosion) [3]. This produces a heterogeneous mixture of sharp, blunt and penetrating trauma, which may affect the entire body and is of discontinuous distribution. Primary blast injuries include fractures, amputations, crush injury, burns, cuts, lacerations, acute occlusion of an artery, air embolism-induced injury, compartment syndrome and others. Secondary injuries are the most common extremity blast injuries. Like primary injuries, they may necessitate limb amputation, be life-threatening and produce severe contamination. Tertiary blast injuries of the extremity may result in traumatic amputations, fractures and severe soft-tissue injuries. Quaternary injuries most often are burns. The presence of multiple injuries often leads to competing priorities of care and these are discussed at daily planning meetings by the role 4 multidisciplinary team.
2. Surgical wound care
(a). Wound debridement
A meticulous debridement is the sine qua non for successful reconstruction. At the first and all subsequent theatre sessions, all wounds, no matter how small or inaccessible are explored and debrided.
The best evidence on timing of debridement comes from the studies of open tibial fracture management. Debridement of open fractures has been performed emergently as exposed bone and deep tissues are likely to have been inoculated with bacteria and other micro-organisms. Surgical treatment is centred on wound irrigation and surgical debridement in the operating theatre within 6 h of injury. This 6 h time limit is based on an extrapolation of knowledge of bacterial doubling times and the clinical evidence for this time limit is conflicting. Kindsfater et al. [4] showed a statistically significant reduction in the rate of osteomyelitis when patients were taken to the operating theatre within 5 h; conversely, there are many studies reporting no significant difference in infection rates when irrigation and debridement took place either side of the 6 h cut-off [5]. All military patients with open fractures will go to theatre immediately upon admission to role 3 facilities for an initial debridement and washout, this is repeated upon the day of arrival at role 4. When debriding wounds, it is helpful to bear in mind the classification system described by Granick & Chehade [6]. This system is based on a concept of tissue injury similar to that described by Jackson when discussing his burn wound model [7]. This concept describes a central area comprised of dead and devitalized tissue, surrounded by an area of living but injured tissue. Surrounding this area is normal tissue. Based on these zones, Granick & Chehade [6] classified wounds according to the level of debridement into:
— Non-debrided wound.
— Incomplete debridement: in which not all of the necrotic material has been removed.
— Marginal debridement: in which all the necrotic tissue has been removed but injured and potentially viable tissue is retained.
— Complete debridement: removal of both necrosed and marginal tissue.
— Radical debridement: which includes normal tissue within the field of excision.
We favour meticulous repeated marginal debridements in military wounds in order to preserve as much tissue as possible and maximize functional outcomes. A radical debridement would necessitate the removal of healthy functional tissue which may otherwise survive. Our protocol for repeated debridements is for a return to theatre every 48–72 h until no further sign of necrosis or infection has occurred. This protocol contrasts with the International Red Cross (IRC) guidelines that recommend an initial debridement followed by application of gauze dressings, which are then left undisturbed until a delayed primary closure is performed at 5 days. However, we have observed progressive necrosis of soft tissues in blast-related wounds.
Limb debridement should always be performed under a tourniquet if possible. Large, complex wounds are often uneven and contain numerous pockets. Without tourniquet control, these areas are quickly obscured with blood so that effective excision of necrotic and contaminated tissue can never be reliably achieved. Furthermore, blood loss can be significant.
It is important not to forget wounds of the buttocks because they are difficult to access with the patient in the supine position; such wounds are ignored at the patient's peril. Foreign material can be driven far up into the tissue planes, far removed from the original injury. All such potentially heavily contaminated material must be removed if subsequent infections are to be avoided. The use of the Versajet hydrosurgery system (Smith & Nephew, Hull, UK) is a particularly useful adjunct in ensuring the removal of all ingrained mud, sand and dirt from the wound surface. High-pressure irrigation is not recommended as this may drive contamination deeper into the tissues and cause additional soft-tissue damage [8,9].
Wound swabs as well as samples of tissue are taken and sent for microbiological assessment, by both culture and histological examination. In heavily contaminated wounds, samples of healthy and dead muscle should be taken. The results of these cultures may be used subsequently to tailor antimicrobial drug use in the individual patient. With sufficient experience and numbers of specimens, these results also guide the empirical use of antimicrobials before an individual patient's results are known. After complete wound excision, all wounds are irrigated with hydrogen peroxide and then with copious amounts of warmed saline.
Photographic documentation of each theatre session facilitates the formation of a surgical reconstruction plan. Such imaging will prevent unnecessary disturbance of dressings by subsequent medical teams, and will ultimately aid classification of wounds and the retrospective analysis of injuries, which will allow the ongoing development of best practice.
(b). Dressings
There is lack of published evidence on the use of negative pressure wound therapy (NPWT) in military injuries [10], but the authors feel that these dressings should be used for all but the most minor military wounds after they have been completely debrided. Sub-atmospheric pressure is applied to the wound bed through a wound filler material, which is covered with an airtight seal. Exudate is drawn off into a collection device, allowing for wound isolation and containment. Until recently, the only filler material available was open-cell polyurethane foam, as part of the VAC system (KCI, San Antonio, Texas). Subsequently, a gauze filler impregnated with polyhexamethylene biguanide has become available (Smith & Nephew, UK). This is laid directly onto the wound bed, a drain is then connected to the dressing and an occlusive film applied. We have found that the gauze fits the contours of the wounds much better than sponge, and it has become invaluable in the management of these irregularly shaped wounds. We have also found that the use of gauze prevents the problems of retained pieces of sponge, and reduces the problem of tissue ingrowth into the dressing compared with sponge. We try not to use a wound contact layer, as this reduces the efficacy of the negative pressure. A wound contact layer is essential however if there are exposed vessels or tendons, and when NPWT is applied over a skin graft.
It is not unusual for some of the large wounds to produce many litres of exudate per day, and such fluid loss needs to be compensated for when calculating overall fluid requirements. Such big wounds may also require the use of more than one pump.
Using NPWT dressings for the limbs also acts as a splint, so that no further splints need to be applied.
3. Military trauma-related infections
(a). Risk factors for infection
In many instances, the risk factors for the wounded soldier developing infection are similar to those of the civilian trauma patient. The patients' microbial flora such as the carriage of Staphylococcus aureus may predispose to infection, as has been demonstrated in elective surgery [11]. There is also a risk of infection from the many necessary medical interventions required to save and maintain life: insertion of chest drains, intravenous cannulae, urinary catheters and endotracheal intubation.
Finally, the nature and extent of the military blast injury contributes to the likelihood of infection through the breaching of physical host defences, hypoxic tissue damage resulting in necrosis, haematoma formation and the implantation of foreign bodies; the latter three of these factors provide a rich, often anaerobic, environment in which micro-organisms may grow.
The blast injury causes these environmental organisms to be implanted deep into devitalized tissues, where they may grow. Often, these environmental organisms are of low pathogenicity and are unusual causes of infection in immunocompetent patients. However, while on operations, soldiers may only have been able to maintain basic hygiene, and as a result of the austere environment of forward operations may already be in a catabolic state before injury, which is then exacerbated by severe trauma. The relative nutritional deficiency from this catabolism may cause some degree of immunocompromise in the severely wounded soldier. Following injury, such a patient often receives a massive blood transfusion (more than eight units), which may cause a direct or indirect reduction in immune function, and is associated with increased risk of healthcare-associated infection [12].
An understanding of prevalence of resistant organisms in other parts of the world is important when considering second-line antibiotics for treatment, and when considering infection control precautions. Repatriated patients may have been treated in healthcare facilities in other parts of the world and may have acquired a microbial flora, sometimes with multi-drug resistant organisms, particular to that facility.
Finally, in addition to trauma-related infection, the injured soldier may manifest infectious diseases acquired overseas, e.g. malaria, after return to the UK. Imported infections must always be considered when infection associated with the primary injury or its treatment, are unlikely, or the patient continues to have symptoms of infection in spite of apparently adequate treatment.
