Abstract
Blast injuries are an increasing problem in both military and civilian practice. Primary blast injury to the lungs (blast lung) is found in a clinically significant proportion of casualties from explosions even in an open environment, and in a high proportion of severely injured casualties following explosions in confined spaces. Blast casualties also commonly suffer secondary and tertiary blast injuries resulting in significant blood loss. The presence of hypoxaemia owing to blast lung complicates the process of fluid resuscitation. Consequently, prolonged hypotensive resuscitation was found to be incompatible with survival after combined blast lung and haemorrhage. This article describes studies addressing new forward resuscitation strategies involving a hybrid blood pressure profile (initially hypotensive followed later by normotensive resuscitation) and the use of supplemental oxygen to increase survival and reduce physiological deterioration during prolonged resuscitation. Surprisingly, hypertonic saline dextran was found to be inferior to normal saline after combined blast injury and haemorrhage. New strategies have therefore been developed to address the needs of blast-injured casualties and are likely to be particularly useful under circumstances of enforced delayed evacuation to surgical care.
Keywords: blast, explosion, haemorrhage, trauma, resuscitation, oxygen
1. Introduction
Blast injuries are an increasing problem in both military and civilian practice [1]. To understand and model blast injury we need an appreciation of the forces and threats acting on a person exposed to an explosion. Broadly, the casualty may be exposed to rapid pressure changes (shock wave), fragment and debris (ballistic/projectile threat) and bodily displacement resulting in blunt injuries and shearing effects. The magnitude, incidence and clinical significance of the various types of injury depend on the nature and environment (e.g. open or closed) of the explosive device, protection worn by the casualty (which may give greater protection against one aspect of the threat) and distance from the explosion.
When an explosive detonates it generates an extremely rapid (effectively instantaneous) increase in pressure in the immediate vicinity of the explosion which travels outwards from the site of the explosion as a high-pressure wave. The high pressure (peak overpressure) usually lasts only for a very short time (milliseconds) and is followed by a fall in pressure, often to sub-atmospheric levels before returning to ambient pressure. This is defined as the ‘shock wave’. The magnitude of the peak overpressure falls as it travels away from the site of the explosion, initially by an inverse cube relation. This is not the component of an explosion that causes the target to move any great distance. Fragments (of the munition casing and pre-formed fragments contained within the device) and surrounding debris energized by the explosion are propelled outwards forming a ballistic/projectile threat. In addition, the explosion usually gives rise to a very large volume of gas, pushing air and debris outwards and acts over a sufficiently long time course to physically throw casualties against other objects. This is the ‘blast wind’ (dynamic overpressure). The shock wave and the blast wind are sometimes collectively called the ‘blast wave’. Finally, for those close to the explosion there is also a large amount of heat which can also cause injury.
2. Classification of blast injuries
Blast injuries are classified according to the forces causing the injury (see above). There are four main categories [2,3]: primary, secondary and tertiary, with miscellaneous additional injuries forming a further (quaternary) group:
Primary blast injuries result from the interaction of a shock wave with the body. Injury is largely confined to the air-containing organs, such as the lungs, bowel and ears, often without external signs of injury [4], although recently there is heightened suspicion that primary blast may also cause brain injury [5–7].
Secondary blast injury results from the impact of fragments and larger missiles accelerated by the blast. Injuries caused by these fragments can be further categorized as penetrating or non-penetrating. This group accounts for the majority of blast injuries, particularly in open spaces.
Tertiary blast injury results from the acceleration of the whole body or parts of the body by the blast wave causing translational impacts of the body with the ground or other fixed objects, and/or traumatic amputation of body parts and stripping of tissue.
Quaternary blast injury represent a further group of miscellaneous injuries includes flash burns, caused by the radiant and convective heat of the explosion, burns caused by the combustion of the environment, crush syndrome, the effects of noxious gaseous products liberated in enclosed spaces, especially carbon monoxide, and psychological effects.
An analysis of military casualties from terrorist bombings [8] found that blast lung was frequently identified at post-mortem examination but, in the majority of cases, that death could also be attributed to other causes such as penetrating wounds, head injuries or traumatic amputations. In general, the incidence of blast lung in survivors admitted to hospitals was low (approx. 1–2%) [8], but higher percentages were reported for explosions in confined spaces (e.g. figures ranging from 63% to 94% of critically injured civilian survivors in the Madrid train bombings [9,10]). More recently, authors have quoted a report by Ramasamy et al. [11] as evidence that the incidence of primary blast injury is very small (approx. 3.8% of casualties sustained in explosive events). However, extreme caution should be exercised in general application of this headline data since Ramasamy et al. [11] focused almost exclusively (91.3% of cases reported in the paper) on a very ‘special’ type of ‘explosive device’, the explosively formed projectile improvised explosive device (EFP-IED). This device is very focused in its action and produces a directional projectile threat. Ramasamy et al. [11] themselves state very explicitly ‘We report a different pattern of injury caused by the EFP-IED compared with conventional explosive devices’. A very recent review of military casualties [12] concluded that 71 per cent of combat casualties admitted to medical treatment facilities during 2003–2006 were the result of explosions, and the proportion of these suffering blast lung injury was very small (3.6%). However, the definition of blast lung (a primary blast injury) used in this study could have significantly underestimated the true incidence since ‘Patients found to have rib-fractures, scapula fractures, or open wounds to the chest were included in the study but were considered to have injuries caused by secondary or tertiary explosive mechanisms’ [12]. While this is a very ‘safe’ definition of blast lung where it is essential to exclude all other possibilities, it will exclude blast lung injuries when it co-exists with other injury types, which is common with conventional munitions and terrorist bombs and hence may underestimate the occurrence of blast lung in current in military casualties. Perhaps the most recent assessment of the incidence is given by Smith et al. [13], where the incidence was found to be approximately 11 per cent in casualties who also suffered other (particularly penetrating) injuries.
These differences in reported incidence emphasize the importance of the context of the explosion, which has a profound effect on the nature, type and frequency of the injuries. Primary blast injury has a higher incidence in a number of different scenarios [14]:
— Explosions in confined spaces increase the effective loading to the body because of blast wave reflection, and, under certain circumstances, can increase the risk of blast lung.
— Unconfined bare charges or explosions of gas or vapour clouds, which generate very few secondary fragments, produce a higher incidence of blast lung injury.
— If the risk of fragmentation injury is reduced by personal body armour, antifragmentation liners in military vehicles, or by field defences (e.g. trenches), the incidence of penetrating injuries (and death) declines.
Because materials used in ballistic body armour will not decouple blast waves, blast lung still occurs and is relatively more common in survivors protected from fragmentation [14]. A threat analysis highlighted the dangers from novel blast weapons ‘New technologies are now being integrated into warheads that claim to have enhanced blast performance. Blast weapons could have been designed to fill a gap in capability; they are generally used for the attack of ‘soft’ targets including personnel, both in the open and within protective structures. With the increased number and range of these weapons, it is probable that UK forces will have to face them in future conflicts’ [15]. Because of these considerations it was important that we increased our ability to protect against weapons causing primary blast injuries and our ability to effectively treat casualties with combined injuries that included a significant component of primary blast injury such as blast lung.
Several strategies were undertaken simultaneously in the UK and by other groups worldwide to address the knowledge and capability gap. Significant progress was made in the understanding of the physical mechanisms underlying primary blast injury (predominantly contributing to improved protection) and the pathophysiological response of the casualty to blast injury (designed to improve treatment strategies). Wherever possible physical (non-animal) models were developed and used to reduce and replace the use of living animals in these studies. However, investigations of the pathophysiology of blast injuries relied on complex interactions between a variety of body systems, e.g. pulmonary, cardiovascular and inflammatory, that could not be modelled without recourse to living animals. In the UK, studies of this nature are conducted in strict accordance with the Animals (Scientific Procedures) Act 1986. The process includes an ethical review of proposed studies prior to submission of an application to the Home Office for a mandatory licence to conduct the study. At each stage there is an assessment of whether the work can be achieved by means other than in vivo studies and a close scrutiny of the scientific hypothesis and proposed methodology to ensure that the work is necessary and conducted humanely.
To assist in the evaluation of protective mechanisms, physical models were developed [16] and contributed to the development of decoupling strategies using improved armour systems to protect against blast shock waves [14]. A detailed discussion of blast protection is beyond the scope of this review, which is intended to focus on the pathophysiology of the response to blast and, in particular, the implications of these responses to resuscitation of casualties with combined blast injuries.