(b). Wound microbiology and antibiotic use
For the soldier wounded by a ‘simple’ gunshot, fragments from a rocket-propelled grenade, or a blast while travelling in a vehicle, surgical debridement and simple, short-course antibiotic use (e.g. co-amoxiclav) is generally all that is required to prevent infection. The infections which should be prevented are those owing to S. aureus, the beta-haemolytic streptococci and clostridia (gas gangrene and tetanus). Surgery is particularly important in removing the risk factors (dead tissue, foreign bodies) for developing the anaerobic conditions required for clostridial growth. In addition, soldiers will be up-to-date with active immunization against tetanus. The majority of military trauma may be managed by surgical debridement and simple antibiotic regimes, and the incidence of infection is relatively low.
Soldiers injured while on foot by an IED blast may have extensive wounds and also be heavily contaminated with their own flora and environmental organisms. Before and during their return to the UK, these patients receive the simple antibiotics (e.g. co-amoxiclav) that prevent the infections described above. However, once in the UK and following microbiological wound sampling, they are given ‘pre-emptive’ broad-spectrum antibiotics (e.g. meropenem) and antifungal drugs (see below) that cover most organisms they may have acquired, often deeply, in their wounds. Some organisms, e.g. Comomonas spp., may be resistant to meropenem; they are often dealt with by the addition of ciprofloxacin or gentamicin.
The list of organisms that may be grown from the wounds is extensive: Aeromonas spp., Acinetobacter spp., Achromobacter spp., Comomonas spp., Clostridium spp., coliforms (Enterobacter spp., Escherichia coli and Klebsiella spp. (including extended-spectrum beta-lactamase producers)), enterococci (including those that are vancomycin-resistant), Pseudomonas spp., beta-haemolytic streptococci and S. aureus and fungi (see below). Bacillus spp., (not B. anthracis) are frequently grown from wounds, but they are considered colonizers or contaminants, and not treated with specific antibiotics. However, although many of the other organisms may simply be colonizing the patients' wounds, they may be trapped deep in tissues and require multiple and extensive debridements to physically remove. On occasions, complete debridement is technically impossible. For this reason, the ‘pre-emptive’ antimicrobials described above are used until the surgeons are satisfied that no further debridements are required, there is no clinical evidence of active infection, and the patient's initial inflammatory response is subsiding.
(c). Invasive fungal infection
In a small number of patients returning from Afghanistan, wounds with heavy environmental contamination have been associated with soft-tissue infection by invasive fungi. Species of fungi that have been associated with such infections include: zygomycetes such as Rhizopus spp., Apophysomyces spp., Mucor spp., Saksenaea spp., and Absidia spp., the environmental fungi such as Chaetomium spp., and finally the parasitic oomycete (once considered a fungus), Pythium spp. These organisms are ubiquitous in the environment and are part of normal soil flora being associated with decaying vegetation. Cutaneous infections from zygomycetes are rare but well-recognized in profoundly immunocompromised patients such as those receiving bone marrow transplants. In addition, they have been described rarely in civilian trauma [13].
Analysis of the patients who have developed invasive fungal soft-tissue infections revealed the following common factors:
— They were all injured in the ‘Green Zone’, Helmand Province, Afghanistan, in an area where there is extensive exposure to decaying vegetation;
— They received a blast or other severe injury, usually on foot patrol;
— They all sustained extensive wounds/amputations, which were heavily contaminated with environmental debris; and
— They all required a massive blood transfusion (more than eight units of blood within the first 24 h after wounding).
These risk factors are used as criteria for antifungal prophylaxis (see below).
Fungal infection was not seen in wounded soldiers from Iraq, and probably reflects differences in combat, mode of injury and survival, rather than a unique feature of Afghanistan environmental flora, since these fungi are distributed worldwide. Invasive fungal infection may present several days to several weeks after return to the UK usually with non-specific features of infection, such as fever and raised inflammatory markers. In such cases, wounds are urgently inspected for signs of necrosis and non-viable fat and muscle, which might suggest invasive fungal infection. There have been rare cases of invasive fungal infection where a patient's mode of injury and initial assessment did not indicate that such an infection was likely. Therefore, all patients who have had major injuries have this diagnosis considered if clinical symptoms and signs are suggestive.
Fungi grown from superficial swabs may merely represent environmental contamination from spores on the external surface of the patient. In contrast, fungi grown from deep tissue or normally sterile sites are much more likely to be significant. Laboratory diagnosis of invasive fungal infection is by three means. Tissues obtained from ‘routine’ debridements, or those in which fungal infection is suspected, are stained with the fluorescent calcofluor white stain. This staining allows a relatively large quantity of tissue to be easily and rapidly examined for fungal hyphae. The morphology of fungal hyphae present may suggest a zygomycete. However, not all fungi causing invasive soft-tissue infection are zygomycetes. In addition, this method does not differentiate between fungal colonization/contamination and invasive infection.
The second means of laboratory diagnosis is by culture. This method is relatively slow, but it does allow identification of the fungi grown, and antifungal drug-susceptibility testing. Tissues will not necessarily grow fungi, even if microscopy with calcofluor white detects their presence. This is because the hyphae may be damaged on sampling, leading to cell death before cultures are started in the laboratory. While the results of culture are not received in sufficient time to influence individual patient management directly, they guide empirical use of antifungals on an epidemiological basis. A wide variety of fungi have been grown from wounds. In addition to those above associated with invasive infection, tissues have frequently grown other environmental mould species, such as Aspergillus spp., Acremonium spp., Alternaria spp., Fusarium spp., and Penicillium spp.; these fungi do not appear to have caused invasive soft-tissue infection in our patients.
The third method of laboratory diagnosis is histopathological examination, including silver staining. This microscopy can demonstrate fungal hyphae in tissues, and zygomycetes may be inferred from morphology of hyphae. In addition, histopathology may demonstrate features suggestive of invasive infection: tissue necrosis and micro-vascular invasion. However, since only a small amount of tissue may be examined by this method, the absence of fungi does not exclude the possibility of invasive infection.
Surgical debridement remains the main treatment and preventative measure for this type of invasive fungal infection. However, in addition to surgery, the antifungal drugs ambisome (Gilead Sciences Europe Ltd., Uxbridge, Middlesex, UK; at 5 mg kg−1) and posaconazole (Merck Sharp & Dohme Limited; Hoddesdon, Hertfordshire, UK) are used in combination, both for treatment and antifungal ‘prophylaxis’ in patients who fulfil the criteria listed above.
(d). Aeromonas infection
Soldiers injured in watery environments, and whose wounds are contaminated with groundwater, may get soft-tissue infections with Aeromonas spp., most commonly A. hydrophila or A. sobria. Since they receive pre-emptive meropenem (active against most Aeromonas spp., encountered), the soldiers who receive the most severe IED injuries do not appear to develop these infections. The small numbers of soldiers who do develop this infection have less severe injuries for which antibiotics active against Aeromonas spp., are not used as first-line prophylaxis. Infection usually presents within a week of arrival in the UK, and is treated with ciprofloxacin (depending on laboratory confirmation of sensitivity).
4. Principles of reconstruction
(a). Timing of reconstruction
Early total care (ETC) is the standard of care for all trauma patients once control of haemorrhage has been established. ETC dramatically reduces the incidence of pulmonary failure and systemic complications following polytrauma, and it is in the most severely injured patients that these improvements are most apparent [14,15].
The ultimate goal of the reconstructive surgeon is to restore normal function and aesthetics. Sadly, our ability to achieve the first is often limited, and the second is often impossible. The desire to achieve ‘gold standard’ reconstructive goals must be tempered by clinical realities.
A hierarchy of reconstructive goals exists, the aim is to achieve:
— a clean non-contaminated wound;
— the removal of all dead tissue;
— timely coverage of exposed tendon and bone;
— timely coverage of open fractures;
— provision of robust well-vascularized coverage over nerve grafts and musculotendinous units; and
— provision of robust soft-tissue coverage of amputation stumps.
There is, however, a subset of trauma patients, who do poorly following ETC. This group was first recognized in young patients who developed significant pulmonary complications following ETC [14,15]. Further studies have shown that this ‘borderline’ patient group, have received a trauma sufficient to induce a ‘systemic inflammatory response syndrome’ (SIRS), such that a ‘second hit’ of definitive operative stabilization is sufficient to tip the patients into multi-organ failure. Soft-tissue damage, fractures or hypoxia cause local and systemic inflammatory and immunological responses (SIRS), which are characterized by systemic activation of endothelial cells, platelets and neutrophils, producing capillary leakage, disseminated intravascular coagulation and end-organ damage leading to multi-organ failure and acute respiratory distress syndrome [16]. Efforts have been made to further identify this ‘borderline’ patient group. Giannoudis and co-workers [17] have proposed four pathophysiological cascades that lead to inflammatory mediator release and the development of SIRS. These are: hypothermia, shock, coagulopathy and soft-tissue injury. Within these they have suggested clinically relevant indicators of physiological exhaustion that predict a borderline state and suggest conversion to a damage control strategy (table 1). These clinical parameters are often seen in severely injured servicemen during their first week post-injury.
Table 1.
Clinically relevant indicators of physiological exhaustion that predict a borderline state and suggest conversion to a damage control strategy. From Pape et al. [17].
| parameter | threshold value | intraoperative assessment available? |
|---|---|---|
| platelet count | <90 000 µl−1 | yes |
| I/O ratio | >+5 l per 6 h after the initial injury | limited |
| urinary output | <50 ml h−1 | yes |
| lactate levels | >2.5 mmol l−1 | yes |
| base excess | >8 mmol l−1 | yes |
| body temperature | <33°C | yes |
| transfusion | >3 U h−1 | yes |
| PaO2/FiO2 | <250 | yes |
| age (yr) | >55 | yes |
There are three factors influencing the development of SIRS. These are:
— the size of the initial traumatic insult—‘the first hit’;
— the individual biological response;
— the additional insults (‘second hits’) caused by surgical intervention and/or complications.
Of these, only the third is modifiable by medical management.
It is important to consider whether the patient may tolerate prolonged reconstructive procedures as there is evidence to suggest that procedures lasting longer than 3 h are associated with the development of multiple organ dysfunction [17].
Recent developments in military prehospital care have led to an increasing number of ‘unexpected survivors’ in our wounded personnel. We are routinely presented with patients with injuries so severe that, had they occurred even a few years ago, would have been fatal as a result of immediate haemorrhage. These individuals present to role 4 with evidence of organ dysfunction and coagulopathy, which can persist for many days. Often, the clinical decision for the reconstructive team is not ‘what could be done’ but ‘what should be done’. The reconstructive surgeon has to be aware that early surgery carries a risk of inducing a surgical ‘second hit’ and precipitating multi-organ failure. The timing of reconstruction and its technical complexity must be carefully considered.
(b). The treatment of open fractures
There is a wealth of evidence of varying levels to guide the treatment of open fractures in the civilian setting. Open lower limb fractures do occur in military injuries, although more often in the upper limb and femur as the commonest presentation of IED blast is a traumatic amputation at either transfemoral or transtibial level.
The appropriate method of bony stabilization and soft-tissue reconstruction to be employed is chosen on a case-by-case basis following close collaboration between orthopaedic and plastic surgical teams.
There is clear body of evidence in the literature supporting the early soft-tissue coverage of open tibial fractures. Coverage within a week, and considerably sooner in some studies, has been shown to produce fracture union sooner, have a lower infection rate and require fewer operative procedures [18–20]. The Guidelines of the British Association of Plastic, Reconstructive and Aesthetic Surgeons and British Orthopaedic Association support this in recommending soft-tissue reconstruction within seven days of injury [21]. Our clinical experience is that it is not possible to reliably debride highly contaminated blast wounds in a single sitting, and we have seen severe infective complications resulting from, what was in hindsight, premature flap closure of open fractures in our military patient group. We therefore delay soft-tissue reconstruction until the wounds are macroscopically clean, and remain so on two successive washouts and the patient requiring minimal physiological support.
(c). Patterns of injury
The modern IED produces a characteristic pattern of injury. All body areas can be affected, but in general, the thoracic and abdominal cavities are well protected by combat body armour. Similarly, the cranium is well protected by the combat helmet if worn properly. The principle areas affected are the face, limbs and perineum.
Face: the neck is rarely significantly injured, there can be soft tissue loss of the lips, tip of the nose, and infraorbital rim, injury to the eye and thence into the anterior cranial fossa.
Upper limb: Severe upper limb injuries are rarely life-threatening, however they are resource-intensive and impairment of the function of the upper limb causes significant alteration of lifestyle [22,23]. The spectrum of upper limb injuries includes amputation of the distal phalanges, traumatic thumb amputation; injury to the dorso-ulnar surface of the forearm extending to the posterior surface of the elbow with segmental fractures of the ulna with bone loss, ulnar nerve injuries and damage to, or loss of, the long flexor muscles with loss of soft-tissue coverage. Nerve injuries are often partial with significant contusion of the remaining in-continuity segment of the nerve. Complex reconstruction is commonly required in upper limb injuries. If required, internal fixation of open fractures, cable-grafting to segmental nerve injuries and flap coverage are performed at one sitting. Similar to Kumar et al. [24], we have a preference for distant or free fasciocutaneous flaps in the upper limb. Bermudez and co-workers reported a 12.5 per cent free flap failure rate and our unreported acute free flap failure rate is similar to this. Distant flaps reduce further sacrifice of tissue from an already damaged limb, facilitate later tendon transfers and reconstructive procedures, and are mandatory over exposed fractures and nerve grafts. For large surface area skin loss, we have a preference for immediate split skin grafting of exposed muscle bellies and rapid wound closure. Exposed muscles are often denuded of epimysium and tethering of muscles often occurs following skin grafting; our preference is to perform a late scar-release and flap resurfacing of the limb as an elective procedure, when microvascular reconstruction may have a better success rate. With regards to elbow coverage, although the pedicled latissimus dorsi is a well-described option for open elbow fractures [24], we prefer free flaps in this situation or a pedicled abdominal flap owing to concerns regarding donor morbidity in amputees.
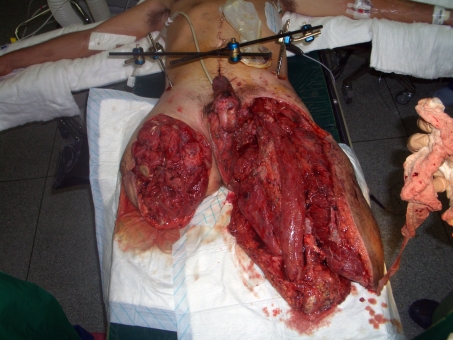
Lower limb: The characteristic IED injury is the bilateral above-knee amputation (figure 1). However, unilateral traumatic amputations at below-knee or through-knee levels also occur, often with inadequate skin coverage for stump. Proximal femoral fractures in association with pelvic fractures have also been seen. Amputation stumps are often produced without adequate skin coverage necessitating skin grafting of muscle-coverage over bone ends. With modern prosthetic fitting, skin-grafted stumps have not produced problems of ulceration to the degree we had expected. There is rarely sufficient tissue for fillet flaps or local flaps [25]. However, our early experience of resurfacing amputation stumps with integra dermal regeneration template has been very positive and we are prospectively evaluating the medium to long-term outcomes. Notwithstanding this, a lack of skin and subcutaneous tissue does predispose to ulceration following the development of heterotopic ossification (HO) (see below).
Figure 1.
Typical presentation of IED blast lower limb injury: high above-knee amputations with inadequate skin coverage, complex defect of perineum, urethral defect and bilateral traumatic orchidectomy.
Perineal injuries can be associated with open pelvic fractures and wound cavities extending to the sacro-iliac joint. Injuries to the external genitalia can occur, with degloving injuries to the penis, segmental loss of penile and/or bulbar urethra with inadequate soft-tissue coverage. Open injury to the corpora with loss of tunica albuginea has been observed. Traumatic orchidectomies have occurred and well as total traumatic penile amputation.