3. Forward (pre-hospital) resuscitation
Haemorrhage remains the leading cause of battlefield deaths [17] and the second leading cause of early death after civilian trauma [18]. Currently, a high proportion of military battlefield injuries are the consequence of explosive events, e.g. detonation of IEDs [11,19], with recent reports indicating that 70–80% of casualties result from explosions. There are also instances of civilians being injured by explosives, with mass casualties resulting from terrorist attacks [9,10,20,21]. Once catastrophic haemorrhage has been arrested [22], fluid resuscitation is often needed to sustain life until the casualty is evacuated to surgical care. Military evacuation timelines can differ significantly from those normally found in civilian settings. Although evacuation times in mature military operations are predominantly short, as are now being seen in Afghanistan, timelines in less mature settings (as we have seen recently [23,24]) can be considerably longer. Responsive resuscitation strategies such as the UK battlefield advanced trauma life support (BATLS [25]) are therefore needed to accommodate situations where evacuation timelines are extended and accounting for military injuries. In addition, these concepts may be applicable to civilian settings, e.g. after terrorist bombings, where there is often disruption to infrastructure and security issues relating to secondary devices resulting in delayed evacuation.
A key feature of far-forward fluid resuscitation of hypovolaemic casualties is the maintenance of an acceptable oxygen delivery to sustain life, and if possible to limit physiological deterioration while minimizing the risk of disrupting nascent blood clots which could cause re-bleeding as the casualty is evacuated to surgical care. A number of authorities advocate the use of ‘hypotensive’ resuscitation where fluid is limited or withheld to deliberately allow blood pressure to remain below normal levels, and many military and civilian medical services now resuscitate casualties to a target systolic blood pressure of approximately 80 mmHg (a palpable radial pulse in humans) [25,26].
The basic scientific evidence supporting hypotensive resuscitation is predominantly based on models of uncontrolled haemorrhage involving major arterial lesions [27–29], e.g. aortotomy, that are among the most susceptible to re-bleeding when arterial pressure is elevated during resuscitation. In addition, the strategies that were compared with hypotensive resuscitation involved immediate and aggressive fluid resuscitation [27–29], again maximizing the likelihood of re-bleeding. Finally, the timelines of the studies were predominantly short (1–2 h), minimizing the impact of hypoperfusion owing to hypotensive resuscitation. Consequently, the models used, while providing valuable information for a debate regarding civilian resuscitation, were less relevant to a potential military situation since the injury models are considered unsurvivable in a battlefield setting and military evacuation timelines can be considerably longer. With longer resuscitation timelines, tissue hypoperfusion, a serious limitation of the hypotensive strategy, assumes greater clinical significance ultimately leading to ischaemic damage [30,31]. The clinical evidence supporting hypotensive resuscitation is limited to short evacuation times to surgical care, e.g. 75 min, reported by Bickell et al. [32].
4. Physiology of resuscitation after combined primary blast injury and haemorrhage
British military doctrine for resuscitation advocated a hypotensive strategy until evacuation to surgical care [33] at the time the initial studies into hypotensive resuscitation after blast injury and haemorrhage were commissioned at Dstl Porton Down. This was in accordance with the still current NICE (UK National Institute of Health and Clinical Excellence) guidelines and US military practice [25,26]. However, there were serious concerns regarding this approach when delayed evacuation was likely to be imposed because of operational issues e.g. intense local hostile action. These concerns were amplified when blast injuries were considered. Primary blast injury to the lungs result in a series of pathophysiological changes culminating in hypoxaemia [34]. In addition, there are a range of cardiovascular [35–37] and microcirculatory [38] disturbances which may further compromise nutritative tissue blood flow. There was therefore a concern that a combination of the low tissue blood flow state inherent in hypotensive resuscitation might be compounded by poor arterial oxygenation leading to an overwhelmingly inadequate tissue oxygen delivery. Conversely, it was also known that blast injury could lead to a degree of myocardial compromise [36], which could limit the response to a more aggressive resuscitation strategy. There was therefore no evidence to promote either a hypotensive or a normotensive resuscitation strategy after blast injury. The first step to resolve the problem was to investigate the physiological response to resuscitation after combined blast injury and haemorrhage. A model was developed to mimic a casualty injured in an explosive event and hence sustaining a primary blast injury to the lungs and a significant haemorrhage as a consequence of a secondary blast injury [39].
The aim of the initial study was a comparison between normotensive and hypotensive strategies and the implication for survival over an 8 h period of resuscitation. Controlled haemorrhage was used in this study to avoid a systematic bias that would be introduced in an uncontrolled haemorrhage model by an anticipated difference in amounts of blood loss in blast and non-blast groups. This study was not intended to address the issue of re-bleeding as this is likely to be highly dependent on the nature of the model (or clinical injury) and would have confounded the interpretation of the physiological data.
The study was conducted in terminally anaesthetized pigs. Two injury patterns were included: haemorrhage (loss of 30% estimated total blood volume (BV), consistent across all groups) either alone or preceded by a survivable primary blast injury. The study compared the consequences of prolonged hypotensive1 and normotensive2 resuscitation after each injury type. All resuscitation was conducted with 0.9 per cent saline at an infusion rate of 3 ml kg−1 min−1 as this was the most commonly available fluid and probable infusion rate in a military pre-hospital setting.
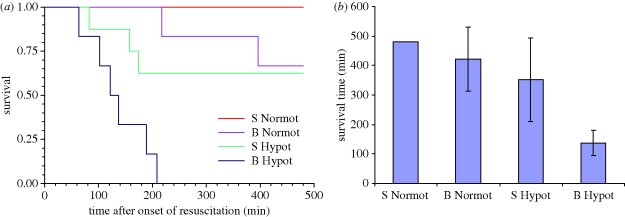
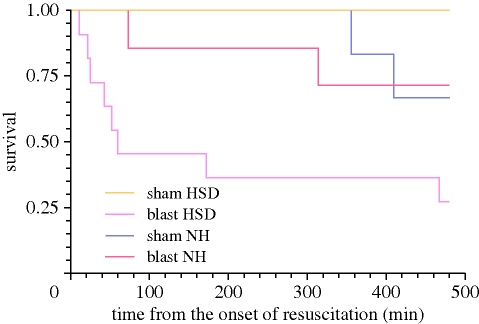
Blast injury was found to modify the response to subsequent haemorrhage, with blood pressure falling to lower levels in blast-exposed animals and remaining lower during the pre-resuscitation shock phase. In addition, blast injury led to a significant and persistent fall in PaO2, such that PaO2 in animals subjected to blast was significantly below that seen in those given no blast. The most important finding of this study [31] was a clear, statistically and clinically significant difference in survival times between groups. All animals (8/8) given normotensive resuscitation after 30 per cent BV haemorrhage in the absence of blast injury survived until the end of the study. Approximately 67 per cent (4/6) of those given normotensive resuscitation after blast injury and haemorrhage survived for the full 480 min after the onset of resuscitation. Similarly, approximately 62 per cent (5/8) of those given hypotensive resuscitation after haemorrhage in the absence of blast injury survived for 480 min. By contrast, none (0/6) of the animals given hypotensive resuscitation after blast and haemorrhage survived beyond 209 min after the onset of resuscitation (figure 1a). The mean survival times in each of the groups are shown in figure 1b. Survival times were therefore significantly shorter in the groups given prolonged hypotensive resuscitation when compared with those given normotensive resuscitation (p < 0.0001). When the data were stratified into those subjected to blast and sham blast before haemorrhage the significant difference in survival between hypotensive and normotensive resuscitation was found to reside in the blast groups (p = 0.0005), but not in the sham blast groups (p = 0.0628).
Figure 1.
(a) Kaplan–Meier survival plot, (b) mean survival times (95% CI) for four groups of animals subjected to either sham blast (S) or blast (B), haemorrhagic shock and resuscitation to either a normotensive (Normot) or hypotensive (Hypot) arterial blood pressure. No 95% CI is shown for S Normot group as all survived to the end of the observation period, 480 min after the start of resuscitation.