(d). Soft-tissue reconstruction
We use the entire gamut of reconstructive techniques in treating these difficult injuries. Local flap options are often not available owing to extensive discontinuous multiple fragmentation injury. Should skin grafting not be considered appropriate, our preference is for free tissue transfer. All patients undergo angiography prior to free tissue transfer as we have seen unpredictable fragmentation injuries to proximal pedicles and thrombosis of recipient vessels. The nature of blast-fragmentation injury means it is often not possible to get proximal to the zone of trauma and microanastamosis must be performed within the zone of trauma. However, if blood flow is good and the intima shows no sign of trauma, we have found free tissue transfer to be safe and reliable in these patients.
Flap selection is a challenge in military patients. Soldiers are athletes and potential donor site morbidity is a major factor in our flap selection. We favour low donor morbidity flaps in the multiply injured patient avoiding core stability muscles, such as latissimus dorsi or rectus abdominis free flaps if at all possible in the soldier owing to the physical high-demand status of these patients.
Latissimus dorsi flap harvest produces an initial reduction in shoulder strength, shoulder disability scores, impaired neural glide and discomfort at six months. These seem to normalize at one year in otherwise unimpaired individuals [26]. Salmi showed that shoulder extension strength deteriorated permanently after part of the latissimus dorsi muscle had been removed even though subjective morbidity was minimal [27]. A prospective study of patients who underwent segmental rectus abdominis harvest showed a clinically significant functional donor-site defect when measuring abdominal wall functional status, albeit with high patient satisfaction rates [28]. However, these were studies of civilian patients and contained no amputees. Bilateral amputees expend almost three times more energy than non-amputees when walking on prostheses [29] and pectoral girdle strength is of great importance for the multiple amputee who will spend much of his time in a wheelchair. We feel that this, plus the increased requirement for core stability in amputees, precludes the routine use of latissimus dorsi and rectus abdominis flaps for reconstruction.
The anterolateral thigh flap is our first choice fasciocutaneous flap [30]. However, it is often not available owing to either the concurrent thigh trauma or the presence of above-knee amputations. We have used thoracodorsal artery perforator flaps and parascapular flaps, the disadvantage of these being the need to turn the patient during the operation.
We favour muscle flaps for open lower limb fractures and our first preference is to use the gracilis or serratus anterior muscle flaps if possible; the gracilis is an expendable muscle and has almost no functional deficit following its harvest. We have succesfully harvested it from a contralateral traumatic above-knee amputation stump for coverage of an open tibial fracture. The disadvantages of gracilis include a short pedicle and small vessel calibre. Following harvest of the lower two slips of serratus anterior there is a theoretical risk of winging of the scapula; our impression is that this is very well-tolerated with no reported weakness from patients. We are currently performing a prospective trial of functional morbidity in soldiers following serratus anterior flap harvest to confirm our clinical impression regarding the low donor morbidity of this flap.
(e). Peripheral nerve injuries
Data from modern conflicts have shown significant numbers of peripheral nerve injuries (PNIs). American data from the Joint Theater Trauma Registry for the period 2001–2005 measures the incidence as 4 per cent [31].
In part, owing to body armour and the nature of the threat, the rate of extremity injury from recent conflicts is reported as 54 per cent [31]. In the setting of extremity trauma, the preservation of peripheral nerve function and therefore utility of the salvaged limb is paramount. Peripheral nerve function is the major determinant of long-term disability: in one study of patients requiring a vascular repair following a gunshot injury, 39 per cent achieved a normal extremity, compared with only 7 per cent if there was an associated PNI in the same limb [32].
(f). Pathophysiology of peripheral nerve injuries
Following nerve division, the peripheral nerve undergoes a series of events that have become known as Wallerian degeneration. This results in the loss of the cellular architecture of the nerve distal to the transection and if satisfactory re-innervation does not occur in a timely fashion then irreversible end-organ dysfunction follows.
In denervated muscle this process begins within one week [33] and continues over a period of approximately three years with fibrosis evident in muscles after three months, but functional recovery possible up to one year [34]. Late recovery of sensation can occur and has been demonstrated in the median nerve many years after PNI if appropriate techniques are well executed. [35].
Nerve injuries have been classified by both Seddon [36] and Sunderland [37] (table 2).
Table 2.
Common classification systems relating to peripheral nerve injuries.
| Sunderland [37] | Seddon [75] | injury | recovery potential |
|---|---|---|---|
| I | neuropraxia | ionic block; possible segmental demyelinization | full |
| II | axonotmesis | axon-severed, endoneural tube intact | full |
| III | endoneural tube torn | slow, incomplete | |
| IV | only epineurium intact | neuroma-in-continuity | |
| V | neurotmesis | loss of continuity | none |
| VI | combination of above |
(g). Outcome of nerve repair
Regenerated axons must reach and re-innervate their end organs in a timely fashion in order to attain the best possible result in PNI. The outcome is also influenced by the level of the injury, attaining a tension-free repair [38] and the division of a named nerve [39]. In particular, high ulnar nerve divisions perform poorly following repair of a penetrating injury [40,41].
The single most important determinant of outcome is the violence of injury to the nerve and the limb, and the extent of destruction of nerve tissue is a reflection of this. All peripheral nerves within the zone of a penetrating injury are explored at surgery and their status documented. If macroscopically intact, then expectant treatment is indicated. The repeated debridement also allows time for serial assessment of the zone of injury and damage to the peripheral nerve that may not be initially apparent. Sepsis is a significant problem in military wounds; we have approached this problem by delaying nerve repair until the wound is ready for closure.
Once a clean wound environment has been achieved, then a tension-free microsurgical epineural repair of correctly orientated nerve ends performed primarily is ideal. A primary repair is defined as one completed within one week, with delayed primary repair between one week and three weeks post-injury [38].
We do not advocate tacking of nerve ends at the first debridement nerve stumps in an effort to prevent retraction and aid future localization [38,42,43]. We have not found locating nerve ends to be a problem when performing primary or delayed primary nerve repair. How much nerve can be safely mobilized to allow a tension-free coaptation is open for debate. Mobilization does not affect nerve regeneration in a monkey model [44] and extensive mobilization of the sciatic nerve did not compromise intraneural blood flow in a rabbit model [45]. As an intra-operative guide to acceptable tension, the two ends should be able to be held together with a single 8/0 nylon suture [46]. Tension adversely affects the repair by impairing blood flow and causing gaping at the repair site [47].
In a prospective randomized trial, no difference was found between epineural repairs and fascicular repairs [48], but all reasonable attempts should be made to correctly orient the proximal and distal components of the repair.
Roganovic's group in Belgrade has published the most well-organized and impressive data regarding the outcomes of peripheral nerve surgery following military injury. They have looked at the level of injury, type of repair, time to repair and age in missile injuries of the major nerves and related this to the outcome after four years' follow-up.
High-level ulnar nerve lesions did abysmally with no successful results, whereas low-level ulnar nerve lesions did well with 77 per cent having a ‘good’ outcome [40]. Poor results were more common in those older than 23, with a defect greater than 4.5 cm and with a delay of greater than 5.5 months before repair. Forty-eight per cent of primary coaptations had a successful outcome when compared with 41 per cent that used nerve graft. Median and radial nerve injuries performed in a similar manner [49,50].
In the lower limb only 10.8 per cent of sciatic nerve transections above the mid-thigh level had a ‘good’ outcome when compared with 57 per cent below the popliteal crease. Nerve gap greater than 4 cm and delay of greater than three months were associated with a poorer outcome [51].
(i). Nerve grafting
In high-energy transfer military wounds trimming of damaged fascicles is usually required such that a primary repair is often not possible and nerve grafting is often required.
The ‘gold standard’ in the management of a gap in a peripheral nerve is autograft. Bunnell originally described nerve grafting in 1927 with ‘cable grafting’ published by Seddon in 1947 [52]. The term cable graft is a misnomer; the individual strands of the cable are not bound tightly together but spread out throughout the wound. Initially all grafts are dead and need to re-establish a blood flow from the adjacent tissue bed. In nerve grafting blood flow would appear to be re-established in a longitudinal fashion; however, a well-vascularized graft bed is essential [53].
The workhorse nerve donor graft is the sural nerve. 30–40 cm of sural nerve can be harvested with little donor site morbidity [54]. However, in our patient population, who have often suffered bilateral traumatic lower limb amputations, the sural nerve is not available and other donor sites for nerve graft need to be considered. These include the lateral/medial antebrachial cutaneous nerves of the forearm [55] and in highly selected cases the superficial branch of the radial nerve [56].
The graft must be reversed and interposed in a tension-free manner. Estimates of 10–20% graft excess are mentioned in the literature to ensure a tension-free coaptation. Nerve graft can be interposed as a trunk, interfascicular or in a ‘cable’ manner as previously described. We have found fibrin glue fixation of nerve grafts to be an excellent technique which reduces operative time considerably [57].
Finding suitable nerve conduits as alternatives to nerve autograft is a topic of intense interest.
(ii). Nerve conduits
Nerve conduits seek to create the ideal micro-environment to promote and guide nerve regeneration from the proximal to distal stump (table 3). They are generally successful in managing gaps of less than 3 cm and are a valuable addition to the armamentarium when donor sites for autograft are inadequate.
Table 3.
Descriptive classification of peripheral nerve conduits.
| biological |
non-biological |
||
|---|---|---|---|
| autogenous | non-autogenous | absorbable | non-absorbable |
| vein | collagen | polyglactin acid | expanded polytetrafluoroethylene (PTFE) |
| muscle | allograft | polyglycolic acid | silicone |
The most popular conduit is vein which is readily available and proven in the management of nerve gaps of less than or equal to 3 cm in sensory nerves of the upper limb [58]. Denatured muscle has been found to perform poorly when compared with nerve graft in the repair of superficial branch of the radial nerve defects following traumatic missile division [59].
Non-absorbable synthetic conduits have shown some promise in the management of small nerve gaps [60] but have been complicated by irritative complications requiring their removal [61].
Nerve allograft is an exciting development in animal modelling but with the requirement for initial immune suppression [62] its future applicability in the military casualty should be looked upon with great caution.
(iii). Nerve transfers
This technique attempts to convert a high nerve lesion to a low nerve lesion and is useful when regeneration across a long nerve graft would not produce re-innervation of distal end organs promptly. Fascicles from the uninjured donor nerve are diverted to the distal stump of the injured nerve to re-innervate target end organs, either motor or sensory [63].
The variety of nerve transfers is increasingly evolving and certain eponymous transfers such as the transfer of ulnar nerve fascicles to the nerve to biceps in order to restore elbow flexion as described by Oberlin in 2004 are popular [64]. In the military patient, they may have particular utility in avoiding a nerve graft through a previously operated and scarred area where complications of surgery may be high, or where one may wish to leave a vascular repair or fracture undisturbed.
5. Heterotopic ossification
Heterotopic ossification (HO) is the abnormal deposition of bone in the soft tissues. Histologically this is defined as mature lamella bone in non-osseous tissue. Current classification describes three forms of HO: the paediatric metabolic condition myositis ossificans progressa, neurogenic HO (after burns or neurological injury) and trauma-related HO following injury. This process is well-described after burns, traumatic brain and spinal injury, and complicating hip arthroplasty, but the physiology is poorly understood. HO is also seen following traumatic amputation [65].
The aetiology of traumatic HO is unclear. The migration of bone marrow cells, interstitial haemorrhagic foci within the muscle causing degeneration, periosteal damage, or proliferation of perivascular connective tissue and subsequent bone metaplasia have all been suggested. All these require osteogenic precursor cells, an inductive stimulus and a favourable local tissue environment. It is thought to be an inflammatory process in response to local tissue trauma regulated in some way by bone morphogenic proteins. The heterotopic bone is very metabolically active with an abnormally high number of osteoblasts.
The risks for development of HO include extensive soft-tissue dissection, muscle ischaemia, soft-tissue trauma, bone trauma, and the presence of bone debris, devitalized tissue, haematoma and infection [66]. All these are present in significant amounts in the military casualty.
(a). Heterotopic ossification in modern military casualties
A retrospective study reviewed 187 United States military amputees with 213 amputations that had radiographs taken more than two months after injury and this demonstrated that 63 per cent of the stumps had developed HO [67]. The significant risk factors for HO formation were found to be final amputation level within the zone of trauma and blast mechanism of injury (p < 0.05). If both these risks were present, the rate rose to 80 per cent, while there was no HO in patients with non-blast injury with final levels of amputation higher than the zone of trauma. Other likely risks were repeated debridements, longer time to closure and infection although these can also be related to the severity of the initial injury.
A second retrospective study of 1213 US combat casualties demonstrated a HO rate of 65 per cent in those 243 patients who had undergone orthopaedic procedures and 13 per cent of the total group [68]. The patients in the HO group were more likely to have lower limb injury, an amputation and an ISS of over 16.
Our UK experience has also shown that large numbers of patients are developing HO and this is a considerable problem for rehabilitation in some cases.
(b). Current management of the UK military patient with symptomatic heterotopic ossification
The development of HO can occur in the soft tissues very early and is sometimes found even before primary healing occurs. However, in the majority, it occurs after a number of months. The patient will typically have had an IED injury and sustained bilateral traumatic above-knee amputations (often with other significant injuries), and will have initially done well with limb fitting and mobilization. They may develop increasing pain in their stumps and can often feel a hard lump or spike within the stump. In severe cases, the bone has actually eroded through the soft tissues (figure 1). This may prevent them from mobilizing and poses a significant challenge to the prosthetics team.
We have identified two morphological types of HO in our practice. Type 1 HO presents as spikes of new bone continuous with the end of the amputated bone and often runs between the muscle groups. This appears on the radiographic reconstructions as flame-like opacities coming out of the stump. Type 2 HO forms sheets of new bone that macroscopically resemble a beetle's carapace (shell) within and around muscle, apparently distinct from the bone end. Type 2 can be challenging to remove without significant collateral damage to the remaining soft tissues. Whether these two forms of HO represent distinct entities remains to be seen.
Traditional teaching advocates allowing the HO to mature before excisional surgery. This allows the ossifying tissue to harden and makes the surgery less traumatic to the surrounding muscles. However, in some cases especially where the skin is breached, it is not appropriate to delay surgery to allow the HO to mature.
Initial reports from the USA have suggested that early excision of HO is a reasonable approach to take in selected patients although long-term follow-up data are lacking.
Twenty-four patients with 25 symptomatic limbs underwent excision of HO and all were tolerating prosthetic limbs at 12 months follow-up, although two had asymptomatic minor recurrences of the HO. Prophylactic irradiation or non-steroidal anti-inflammatory (NSAIDs) medications were used in the majority of these patients although it is unclear if this is necessary to control recurrence [67,68].
Our current practice is to intervene when patient is unable to progress with rehabilitation owing to complications of HO. Computerized tomography (CT) allows three-dimensional reconstruction of the area of calcification and facilitates operative planning. Traditional radiographs can be misleading especially in the early stages of the condition; immature bone spikes are not visualized well on plain X-ray films.
During the stump revision, areas of previous skin graft can often be removed at least in part, and this can improve the weight-bearing surface. This technique of staged removal of the skin graft using a serial excision approach can be used to resurface large areas of skin over a prolonged time period.
Although stump revision in this semi-acute phase can be difficult for both surgeon and patient, the results are worthwhile, and allow these very well-motivated individuals to continue to progress with their rehabilitation. We continue to monitor the patients for recurrence of the HO and are performing post-operative CT scanning at regular intervals to identify any recurrence.