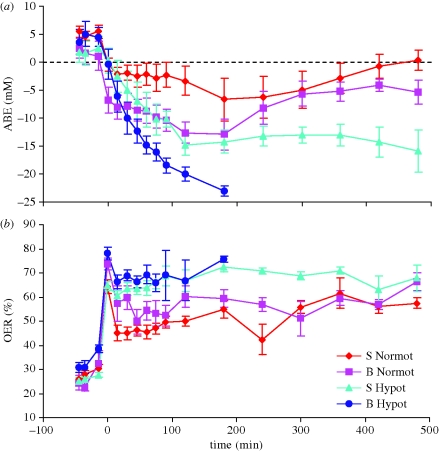
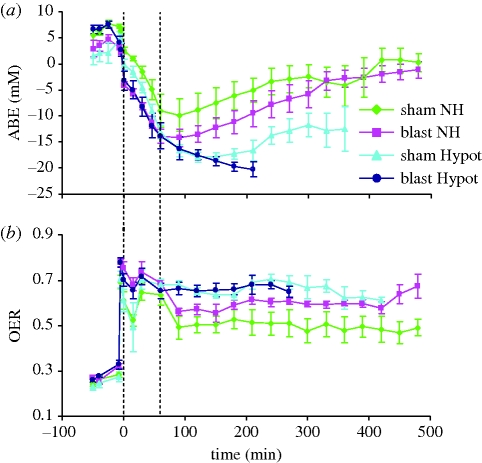
A detailed assessment of oxygen transport suggested that the problem with hypotensive resuscitation after combined blast injury and haemorrhage related to oxygen delivery [31]. Arterial base excess (ABE) was used as a marker of underlying metabolic acidosis. Haemorrhage and shock caused a significant fall in ABE. After the onset of resuscitation there was a significant change in ABE, but the pattern of change differed significantly between groups. In animals given normotensive resuscitation ABE improved (became less negative), while those given hypotensive resuscitation after sham blast and haemorrhage displayed a clinically significant persistent metabolic acidosis. The fall in ABE was significantly greater in animals given the hypotensive resuscitation after blast injury and haemorrhage, culminating in death (figure 2). Oxygen extraction ratio (OER) was significantly increased after haemorrhage and shock. However, animals that were given normotensive resuscitation were able to reduce their OER to levels which were significantly lower than those seen in animals given hypotensive resuscitation (figure 2). Despite maximal oxygen extraction it was clear that oxygen delivery was grossly inadequate during hypotensive resuscitation after combined blast and haemorrhage, and whole body oxygen consumption was found to be reduced in this group [31]. The implications of these data were that normotensive resuscitation restored organ perfusion sufficiently to satisfy demand for oxygen, even in those where blood oxygenation was impaired after blast.
Figure 2.
(a) Arterial actual base excess (ABE) and (b) oxygen extraction ratio (OER) in four groups of animals (for details see legend to figure 1). Time indicates time from the onset of resuscitation. First three values represent baseline 1, baseline 3 and blast (or sham blast). Mean values ± s.e.m.
This physiological study therefore demonstrated that prolonged hypotensive resuscitation is incompatible with survival after primary blast injury and haemorrhage. However, over shorter timescales of up to 1 h, it was possible to sustain life using a hypotensive strategy. Even in the absence of blast injury, hypotensive resuscitation after haemorrhage allows significant physiological deterioration likely to cause later clinical problems including coagulopathy [40] and the development of inflammatory complications [41].
These timescales are of profound military clinical significance, as survival for 1 h would allow evacuation to a Regimental Aid Post (Role 1 Facility) or other facility at which a doctor would be present. Conversely, survival was significantly prolonged if an aggressive resuscitation strategy is used. Although the model used in this study did not allow us to comment on the likelihood of normotensive resuscitation re-initiating haemorrhage which may itself affect survival [32], the study did demonstrate that aggressive resuscitation did not overwhelm the functional capacity of the blast injured myocardium. Normotensive resuscitation could be incorporated into resuscitation protocols following blast, or suspected blast injury, dependent on clinical judgement regarding the risk of rebleeding.
5. Developing an improved resuscitation strategy: novel hybrid resuscitation
The utility of hypotensive resuscitation is clearly limited to short evacuation times. However, although the aim is always to minimize evacuation times for battlefield casualties, provision has to be made for longer evacuation times as a contingency measure. The underlying cause of poor survival with prolonged hypotensive resuscitation was found to be inadequate oxygen delivery to tissues. Tissue oxygen delivery is the product of two factors: the oxygen content of arterial blood and the amount of blood perfusing the organs (equation (5.1)). Arterial oxygen content, in turn is dependent on both the concentration of haemoglobin in blood and the degree of saturation of haemoglobin with oxygen (equation (5.2)).
| 5.1 |
The above equation gives the relationship between whole body oxygen delivery (DO2), arterial oxygen content (CaO2) and cardiac output (CO).
| 5.2 |
The above equation gives the relationship between arterial oxygen content (CaO2), haemoglobin concentration [Hb] and arterial saturation with oxygen (SaO2); an additional term for the amount of oxygen dissolved in plasma has been omitted since this is small under normobaric conditions.
Achieving adequate oxygen delivery is therefore a balance of a number of factors, e.g. administration of clear fluids will improve cardiac output, initially reduce viscosity (improving microvascular flow) but will eventually dilute the haemoglobin and become counter-productive. Oxygen administration will make a significant impact when initial arterial saturation is low (e.g. in blast lung) but less so when initial saturation is approaching 100 per cent. Additional considerations are superimposed on this simple drive to maximize oxygen delivery e.g. the need to avoid excessive increases in arterial blood pressure which may disrupt nascent blood clots (see earlier). This results in a compromise between several variables and has to evolve as the balance of risk changes. These considerations led to the proposal of a new resuscitation strategy termed ‘novel hybrid resuscitation’ [42].
The novel hybrid strategy involves initial hypotensive resuscitation allowing time for clot stabilization in cases with incompressible haemorrhage, followed by normotensive resuscitation before the physiological penalties of hypotensive resuscitation become overwhelming3. To allow a systematic investigation of the new strategy, the model of explosive injury had to be developed further. An in vivo experimental model was unavoidable since the responses being investigated represented a complex interaction between the cardiovascular system, the lungs, metabolism, clotting mechanisms and physical risk of clot disruption and consequent re-bleeding. A model to assess the effectiveness of the novel resuscitation strategy therefore had to include an element of uncompressed haemorrhage to allow for the possibility of re-bleeding, and be a realistic model of ‘survivable’ battlefield injury that requires resuscitation to sustain life.
6. Model requirements
The model of incompressible haemorrhage used to assess new resuscitation protocols had to be severe enough to be life threatening and require resuscitation. In addition, the model needed to be representative of battlefield injuries that are potentially survivable given the level of care available to battlefield casualties, within realistic timescales, including extended evacuation times such as those recently seen in early phases of operations in Afghanistan and Iraq. The key features of the model included:
— an injury severe enough to require resuscitation to maintain life for up to 8 h;
— must be representative of a realistic, survivable, battlefield injury;
— assumes no surgical intervention for up to 8 h;
— provides data on survival and physiological state from the onset of resuscitation up to a maximum of 8 h; and
— must be reproducible, quantifiable and not introduce bias by being sensitive to confounding factors.
Several established models of uncontrolled and incompressible haemorrhage were in use by various research groups when this work was initiated and these were critically evaluated to determine if any were suitable for the programme's needs.
7. Established models of uncontrolled and incompressible haemorrhage
Uncontrolled haemorrhage refers to blood loss from a damaged blood vessel that has not been arrested by intrinsic haemostatic mechanisms or external intervention. Incompressible haemorrhage, in clinical terms, refers to a source of bleeding that is in an anatomical location where it is impossible to apply direct pressure to adequately control blood loss. Lesions to blood vessels where it is possible to apply direct pressure can be used as a model of incompressible haemorrhage if the experimental protocol excludes the use of direct pressure.
Established models of haemorrhage fall into three main categories:
— high pressure/high volume owing to a lesion in a major artery;
— low pressure/high volume owing to a lesion in a major vein;
— a mixed model owing to a lesion in both arteries and veins.
The rate of blood loss will depend on the magnitude of the vessel and the size of the lesion. In addition, arterial models will tend to have an initial higher rate of blood loss that will fall exponentially as arterial blood pressure falls [29] until either death or the intrinsic haemostatic mechanisms stop the bleeding. This type of model is particularly sensitive to re-bleeding when arterial pressure is elevated e.g. owing to dislodging a haemostatic clot during resuscitation.
Venous models have a lower initial rate of bleeding since intravascular pressure is much less in veins than arteries. However, venous bleeding can be substantial, prolonged and life threatening.
In mixed arterial/venous models the greatest volume of blood loss is initially arterial. However, as the haemorrhage progresses and arterial pressure falls the relative venous contribution becomes more important. Susceptibility to re-bleeding upon resuscitation is intermediate between pure arterial and venous models.
Other models of haemorrhage involve small, standardized, cuts to organs e.g. skin or spleen [43] to determine the duration of bleeding in response to various treatments. These latter models were not considered further since the degree of blood loss is not great or life threatening, and hence the models are irrelevant as a means of assessing resuscitation following battlefield injuries.
8. Potential models of haemorrhage relevant to battlefield casualties
(a). Arterial bleeding
A model incorporating aortotomy has been used extensively in studies investigating re-bleeding during resuscitation [27–29,44–46] Advice from senior surgical colleagues from the UK Defence Medical Services indicated that significant arterial injury of this nature was simply non-survivable in most battlefield casualties. Therefore, although an aortotomy model is one of the most stringent tests of re-bleeding during resuscitation, it is simply inappropriate in the context of assessing forward resuscitation strategies as the conclusions may be biased towards selecting a protocol only applicable to a minority of casualties.
(b). Venous bleeding
Grade V liver injury [47] is a well-established model that involves massive venous bleeding. This model has been used to assess the haemostatic potential of rFVIIa and topical agents [48–51]. However, it would not be a satisfactory model of a survivable battlefield injury in the context of assessing resuscitation strategies as survival is dependent on early surgical packing of the liver [49,51]. This could not happen in an austere pre-hospital environment for battlefield casualties. Consequently, a model involving a very severe venous haemorrhage was rejected.
(c). Mixed arterial and venous bleeding
Surgical opinion suggested that a mixed model of arterial and venous bleeding would be the most appropriate to assess any resuscitation strategy. Ideally this model should involve bleeding into a body cavity for face-validity4. A grade IV liver injury [47] has been used by others and has been shown to involve significant bleeding that is sensitive to therapy [52]. This is viewed as a mixed model since although it is predominantly venous it does also involve section of significant arteries as well as veins [52].
Grade IV liver injury was shown to be capable of producing a substantial haemorrhage, but relying solely on this injury as the source of blood loss could lead to increased variability in volume of initial haemorrhage and to potential bias between groups. Bias was likely to arise between blast and non-blast injured groups because haemorrhage after blast results in more rapid early falls in arterial blood pressure [53], resulting in greater uncontrolled blood loss from those subjected to haemorrhage without blast injury. The problem was solved by incorporating an initial phase of controlled haemorrhage (30% total estimated BV), at the conclusion of which arterial blood pressure had diminished to similar levels regardless of prior blast exposure. At the end of the controlled haemorrhage the grade IV liver injury was created from which further uncontrolled haemorrhage could ensue in all groups without systematic bias. It was found that animals' haemostatic mechanisms could arrest the uncontrolled haemorrhage and, by leaving the injury uncompressed, re-bleeding could potentially ensue. This model has face validity since a grade IV liver injury is viewed as serious, and is associated with significant bleeding in casualties and is the type of initially survivable injury where there would be concern regarding clot disruption and re-bleeding. The model required fluid resuscitation to sustain life for up to 8 h without surgical intervention.
9. Novel hybrid resuscitation strategy
The aim of the next study was to evaluate the novel hybrid resuscitation strategy whereby an initial period of hypotensive resuscitation (1 h), to sustain life and allow time for clot stabilization, is followed by normotensive resuscitation in an attempt to limit (or preferably reverse) the physiological deterioration that developed during the hypotensive phase. The primary endpoint was survival over an 8 h period simulating prolonged evacuation. Secondary outcome variables included physiological indices of deterioration such as metabolic acidosis and inflammatory state. The study was conducted on terminally anaesthetized Large White pigs. The animals were randomly allocated to one of four groups at the outset:
— group 1 (n = 6) haemorrhage/NH;
— group 2 (n = 6) blast/haemorrhage/NH;
— group 3 (n = 6) haemorrhage/Hypot;
— group 4 (n = 6) blast/haemorrhage/Hypot
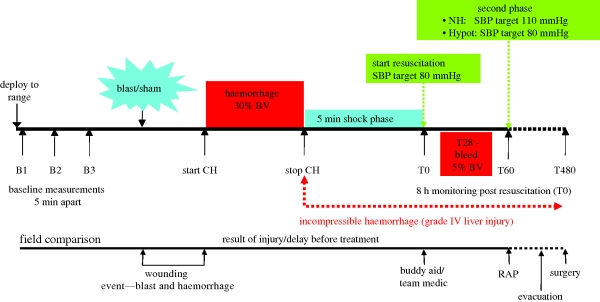
The resuscitation strategies employed were either novel hybrid (NH, target systolic arterial pressure of 80 mmHg for the first hour and 110 mmHg thereafter) or hypotensive (Hypot, target systolic arterial pressure of 80 mmHg throughout). The protocol is summarized in figure 3.
Figure 3.
Experimental protocol which commenced approximately 1 h after the end of surgery. CH, controlled haemorrhage; BV, total estimated blood volume; RAP, Regimental Aid Post; SBP, systolic arterial blood pressure.
(a). Effect of novel hybrid resuscitation on survival
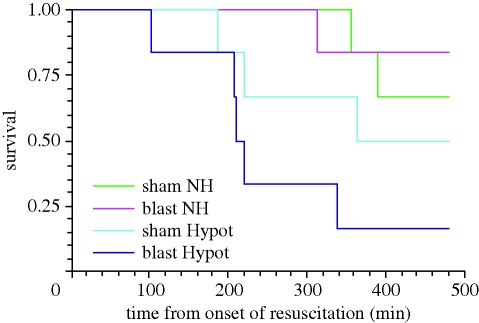
Novel hybrid resuscitation was associated with a significantly increased survival time compared with prolonged hypotensive resuscitation in blast injured groups (figure 4, p = 0.02, Kaplan–Meier survival analysis, Peto's log rank test). By contrast, there was no significant difference in survival time between NH and hypotensive resuscitation in the absence of blast injury (p = 0.45).
Figure 4.
Kaplan–Meier survival plot for four groups of animals subjected to either sham blast (sham) or blast (blast), haemorrhagic shock and novel hybrid (NH) or hypotensive (Hypot) resuscitation with 0.9% saline (NS).
(b). Metabolic effects associated with alterations in oxygen transport
A statistically and clinically significant metabolic acidosis developed in all groups during the hypotensive phase of resuscitation, characterized by a significant fall in arterial base excess (figure 5). In animals given NH resuscitation the metabolic acidosis was reversed after the onset of the normotensive phase of resuscitation. By contrast, animals given prolonged hypotensive resuscitation after blast injury and haemorrhage showed a continued fall in arterial base excess until the animals succumbed. Continued hypotensive resuscitation after haemorrhage in the absence of blast injury resulted in a severe, sustained, metabolic acidosis.
Figure 5.
(a) Arterial base excess (ABE) and (b) oxygen extraction ratio (OER) in four groups of animals subjected to either sham blast (sham) or blast (blast), haemorrhagic shock and resuscitation. The first dotted line represents the onset of resuscitation (hypotensive in all groups). The second dotted line indicates the onset of normotensive resuscitation in NH groups and continued hypotensive resuscitation in the Hypot groups. For further explanation of figure see legend to figure 4.
A detailed analysis of oxygen transport suggested that the improvement seen with the NH resuscitation strategy was associated with enhanced tissue oxygen delivery. Following haemorrhagic shock there was a significant increase in oxygen extraction ratio (OER) from the physiological resting normal value of approximately 25 per cent to the physiological maximum of approximately 80 per cent. OER persisted at the maximal level until the onset of normotensive resuscitation in the NH groups. Thereafter OER was able to fall in both groups given NH resuscitation, but remained maximal in the hypotensive groups, resulting in a statistically significant difference between NH and hypotensive resuscitation groups (figure 5). Clearly, the level of tissue oxygen delivery was grossly inadequate in the group given hypotensive resuscitation after blast injury and haemorrhage since arterial base excess, a marker of metabolic acidosis, continued to fall despite maximal OER. By contrast, in the groups given NH resuscitation the decline in arterial base excess was reversed and base excess returned towards normal. Therefore, NH resuscitation conferred physiological advantage even in the absence of blast injury since it reversed a physiologically damaging metabolic acidosis which has been associated with enhanced inflammatory responses and morbidity [41].