Radiotherapy and NSAIDs have been advocated for prevention of HO after hip arthroplasty for trauma and this is well described in the literature [69]. Following procedures that may be complicated by HO, recommendations indicate that prophylaxis should be given in the form of NSAIDs, such as indomethacin, or aspirin-like drugs that act as non-specific cyclooxygenase inhibitors [70,71]. The duration of treatment is controversial. Some studies have shown that treatment should continue for six weeks after the procedure; others have suggested that it need only be continued for 20, 14 or 7 days after the procedure. Our patients are routinely started on a four-week prophylaxis of indomethacin with a gastric protector agent to minimize the risks of gastric irritation and bleeding.
The development of HO in military patients following traumatic amputation is a significant problem. We have evolved a treatment protocol that attempts to maximize the speed of rehabilitation of these patients while minimizing the risks associated with surgical excision and that of recurrence of HO.
(c). Future directions
The large number of amputees generated by recent conflicts has renewed interest in fully functional neurologically integrated prostheses, especially with regard to the upper limb. In the United States, the Defence Advanced Research Program Agency (DARPA) has a goal of restoring full upper limb functionality via a prosthetic limb and using a technique called ‘targeted re-innervation’ to provide ‘meaningful sensory feedback’ from such a limb [72].
Advances have been made in the integration of prosthetic devices to both the bony skeleton and the skin most noticeably in the UK with the intraosseous transcutaneous amputation prosthesis [73,74]. Perhaps, the final boundary is an implantable medical device within the peripheral nerves acting as an interface with a functioning prosthetic limb? [42].
Acknowledgements
We thank Dr ER Johnson, HPA Mycology Reference Laboratory, Bristol, for her advice and her help in identifying fungal isolates.
Footnotes
One contribution of 20 to a Theme Issue ‘Military medicine in the 21st century: pushing the boundaries of combat casualty care’.
References
- 1.Davenport R. A., et al. 2010. A major trauma centre is a specialty hospital not a hospital of specialties. Br. J. Surg. 97i, 109–117 10.1002/bjs.6806 (doi:10.1002/bjs.6806) [DOI] [PubMed] [Google Scholar]
- 2.O'Reilly D., König T., Tai N. 2008. Field trauma care in the 21st century. J. R. Army Med. Corps 154, 260–264 [PubMed] [Google Scholar]
- 3.Ramasamy A., Hill A. M., Clasper J. C. 2009. Improvised explosive devices: Pathophysiology, injury profiles and current medical management. J. R. Army Med. Corps 155, 265–272 [DOI] [PubMed] [Google Scholar]
- 4.Kindsfater K., Jonassen E. A. 1995. Osteomyelitis in grade II and III open tibia fractures with late debridement. J. Orthop. Trauma 9, 121–127 10.1097/00005131-199504000-00006 (doi:10.1097/00005131-199504000-00006) [DOI] [PubMed] [Google Scholar]
- 5.Khatod M., Botte M. J., Hoyt D. B., Meyer R. S., Smith J. M., Akeson W. H. 2003. Outcomes in open tibia fractures: relationship between delay in treatment and infection. J. Trauma 55, 949–954 10.1097/01.TA.0000092685.80435.63 (doi:10.1097/01.TA.0000092685.80435.63) [DOI] [PubMed] [Google Scholar]
- 6.Granick M. S., Chehade M. 2007. The evolution of surgical wound management: toward a common language. In Surgical wound management (eds Granick M. S., Gamelli R. L.), pp. 17–28 New York, NY: Informa [Google Scholar]
- 7.Jackson D. M. 1983. The William Gissane lecture 1982 the burn wound: its character, closure and complications. Burns 10, 1–8 [DOI] [PubMed] [Google Scholar]
- 8.Boyd J. I., Wongworawat M. D. 2004. High-pressure pulsatile lavage causes soft tissue damage. Clin. Orthop. Relat. Res. 427, 13–17 10.1097/01.blo.0000144859.73074.45 (doi:10.1097/01.blo.0000144859.73074.45) [DOI] [PubMed] [Google Scholar]
- 9.Hassinger S. M., Harding G., Wongworawat M. D. 2005. High-Pressure pulsatile lavage propagates bacteria into soft tissue. Clin. Orthop. Relat. Res. 439, 27–31 10.1097/01.blo.0000182246.37454.b2 (doi:10.1097/01.blo.0000182246.37454.b2) [DOI] [PubMed] [Google Scholar]
- 10.Fries C. A., Jeffery S. L., Kay A. R. 2010. Topical negative pressure and military wounds–a review of the evidence. Injury 41, 708–712 10.1016/j.injury.2010.02.027 (doi:10.1016/j.injury.2010.02.027) [DOI] [PubMed] [Google Scholar]
- 11.Muñoz P., Hortal J., Giannella M., Barrio J. M., Rodríguez-Créixems M., Pérez M. J., Rincón C., Bouza E. 2008. Nasal carriage of S. aureus increases the risk of surgical site infection after major heart surgery. J. Hosp. Infect. 68, 25–31 10.1016/j.jhin.2007.08.010 (doi:10.1016/j.jhin.2007.08.010) [DOI] [PubMed] [Google Scholar]
- 12.Taylor R. W., Manganaro L., O'Brien J., Trottier S. J., Parkar N., Veremakis C. 2002. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit. Care Med. 30, 2249–2254 10.1097/01.CCM.0000030457.48434.17 (doi:10.1097/01.CCM.0000030457.48434.17) [DOI] [PubMed] [Google Scholar]
- 13.Skiada A., Petrikkos G. 2009. Cutaneous zygomycosis. Clin. Microbiol. Infect. 15(Suppl. 5), 41–45 10.1111/j.1469-0691.2009.02979.x (doi:10.1111/j.1469-0691.2009.02979.x) [DOI] [PubMed] [Google Scholar]
- 14.Riska E. B., von Bonsdorff H., Hakkinen S., Jaroma H., Kiviluoto O., Paavilainen T. 1977. Primary operative fixation of long bone fractures in patients with multiple injuries. J. Trauma 17, 111–121 10.1097/00005373-197702000-00005 (doi:10.1097/00005373-197702000-00005) [DOI] [PubMed] [Google Scholar]
- 15.Bone L. B., Johnson K. D., Weigelt J., Scheinberg R. 1989. Early versus delayed stabilization of femoral fractures. A prospective randomized study. J. Bone Jt. Surg. Am. 71, 336–340 [PubMed] [Google Scholar]
- 16.Keel M., Trentz O. 2005. Pathophysiology of polytrauma. Injury 36, 691–709 10.1016/j.injury.2004.12.037 (doi:10.1016/j.injury.2004.12.037) [DOI] [PubMed] [Google Scholar]
- 17.Pape H. C., Giannoudis P. V., Krettek C., Trentz O. 2005. Timing of fixation of major fractures in blunt polytrauma: role of conventional indicators in clinical decision making. J. Orthop. Trauma 19, 551–562 10.1097/01.bot.0000161712.87129.80 (doi:10.1097/01.bot.0000161712.87129.80) [DOI] [PubMed] [Google Scholar]
- 18.Francel T. J., Vander Kolk C. A., Hoopes J. E., Manson P. N., Yaremchuk M. J. 1992. Microvascular soft-tissue transplantation for reconstruction of acute open tibial fractures: timing of coverage and long-term functional results. Plast. Reconstr. Surg. 89, 478–487 (discussion 488–489) [PubMed] [Google Scholar]