(c). Evidence of enhanced inflammatory response associated with hypotensive resuscitation
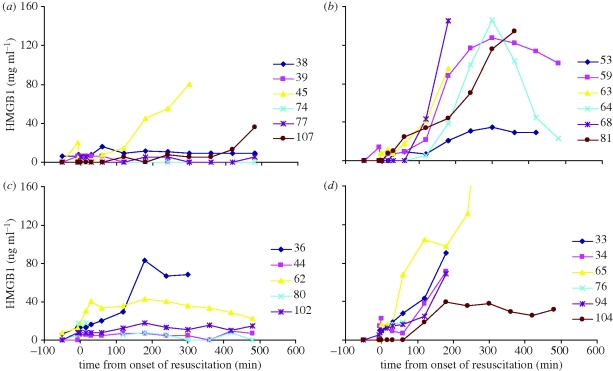
High mobility group box 1 (HMGB1), a highly conserved, ubiquitous protein present in the nuclei and cytoplasm of nearly all cell types, is a necessary and sufficient mediator of inflammation during sterile and infection-associated responses [54]. Significantly elevated levels of HMGB1 were found to be associated with hypotensive resuscitation (p = 0.0156 Friedman analysis of variance) rather than injury type (p = 0.6695, blast versus sham blast, figure 6). Elevated HMGB1 was found to be associated with metabolic acidosis, possibly as a consequence of poor oxygen delivery to tissue rather than blast injury per se.
Figure 6.
Data from individual animals in each of four groups showing arterial plasma levels of high mobility group box 1 (HMGB1). (a) sham NH; (b) sham Hypot; (c) blast NH; (d) blast Hypot.
(d). No evidence of increased uncontrolled haemorrhage (re-bleeding) associated with novel hybrid resuscitation
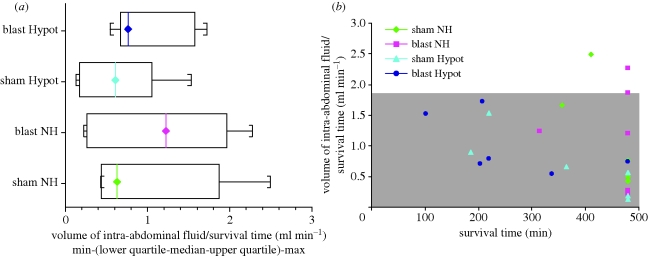
No evidence could be found of increased intra-abdominal blood loss (re-bleeding from the grade IV liver injury) associated with NH resuscitation. There was no significant difference in the volumes of intra-abdominal fluid (normalized for survival time) between groups (p = 0.33, 2 way ANOVA, figure 7a). To evaluate whether early death owing to clinically significant re-bleeding was seen in any individual animal given NH resuscitation, data representing normalized volumes of intra-abdominal fluid were plotted for individual animals as a function of survival time. For illustrative purposes the data overlie the mean ± 2 standard deviation envelope for the animals given hypotensive resuscitation (figure 7b). This envelope corresponds to the volume of intra-abdominal haemorrhage expected in approximately 95 per cent of those given hypotensive resuscitation and hence can be used to determine whether any individual animals exhibited a greater than expected volume of uncontrolled blood loss. Three animals did fall outside of this envelope and exhibited a greater degree of fluid loss into the abdomen, and all three were in the group given NH resuscitation. However, there was no evidence that the degree of fluid loss in these three animals resulted in a fatal compromise: two survived to the end of the study (8 h) and the third survived almost to the end of the study.
Figure 7.
Volume of intra-abdominal fluid assessed immediately post-mortem, normalized for survival time in four groups of animals. (a) Summary data from four groups; (b) data from individual animals, grey shaded area represents mean ± 2 standard deviation envelope for the animals given hypotensive resuscitation.
A notable difference between the present study and previous studies that have been used to support the concept of hypotensive resuscitation is the degree of re-bleeding. There are a number of important differences between our study and previous published work (e.g. [27–29,32]). As described earlier, the model of injury underpinning the re-bleeding element may be significant. Earlier studies (e.g. [27–29]) used a model that is perhaps the most sensitive to re-bleeding (lesion, often a longitudinal tear, in a major artery), while our model is one of a mixed arterial/venous lesion. The result of model choice in previous studies therefore results in a bias towards hypotensive resuscitation. This can be viewed as a strength in that it selects for the ‘safest’ option, unless the model itself is viewed as an unrealistically severe challenge whereupon it becomes a weakness since it may bias against a more effective treatment for the majority of casualties. Consequently, the earlier studies have provided valuable insight into the context of a civilian setting with rapid evacuation to a surgical facility. However, these earlier studies are less relevant for a military setting when there can be extended evacuation times. A second important difference between the present study and earlier work, including the clinical trial [32], is the timing of the normotensive phase of the resuscitation. The earlier studies compared hypotensive resuscitation with immediate and very aggressive fluid resuscitation to restore blood pressure, a time when the nascent clot is most fragile and vulnerable. By contrast, NH involves initial hypotensive resuscitation (for 1 h) allowing time for the clot to stabilize [55] before initiating normotensive resuscitation. These differences may explain why NH is not associated with significant re-bleeding compared with earlier studies that reported re-bleeding with normotensive resuscitation strategies.
Novel hybrid resuscitation was therefore found to be superior to hypotensive resuscitation for extended evacuation times (up to 8 h) in a model of survivable battlefield injury. This should not be viewed as a simple challenge to hypotensive resuscitation but rather sets boundaries to its application when timelines of evacuation are extended, especially if there are concomitant lung injuries that compromise oxygenation. NH resuscitation is a viable solution to a problem that arises when extended evacuation is enforced: after the first hour of resuscitation consider elevating blood pressure towards normal levels to improve tissue perfusion and oxygen delivery before the physiological penalties of under-perfusion associated with hypotensive resuscitation become overwhelming. In this way a compromise is found between hypotensive and normotensive resuscitation by minimizing the likelihood of re-bleeding initially (using the established hypotensive principle) but attempting to reverse the physiological compromise later if the casualties have not yet arrived at an echelon of surgical care. This provides underpinning evidence for the current BATLS strategy for management of hypovolaemic casualties [25]. Many clinical decisions are based on a compromise representing a balance of risk, and resuscitation is no exception. The evaluation of the casualty should be made on a case by case basis. Wherever possible the decision making will include the potential risk of re-bleeding and factors predisposing to physiological compromise (e.g. lung injury) based on nature of injury, together with the imminence of evacuation. However, in some circumstances such evaluation may be impossible owing to the austerity of the environment or lack of a suitably qualified individual to make the judgement. Under those conditions, the NH strategy may represent the safest choice for the majority of surviving battlefield casualties if there is concomitant lung injury.
(e). Hypertonic saline dextran
The initial demonstration of the novel hybrid resuscitation strategy used 0.9 per cent saline as the only resuscitation fluid. It was possible that further advantage could be gained by initiating resuscitation with a hypertonic solution e.g. hypertonic saline dextran (HSD). Hypertonic solutions have been advocated for far-forward military resuscitation [56] since they possess a significant logistical benefit in terms of reduced weight burden; 250 ml of HSD is reputed to be equivalent to 3 l of 0.9 per cent saline with respect to early plasma volume expansion. Hypertonic solutions are also known to dampen the systemic inflammatory response that develops after trauma and which is thought to be part of the aetiology underlying later complications [57–60]. This benefit of HSD has been shown in a number of clinical studies [59–61]. In addition, hypertonic solutions have been shown to confer microcirculatory benefit, possibly improving the distribution of blood flow in tissue [62]. Consistent with this, in an early study we found that HSD reduced the metabolic acidosis during hypotensive resuscitation after haemorrhage alone [42]. Unfortunately this beneficial effect was not apparent after combined blast injury and haemorrhage. A possible explanation for this at the time was that the microcirculatory effect of HSD was simply not sufficient to overcome the very poor tissue oxygen delivery owing to combined low arterial oxygen content (owing to blast lung) and low flow (owing to the hypotension). [42] However, a resuscitation strategy that promotes better tissue perfusion might allow the beneficial effects of HSD to become apparent after combined blast injury and haemorrhage.
An extension of the novel hybrid study therefore evaluated whether HSD, as part of a resuscitation strategy employing the novel hybrid blood pressure profile, was at least as effective as 0.9 per cent saline in relation to survival. Furthermore, we sought to determine whether HSD was superior to 0.9 per cent saline with respect to physiological changes by limiting the initial deterioration in acid base status during the hypotensive phase of resuscitation and enhance the acid base improvement during the normotensive phase. This study therefore compared the effectiveness of novel hybrid resuscitation when the resuscitation was commenced with HSD versus normal saline. The maximum amount of HSD was capped at 500 ml per 70 kg and the fluid was given in controlled aliquots to avoid over-shooting of the target blood pressure and increasing the risk of re-bleeding from an uncompressed haemorrhage model.
(f). Effect of HSD on survival
Surprisingly, HSD was associated with a significantly reduced survival time in the blast/haemorrhage groups (Kaplan–Meier survival analysis, Wilcoxon method, p = 0.04, figure 8) but not in the groups subjected to haemorrhage alone (p = 0.11).
Figure 8.
Kaplan–Meier survival plot for four groups of animals subjected to either sham blast (S) or blast (B), haemorrhagic shock and resuscitation initiated with either hypertonic saline dextran (HSD) or 0.9% saline (NS).
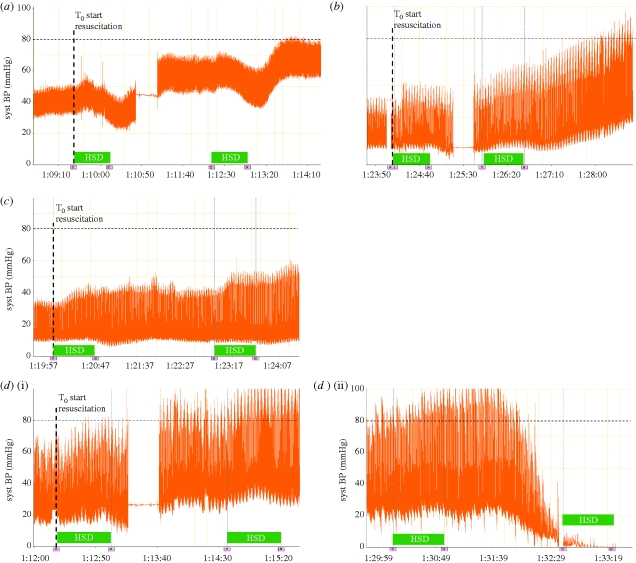
Poor survival in the group subjected to combined blast injury and haemorrhage and subsequently resuscitation with HSD was owing to poor responsiveness to HSD. An example of a ‘good’ response to HSD is given in figure 9a,b. HSD can be seen to cause an initial, brief, fall in arterial blood pressure followed by a significant rise. In both these cases two doses were required to attain the target systolic arterial pressure (SBP). The individual animals shown in figure 9a,b continued to respond well to HSD and survived, respectively, for 480 and 467 min after the onset of resuscitation. By contrast, an example of ‘poor’ response to HSD is shown in figure 9c. This animal showed little response to HSD, the target SBP was not met despite repeated doses of HSD and the animal eventually succumbed within 21 min of the onset of resuscitation. A more dramatic HSD failure is shown in figure 9d(i) and 9d(ii). Here the response to HSD was initially good and the target SBP was attained. However, this was followed by a sudden collapse and death approximately 15 min after the onset of resuscitation, despite further attempts at fluid resuscitation as directed by the protocol. Of 11 animals in the group given combined blast and haemorrhage and resuscitated with HSD, six showed a good response to HSD and survived beyond the first hour of the study, three failed to respond (did not attain the target SBP) and two showed the catastrophic collapse shown in figure 9d. Furthermore, one of the animals that failed to attain the target SBP also showed a sudden collapse in blood pressure prior to death. In the absence of blast injury all animals given HSD responded well and survived the duration of the study (8 h from the onset of resuscitation).
Figure 9.
Systemic arterial blood pressure response to infusion of hypertonic saline dextran (HSD) in four individual animals. All animals had received a controlled haemorrhage followed by a grade IV liver injury. Preceding the haemorrhage animals were either subject to blast injury or no blast injury as follows: (a) no blast injury, good response to HSD; (b) blast injury, good response to HSD; (c) blast injury, poor response to HSD, target blood pressure was not attained before animal died; (d)(i) blast injury, initial good response to HSD and attainment of target blood pressure followed approximately 15 min later by (d)(ii) catastrophic collapse and death. Blocks labelled HSD indicate periods of HSD infusion.
The mechanism(s) whereby HSD fails after combined primary blast injury and haemorrhage is of particular interest. Unfortunately, although the study identified clear exclusions of potential mechanisms it did not identify a clear mechanism of failure. Three plausible possibilities were excluded based on the study: catastrophic re-bleeding, increased vascular permeability attenuating the ability of HSD to expand plasma volume and acute sodium toxicity.
HSD (for initial resuscitation) was therefore inferior to 0.9 per cent saline when haemorrhage was complicated with primary blast injury. This was owing to early failure to respond adequately to HSD, or sudden collapse during its use, in a number of cases. By contrast HSD did show a significant physiological benefit when used after haemorrhage in the absence of primary blast injury. However, this benefit may not outweigh the risk of overshooting the target blood pressure during resuscitation especially in austere circumstances where accurate, continuous measurement of arterial blood pressure is impossible. Nonetheless HSD may have a role (in circumstances where the logistical benefit of weight reduction is very important) for resuscitating casualties where primary blast injury can definitely be excluded e.g. injuries not associated with explosions. Should HSD be used in these circumstances very careful titration of HSD to target blood pressures would be necessary, imposing its own burden on those responsible for resuscitating the casualty under difficult circumstances.
(g). Supplementary oxygen
The strategies described hitherto focused on improving tissue perfusion. These were investigated first because forward deployment of oxygen in a military setting is especially problematic: pressurized cylinders represent a substantial additional hazard in an environment where there is a ballistic threat, although newer technologies to generate oxygen in situ may provide a solution in the future. In theory oxygen administration should make a significant impact when initial arterial saturation is low (e.g. in blast lung) but less so when initial saturation is approaching 100 per cent. Additional considerations are superimposed on this simple drive to maximize oxygen delivery e.g. the need to avoid excessive increases in arterial blood pressure which may disrupt nascent blood clots (see earlier). A further study was therefore undertaken to assess the effects of elevated FiO2 in combination with hypotensive resuscitation on survival after combined blast injury and haemorrhage. The primary outcome variable was again survival and secondary outcomes included physiological state.
The study was conducted on two groups of terminally anaesthetized Large White pigs. Both groups were subjected to primary blast injury followed by the model of controlled/uncontrolled haemorrhage (grade IV liver injury). A hypotensive resuscitation strategy using normal saline was used throughout. Thirty minutes after the onset of resuscitation one group of animals were given elevated inspired oxygen, titrated to increase arterial oxygen saturation to 95 per cent. The second (control) group continued to breathe air throughout.
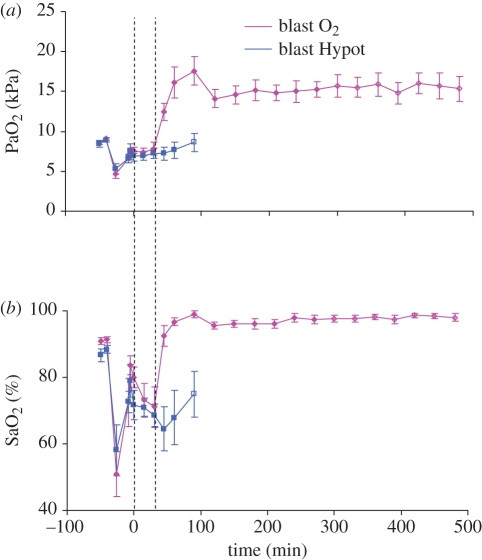
Administration of supplementary oxygen successfully increased PaO2 and SaO2, and there were no difficulties in titrating SaO2 to a target value of 95 per cent (figure 10). In a field situation this could be achieved using pulse oximetry.
Figure 10.
(a) Arterial oxygen tension (PaO2) and (b) saturation (SaO2) in two groups of animals subjected to blast injury, haemorrhage and hypotensive resuscitation. Time indicates time from the onset of resuscitation which was initiated at the first dotted line. Second dotted line (30 min after the onset of resuscitation) represents the divergence of treatment groups with supplemental inspired oxygen being introduced into the blast O2 group while the blast Hypot group continue to breathe air. First two values represent baseline, followed by post blast, pre and post haemorrhage and then the onset of resuscitation. Open symbols indicate 66% of animals surviving. No data plotted when proportion surviving fell to 50% or below. Mean values ± s.e.m.