- 19.Small J. O., Mollan R. A. 1992. Management of the soft tissues in open tibial fractures. Br. J. Plast. Surg. 45, 571–577 10.1016/0007-1226(92)90022-P (doi:10.1016/0007-1226(92)90022-P) [DOI] [PubMed] [Google Scholar]
- 20.Sinclair J. S., McNally M. A., Small J. O., Yeates H. A. 1997. Primary free-flap cover of open tibial fractures. Injury 28, 581–587 10.1016/S0020-1383(97)00093-4 (doi:10.1016/S0020-1383(97)00093-4) [DOI] [PubMed] [Google Scholar]
- 21.Nanchahal J., Nayagam D., Khan U., Moran C., Barrett S., Sanderson F., Pallister I. 2009. Standards for the management of open fractures of the lower limb. See http://www.bapras.org.uk/guide.asp?id=355 [Google Scholar]
- 22.Lutz B. S., Klauke T., Dietrich F. E. 1997. Late results after microvascular reconstruction of severe crush and avulsion injuries of the upper extremity. J. Reconstr. Microsurg. 13, 423–429 10.1055/s-2007-1006423 (doi:10.1055/s-2007-1006423) [DOI] [PubMed] [Google Scholar]
- 23.Pezzin L. E., Dillingham T. R., MacKenzie E. J. 2000. Rehabilitation and the long-term outcomes of persons with trauma-related amputations. Arch. Phys. Med. Rehabil. 81, 292–300 10.1016/S0003-9993(00)90074-1 (doi:10.1016/S0003-9993(00)90074-1) [DOI] [PubMed] [Google Scholar]
- 24.Kumar A. R., Grewal N. S., Chung T. L., Bradley J. P. 2009. Lessons from the modern battlefield: Successful upper extremity injury reconstruction in the subacute period. J. Trauma 67, 752–757 10.1097/TA.0b013e3181808115 (doi:10.1097/TA.0b013e3181808115) [DOI] [PubMed] [Google Scholar]
- 25.Küntscher M. V., Erdmann D., Homann H. H., Steinau H. U., Levin S. L., Germann G. 2001. The concept of fillet flaps: classification, indications, and analysis of their clinical value. Plast. Reconstr. Surg. 108, 885–896 [DOI] [PubMed] [Google Scholar]
- 26.Glassey N., Perks G. B., McCulley S. J. 2008. A prospective assessment of shoulder morbidity and recovery time scales following latissimus dorsi breast reconstruction. Plast. Reconstr. Surg. 122, 1334–1340 10.1097/PRS.0b013e3181881ffe (doi:10.1097/PRS.0b013e3181881ffe) [DOI] [PubMed] [Google Scholar]
- 27.Salmi A., Tuominen R., Tukiainen E., Asko-Seljavaara S. 1995. Morbidity of donor and recipient sites after free flap surgery. A prospective study. Scand. J. Plast. Reconstr. Surg. Hand Surg. 29, 337–341 10.3109/02844319509008969 (doi:10.3109/02844319509008969) [DOI] [PubMed] [Google Scholar]
- 28.Geishauser M., Staudenmaier R. W., Biemer E. 1998. Donor-site morbidity of the segmental rectus abdominis muscle flap. Br. J. Plast. Surg. 51, 603–607 [DOI] [PubMed] [Google Scholar]
- 29.Huang C. T., Jackson J. R., Moore N. B., Fine P. R., Kuhlemeier K. V., Traugh G. H., Saunders P. T. 1979. Amputation: energy cost of ambulation. Arch. Phys. Med. Rehabil. 60, 18–24 [PubMed] [Google Scholar]
- 30.Song Y. G., Chen G. Z., Song Y. L. 1984. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br. J. Plast. Surg. 37, 149–159 10.1016/0007-1226(84)90002-X (doi:10.1016/0007-1226(84)90002-X) [DOI] [PubMed] [Google Scholar]
- 31.Owens B. D., Kragh J. F., Macaitis J., Svoboda S. J., Wenke J. C. 2007. Characterization of extremity wounds in operation Iraqi freedom and operation enduring freedom. J. Orthop. Trauma 21, 254–257 10.1097/BOT.0b013e31802f78fb (doi:10.1097/BOT.0b013e31802f78fb) [DOI] [PubMed] [Google Scholar]
- 32.Visser P. A., Hermreck A. S., Pierce G. E., Thomas J. H., Hardin C. A. 1980. Prognosis of nerve injuries incurred during acute trauma to peripheral arteries. Am. J. Surg. 140, 596–599 [DOI] [PubMed] [Google Scholar]
- 33.Gutmann E. 1948. Effect of delay of innervation on recovery of muscle after nerve lesions. J. Neurophysiol. 11, 279–294 [DOI] [PubMed] [Google Scholar]
- 34.Sunderland S. 1950. Capacity of reinnervated muscles to function efficiently after prolonged denervation. Arch. Neurol. Psychiatry 64, 755–771 [DOI] [PubMed] [Google Scholar]
- 35.Novak C. B., Kelly L., Mackinnon S. E. 1992. Sensory recovery after median nerve grafting. J. Hand Surg. Am. 17, 59–68 10.1016/0363-5023(92)90114-5 (doi:10.1016/0363-5023(92)90114-5) [DOI] [PubMed] [Google Scholar]
- 36.Seddon H. J. 1950. Peripheral nerve injuries in Great Britain during World War II: a review. Arch. Neurol. Psychiatry 63, 171–173 [PubMed] [Google Scholar]
- 37.Sunderland S. 1978. Nerves and nerve injuries, 2nd ed. Edinburgh, UK: Churchill Livingstone [Google Scholar]
- 38.Dvali L., Mackinnon S. 2003. Nerve repair, grafting, and nerve transfers. Clin. Plast. Surg. 30, 203–221 10.1016/S0094-1298(02)00096-2 (doi:10.1016/S0094-1298(02)00096-2) [DOI] [PubMed] [Google Scholar]
- 39.Roganović Z. 1998. Factors influencing the outcome of nerve repair. Vojnosanit Pregl. 55, 119–131 [PubMed] [Google Scholar]
- 40.Roganovic Z. 2004. Missile-caused ulnar nerve injuries: outcomes of 128 repairs. Neurosurgery 55, 1120–1129 10.1227/01.NEU.0000142353.92119.FE (doi:10.1227/01.NEU.0000142353.92119.FE) [DOI] [PubMed] [Google Scholar]
- 41.Stewart M. P., Birch R. 2001. Penetrating missile injuries of the brachial plexus. J. Bone Jt. Surg. Br. 83, 517–524 10.1302/0301-620X.83B4.11583 (doi:10.1302/0301-620X.83B4.11583) [DOI] [PubMed] [Google Scholar]
- 42.Ecklund J. M., Ling G. S. 2009. From the battlefront: peripheral nerve surgery in modern day warfare. Neurosurg. Clin. N. Am. 20, 107–110 10.1016/j.nec.2008.07.022 (doi:10.1016/j.nec.2008.07.022) [DOI] [PubMed] [Google Scholar]
- 43.Isaacs J. 2010. Treatment of acute peripheral nerve injuries: current concepts. J. Hand Surg. Am. 35, 491–497 (quiz 498) 10.1016/j.jhsa.2009.12.009 (doi:10.1016/j.jhsa.2009.12.009) [DOI] [PubMed] [Google Scholar]
- 44.Kline D. G., Hackett E. R., Davis G. D., Myers M. B. 1972. Effect of mobilization on the blood supply and regeneration of injured nerves. J. Surg. Res. 12, 254–266 10.1016/0022-4804(72)90020-0 (doi:10.1016/0022-4804(72)90020-0) [DOI] [PubMed] [Google Scholar]
- 45.Maki Y., Firrell J. C., Breidenbach W. C. 1997. Blood flow in mobilized nerves: results in a rabbit sciatic nerve model. Plast. Reconstr. Surg. 100, 627–633 (discussion 634–635) [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto Y. 1979. Experimental study of results of nerve suture under tension vs. nerve grafting. Plast. Reconstr. Surg. 64, 540–549 [DOI] [PubMed] [Google Scholar]
- 47.Clark W. L., Trumble T. E., Swiontkowski M. F., Tencer A. F. 1992. Nerve tension and blood flow in a rat model of immediate and delayed repairs. J. Hand Surg. Am. 17, 677–687 10.1016/0363-5023(92)90316-H (doi:10.1016/0363-5023(92)90316-H) [DOI] [PubMed] [Google Scholar]