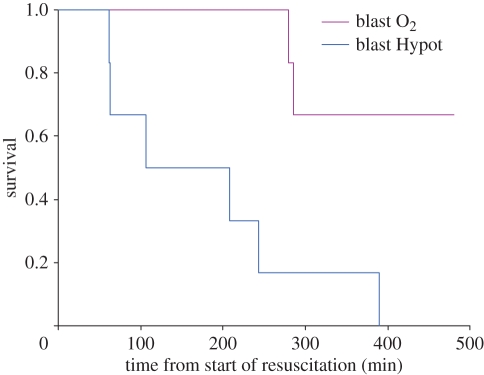
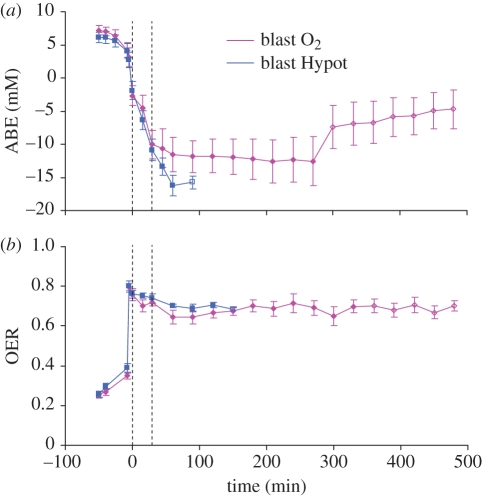
Survival times were significantly longer in the oxygen-treated group compared with animals breathing air (p = 0.014, figure 11). The improved survival was associated with an arrest of the development of metabolic acidosis in the oxygen-treated group (figure 12). By contrast, in the group given air to breathe the reduction in base excess continued with prolonged resuscitation until the animals succumbed (figure 12).
Figure 11.
Kaplan–Meier survival plot for two groups of animals subjected to blast injury, haemorrhage and hypotensive resuscitation. The Blast O2 group was given supplemental oxygen to elevate SaO2 to 95% starting 30 min after the onset of resuscitation, while blast Hypot continued to breathe air throughout.
Figure 12.
(a) Arterial base excess (ABE) and (b) oxygen extraction ratio (OER) in two groups of animals subjected to blast injury, haemorrhage and hypotensive resuscitation. For further explanation see legend to figure 10.
The effect of increased FiO2 was an elevation in arterial oxygen content and it is probable that this resulted in a beneficial effect by enhancing tissue oxygen delivery. This is supported by the differences in OER between the two groups once supplemental oxygen administration had commenced. Initially OER was found to be at the physiological normal of approximately 25 per cent in both groups, and was elevated significantly after blast injury and haemorrhage to the physiological maximum value of approximately 80 per cent (figure 12). OER remained at this level in both groups for the first 30 min of resuscitation, and until this point there were no significant differences between groups. OER remained at the physiological maximum for the reminder of the study in the group given air to breathe. Clearly, the level of tissue oxygen delivery was grossly inadequate in the group given air to breathe since arterial base excess continued to fall despite maximal OER. By contrast, in the group given supplemental oxygen OER fell marginally (although this did not attain statistical significance) and, importantly from a clinical perspective the fall in base excess was arrested. Base excess subsequently remained stable, but low, in the group given supplemental oxygen, suggesting that tissue oxygen delivery was just adequate to sustain life, although in this model not sufficient to pay the ‘oxygen debt’ incurred during the shock phase and the initial period of resuscitation.
Supplementary oxygen, sufficient to restore arterial oxygen saturation to 95 per cent, significantly increased survival during hypotensive resuscitation after primary blast injury and haemorrhage. The beneficial effects of oxygen appear to be due to improved oxygenation (owing to elevated arterial oxygen content). This is clearly a beneficial effect compared with air-breathing and is likely to ‘buy time’ in blast-injured hypovolaemic casualties, but it is likely to be limited by the poor tissue perfusion associated with hypotensive resuscitation. However, in casualties where there is a high risk of re-bleeding and an elevation in arterial blood pressure later in the resuscitation process (novel hybrid resuscitation) poses an unacceptable risk, supplemental oxygen has a clear role to increase survival times during protracted evacuation to surgical care. This use of supplementary oxygen to a pre-defined arterial saturation level is in accordance with published guidelines relating to the use of emergency oxygen in adults, which was the subject of a recent editorial in the British Medical Journal [63].
10. Conclusions
The conclusion of the programme on focused far-forward resuscitation strategies was the provision of objective evidence to describe the limitations of hypotensive resuscitation strategies with particular reference to battlefield injuries, and an understanding of the mechanisms of that limitation. A new strategy, novel hybrid resuscitation, was developed to combine the strengths of hypotensive resuscitation during rapid evacuation with a method of mitigating its weaknesses when delayed evacuation is enforced. An alternative, initially attractive, resuscitation fluid (HSD) was found to be unsuitable after combined blast injury and hypovolaemia, recognizing an important limitation to its usefulness. Finally, a strong evidence base is emerging to drive consideration of forward oxygen deployment. This would be especially attractive for casualties at high risk of re-bleeding where novel hybrid resuscitation may not be suitable, although technological advances will need to be made to solve the logistical and safety issues relating to forward deployment of oxygen.
Acknowledgements
We gratefully acknowledge the essential collaboration with our clinical colleagues the Defence Professors of Anaesthesia & Critical Care (Col P. Mahoney), Emergency Medicine (Col T. Hodgetts) and Surgery (Surg Capt M. Midwinter), Royal Center for Defence Medicine, Birmingham. Also, seconded military clinicians, Lt Col J. Garner, Maj J. Granville-Chapman and Sqn Ldr N. Jacobs, aspects of whose MD theses are presented in this article, and the expert technical support of our colleagues at Porton Down who provide the infrastructure to make the experimental work possible.
© Crown copyright 2011. Published with the permission of the Defence Science and Technology Laboratory on behalf of the Controller of HMSO.
Endnotes
Target systolic arterial pressure (SBP) of 80 mmHg, corresponding to a palpable radial pulse.
Target SBP of 110 mmHg, attained using the ATLS strategy of infusing 2 l/70 kg 0.9 per cent saline followed by further aliquots as necessary to attain and maintain the target SBP.
It is acknowledged that a pressure endpoint is a very poor proxy of tissue blood flow and oxygen delivery. However, pressure endpoints are used in far-forward resuscitation strategies because currently in these austere environments equipment to measure blood flow or tissue oxygenation is simply unavailable. A combat medical technician (military paramedic), who often provides early treatment, has to carry their equipment and fluids over long distances in hostile environments. Hence the equipment available is limited and assessment of blood pressure is reduced to e.g. detection of a palpable radial pulse (corresponding to a systolic arterial pressure of 80–90 mmHg). Identification of parameters to improve casualty assessment and equipment to make this feasible in a forward environment is an area of essential research.
Face validity in this case indicates that the model is both valid and is recognized as being valid by those relying on conclusions drawn using it rather than a model that may fulfil the scientific criteria but is not easily recognized as being a valid model of incompressible bleeding.
One contribution of 20 to a Theme Issue ‘Military medicine in the 21st century: pushing the boundaries of combat casualty care’.