- 48.Young L., Wray R. C., Weeks P. M. 1981. A randomized prospective comparison of fascicular and epineural digital nerve repairs. Plast. Reconstr. Surg. 68, 89–93 [DOI] [PubMed] [Google Scholar]
- 49.Roganovic Z., Petkovic S. 2004. Missile severances of the radial nerve. Results of 131 repairs. Acta Neurochir. (Wien) 146, 1185–1192 10.1007/s00701-004-0361-x (doi:10.1007/s00701-004-0361-x) [DOI] [PubMed] [Google Scholar]
- 50.Roganovic Z. 2005. Missile-caused median nerve injuries: results of 81 repairs. Surg. Neurol. 63, 410–418 (discussion 418–419) 10.1016/j.surneu.2004.08.007 (doi:10.1016/j.surneu.2004.08.007) [DOI] [PubMed] [Google Scholar]
- 51.Roganovic Z. 2005. Missile-caused complete lesions of the peroneal nerve and peroneal division of the sciatic nerve: results of 157 repairs. Neurosurgery 57, 1201–1212 (discussion 1201–1212) [DOI] [PubMed] [Google Scholar]
- 52.Millesi H. 2000. Techniques for nerve grafting. Hand Clin. 16, 73–91 [PubMed] [Google Scholar]
- 53.Best T. J., Mackinnon S. E. 1994. Peripheral nerve revascularization: a current literature review. J. Reconstr. Microsurg. 10, 193–204 10.1055/s-2007-1006587 (doi:10.1055/s-2007-1006587) [DOI] [PubMed] [Google Scholar]
- 54.Hill H. L., Vasconez L. O., Jurkiewicz M. J. 1978. Method for obtaining a sural nerve graft. Plast. Reconstr. Surg. 61, 177–179 [DOI] [PubMed] [Google Scholar]
- 55.Colen K. L., Choi M., Chiu D. T. 2009. Nerve grafts and conduits. Plast. Reconstr. Surg. 124(Suppl. 6), e386–e394 10.1097/PRS.0b013e3181bf8430 (doi:10.1097/PRS.0b013e3181bf8430) [DOI] [PubMed] [Google Scholar]
- 56.Sunderland S., Ray L. J. 1947. The selection and use of autografts for bridging gaps in injured nerves. Brain 70, 75–92 10.1093/brain/70.1.75 (doi:10.1093/brain/70.1.75) [DOI] [PubMed] [Google Scholar]
- 57.Ornelas L., et al. 2006. Fibrin glue: an alternative technique for nerve coaptation—part II. Nerve regeneration and histomorphometric assessment. J. Reconstr. Microsurg. 22, 123–128 10.1055/s-2006-932507 (doi:10.1055/s-2006-932507) [DOI] [PubMed] [Google Scholar]
- 58.Chiu D. T., Strauch B. 1990. A prospective clinical evaluation of autogenous vein grafts used as a nerve conduit for distal sensory nerve defects of 3 cm or less. Plast. Reconstr. Surg. 86, 928–934 [DOI] [PubMed] [Google Scholar]
- 59.Roganovic Z., Ilic S., Savic M. 2007. Radial nerve repair using an autologous denatured muscle graft: comparison with outcomes of nerve graft repair. Acta Neurochir. (Wien) 149, 1033–1038 (discussion 1038–1039). 10.1007/s00701-007-1269-z (doi:10.1007/s00701-007-1269-z) [DOI] [PubMed] [Google Scholar]
- 60.Stanec S., Stanec Z. 1998. Reconstruction of upper-extremity peripheral-nerve injuries with ePTFE conduits. J. Reconstr. Microsurg. 14, 227–232 10.1055/s-2007-1000173 (doi:10.1055/s-2007-1000173) [DOI] [PubMed] [Google Scholar]
- 61.Lundborg G., Rosén B., Dahlin L., Danielsen N., Holmberg J. 1997. Tubular versus conventional repair of median and ulnar nerves in the human forearm: early results from a prospective, randomized, clinical study. J. Hand Surg. Am. 22, 99–106 10.1016/S0363-5023(05)80188-1 (doi:10.1016/S0363-5023(05)80188-1) [DOI] [PubMed] [Google Scholar]
- 62.Mackinnon S. E., Hudson A. R., Bain J. R., Falk R. E., Hunter D. A. 1987. The peripheral nerve allograft: an assessment of regeneration in the immunosuppressed host. Plast. Reconstr. Surg. 79, 436–446 [PubMed] [Google Scholar]
- 63.Narakas A. O. 1984. Thoughts on neurotization or nerve transfers in irreparable nerve lesions. Clin. Plast. Surg. 11, 153–159 [PubMed] [Google Scholar]
- 64.Teboul F., Kakkar R., Ameur N., Beaulieu J. Y., Oberlin C. 2004. Transfer of fascicles from the ulnar nerve to the nerve to the biceps in the treatment of upper brachial plexus palsy. J. Bone Jt. Surg. Am. 86-A, 1485–1490 [DOI] [PubMed] [Google Scholar]
- 65.Henrot P., Stines J., Walter F., Martinet N., Paysant J., Blum A. 2000. Imaging of the painful lower limb stump. Radiographics 20, S219–S235 [DOI] [PubMed] [Google Scholar]
- 66.Stein D. A., Patel R., Egol K. A., Kaplan F. T., Tejwani N. C., Koval K. J. 2003. Prevention of heterotopic ossification at the elbow following trauma using radiation therapy. Bull. Hosp. Jt. Dis. 61, 151–154 [PubMed] [Google Scholar]
- 67.Potter B. K., Burns T. C., Lacap A. P., Granville R. R., Gajewski D. A. 2007. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J. Bone Jt. Surg. Am. 89, 476–486 10.2106/JBJS.F.00412 (doi:10.2106/JBJS.F.00412) [DOI] [PubMed] [Google Scholar]
- 68.Forsberg J. A., et al. 2009. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J. Bone Jt. Surg. Am. 91, 1084–1091 10.2106/JBJS.H.00792 (doi:10.2106/JBJS.H.00792) [DOI] [PubMed] [Google Scholar]
- 69.Pakos E. E., Pitouli E. J., Tsekeris P. G., Papathanasopoulou V., Stafilas K., Xenakis T. H. 2006. Prevention of heterotopic ossification in high-risk patients with total hip arthroplasty: the experience of a combined therapeutic protocol. Int. Orthop. 30, 79–83 10.1007/s00264-005-0054-y (doi:10.1007/s00264-005-0054-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macfarlane R. J., Ng B. H., Gamie Z., El Masry M. A., Velonis S., Schizas C., Tsiridis E. 2008. Pharmacological treatment of heterotopic ossification following hip and acetabular surgery. Exp. Opin. Pharmacother. 9, 767–786 10.1517/14656566.9.5.767 (doi:10.1517/14656566.9.5.767) [DOI] [PubMed] [Google Scholar]
- 71.Baird E. O., Kang Q. K. 2009. Prophylaxis of heterotopic ossification: an updated review. J. Orthop. Surg. Res. 4, 10.1186/1749-799X-4-12 (doi:10.1186/1749-799X-4-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Shaughnessy K. D., Dumanian G. A., Lipschutz R. D., Miller L. A., Stubblefield K., Kuiken T. A. 2008. Targeted reinnervation to improve prosthesis control in transhumeral amputees. A report of three cases. J. Bone Jt. Surg. Am. 90, 393–400 10.2106/JBJS.G.00268 (doi:10.2106/JBJS.G.00268) [DOI] [PubMed] [Google Scholar]
- 73.Pendegrass C. J., Goodship A. E., Blunn G. W. 2006. Development of a soft tissue seal around bone-anchored transcutaneous amputation prostheses. Biomaterials 27, 4183–4191 10.1016/j.biomaterials.2006.03.041 (doi:10.1016/j.biomaterials.2006.03.041) [DOI] [PubMed] [Google Scholar]
- 74.Pendegrass C. J., Goodship A. E., Price J. S., Blunn G. W. 2006. Nature's answer to breaching the skin barrier: an innovative development for amputees. J. Anat. 209, 59–67 10.1111/j.1469-7580.2006.00595.x (doi:10.1111/j.1469-7580.2006.00595.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seddon H. J. 1972. Surgical disorders of peripheral nerves. Baltimore, MD: Williams & Wilkins [Google Scholar]