References
- 1.Nelson T. J., Wall D. B., Stedje-Larsen E. T., Clark R. T., Chambers L. W., Bohman H. R. 2006. Predictors of mortality in close proximity blast injuries during Operation Iraqi Freedom. J. Am. Coll. Surg. 202, 418–422 10.1016/j.jamcollsurg.2005.11.011 (doi:10.1016/j.jamcollsurg.2005.11.011) [DOI] [PubMed] [Google Scholar]
- 2.Maynard R. L., Cooper G. J., Scott R. 1989. Mechanism of injury in bomb blasts and explosions. In Trauma (ed. Westaby S.). London, UK: Heinemann [Google Scholar]
- 3.Zuckerman S. 1941. Discussion on the problem of blast injuries. Proc. R. Soc. Med. 34, 171–188 [PMC free article] [PubMed] [Google Scholar]
- 4.Clemedson C. J. 1956. Shock wave transmission to the central nervous system. Acta Physiol. Scand. 37, 204–214 10.1111/j.1748-1716.1956.tb01356.x (doi:10.1111/j.1748-1716.1956.tb01356.x) [DOI] [PubMed] [Google Scholar]
- 5.Belanger H. G., Scott S. G., Scholten J., Curtiss G., Vanderploeg R. D. 2005. Utility of mechanism-of-injury-based assessment and treatment: blast injury program case illustration. J. Rehab. Res. Dev. 42, 403–412 10.1682/JRRD.2004.08.0095 (doi:10.1682/JRRD.2004.08.0095) [DOI] [PubMed] [Google Scholar]
- 6.Lew H. L., Poole J. H., Guillory S. B., Salerno R. M., Leskin G., Sigford B. 2006. Persistent problems after traumatic brain injury: the need for long-term follow-up and coordinated care—Guest Editorial. J. Rehab. Res. Dev. 43, VII–VIX 10.1682/JRRD.2006.05.0054 (doi:10.1682/JRRD.2006.05.0054) [DOI] [PubMed] [Google Scholar]
- 7.Okie S. 2005. Traumatic brain injury in the war zone. N. Engl. J. Med. 352, 2043–2047 10.1056/NEJMp058102 (doi:10.1056/NEJMp058102) [DOI] [PubMed] [Google Scholar]
- 8.Mellor S. G., Cooper G. J. 1989. Analysis of 828 servicemen killed or injured by explosion in northern Ireland 1970–84: the hostile action casualty system. Br. J. Surg. 76, 1006–1010 10.1002/bjs.1800761006 (doi:10.1002/bjs.1800761006) [DOI] [PubMed] [Google Scholar]
- 9.De Ceballos J. P., Turegano-Fuentes F., Perez-Diaz D., Sanz-Sanchez M., Martin-Llorente C., Guerrero-Sanz J. E. 2005. 11 March 2004: the terrorist bomb explosions in Madrid, Spain—an analysis of the logistics, injuries sustained and clinical management of casualties treated at the closest hospital. Crit. Care 9, 104–111 10.1186/cc2995 (doi:10.1186/cc2995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marti M., Parron M., Baudraxler F., Royo A., Gomez L. N., Varez-sala R. 2006. Blast injuries from Madrid terrorist bombing attacks on March 11, 2004. Emerg. Radiol. 13, 113–122 10.1007/s10140-006-0534-4 (doi:10.1007/s10140-006-0534-4) [DOI] [PubMed] [Google Scholar]
- 11.Ramasamy A., Harrisson S. E., Clasper J. C., Stewart M. P. 2008. Injuries from roadside improvised explosive devices. J. Trauma 65, 910–914 10.1097/TA.0b013e3181848cf6 (doi:10.1097/TA.0b013e3181848cf6) [DOI] [PubMed] [Google Scholar]
- 12.Ritenour A. E., Blackbourne L. H., Kelly J. F., Mclaughlin D. F., Pearse L. A., Holcomb J. B., Wade C. E. 2010. Incidence of primary blast injury in US military overseas contingency operations: a retrospective study. Ann. Surg. 251, 1140–1144 10.1097/SLA.0b013e3181e01270 (doi:10.1097/SLA.0b013e3181e01270) [DOI] [PubMed] [Google Scholar]
- 13.Smith J. E. 2011. The epidemiology of blast lung injury during recent military conflicts: a retrospective database review of cases presenting to deployed military hospitals, 2003–2009. Phil. Trans. R. Soc. B 366, 291–294 10.1098/rstb.2010.0251 (doi:10.1098/rstb.2010.0251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper G. J. 1996. Protection of the lung from blast overpressure by thoracic stress wave decouplers. J. Trauma 40(Suppl. 3), S105–S110 10.1097/00005373-199603001-00024 (doi:10.1097/00005373-199603001-00024) [DOI] [PubMed] [Google Scholar]
- 15.Dearden P. 2001. New blast weapons. J. R. Army Med. Corps 147, 80–86 [DOI] [PubMed] [Google Scholar]
- 16.Cooper G. J., Pearce B. P., Sedman A. J., Bush I. S., Oakley C. W. 1996. Experimental evaluation of a rig to simulate the response of the thorax to blast loading. J. Trauma 40, S38–S41 10.1097/00005373-199603001-00007 (doi:10.1097/00005373-199603001-00007) [DOI] [PubMed] [Google Scholar]
- 17.Champion H. R., Bellamy R. F., Roberts C. P., Leppaniemi A. 2003. A profile of combat injury. J. Trauma 54, S13–S19 [DOI] [PubMed] [Google Scholar]
- 18.Sauaia A., Moore F. A., Moore E. E., Moser K. S., Brennan R., Read R. A., Pons P. T. 1995. Epidemiology of trauma deaths—a reassessment. J. Trauma 38, 185–193 10.1097/00005373-199502000-00006 (doi:10.1097/00005373-199502000-00006) [DOI] [PubMed] [Google Scholar]
- 19.Champion H. R., Holcomb J. B., Young L. A. 2009. Injuries from explosions: physics, biophysics, pathology, and required research focus. J. Trauma 66, 1468–1477 10.1097/TA.0b013e3181a27e7f (doi:10.1097/TA.0b013e3181a27e7f) [DOI] [PubMed] [Google Scholar]
- 20.Almogy G., Luria T., Richter E., Pizov R., Bdolah-Abram T., Mintz Y., Zamir G., Rivkind A. I. 2005. Can external signs of trauma guide management?: lessons learned from suicide bombing attacks in Israel. Arch. Surg. 140, 390–393 [DOI] [PubMed] [Google Scholar]
- 21.Leibovici D., Gofrit O. N., Stein M., Shapira S. C., Noga Y., Heruti R. J., Shemer J. 1996. Blast injuries: bus versus open-air bombings—a comparative study of injuries in survivors of open-air versus confined-space explosions. J. Trauma 41, 1030–1035 10.1097/00005373-199612000-00015 (doi:10.1097/00005373-199612000-00015) [DOI] [PubMed] [Google Scholar]
- 22.Hodgetts T. J., Mahoney P. F., Russell M. Q., Byers M. 2006. ABC to ABC: redefining the military trauma paradigm. Emerg. Med. J. 23, 745–746 10.1136/emj.2006.039610 (doi:10.1136/emj.2006.039610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilski T. R., Baker B. C., Grove J. R., Hinks R. P., Harrison M. J., Sabra J. P., Temerlin S. M., Rhee P. 2003. Battlefield casualties treated at Camp Rhino, Afghanistan: lessons learned. J. Trauma 54, 814–821 10.1097/01.TA.0000046627.87250.1D (doi:10.1097/01.TA.0000046627.87250.1D) [DOI] [PubMed] [Google Scholar]
- 24.Bohman H. R., Stevens R. A., Baker B. C., Chambers L. W. 2005. The US Navy's forward resuscitative surgery system during operation Iraqi freedom. Military Med. 170, 297–301 [DOI] [PubMed] [Google Scholar]
- 25.2006. Battlefield advanced life support. UK: Defence Medical Education and Training Agency. [Google Scholar]
- 26.2004. Pre-hospital initiation of fluid replacement therapy in trauma. NICE Technology Appraisal 74. [Google Scholar]
- 27.Bickell W. H., Bruttig S. P., Wade C. E. 1989. Hemodynamic-response to abdominal aortotomy in the anesthetized swine. Circ. Shock 28, 321–332 [PubMed] [Google Scholar]
- 28.Kowalenko T., Stern S., Dronen S., Xu W. 1992. Improved outcome with hypotensive resuscitation of uncontrolled hemorrhagic-shock in a swine model. J. Trauma 33, 349–353 10.1097/00005373-199209000-00003 (doi:10.1097/00005373-199209000-00003) [DOI] [PubMed] [Google Scholar]
- 29.Stern S. A., Dronen S. C., Birrer P., Wang X. 1993. Effect of blood-pressure on hemorrhage volume and survival in a near-fatal hemorrhage model incorporating a vascular injury. Ann. Emerg. Med. 22, 155–163 10.1016/S0196-0644(05)80195-7 (doi:10.1016/S0196-0644(05)80195-7) [DOI] [PubMed] [Google Scholar]
- 30.Rafie A. D., Rath P. A., Michell M. W., Kirschner R. A., Deyo D. J., Prough D. S., Grady J. J., Kramer G. C. 2004. Hypotensive resuscitation of multiple hemorrhages using crystalloid and colloids. Shock 22, 262–269 10.1097/01.shk.0000135255.59817.8c (doi:10.1097/01.shk.0000135255.59817.8c) [DOI] [PubMed] [Google Scholar]
- 31.Garner J., Watts S., Parry C., Bird J., Cooper G., Kirkman E. 2010. Prolonged permissive hypotensive resuscitation is associated with poor outcome in primary blast injury with controlled hemorrhage. Ann. Surg. 251, 1131–1139 10.1097/SLA.0b013e3181e00fcb (doi:10.1097/SLA.0b013e3181e00fcb) [DOI] [PubMed] [Google Scholar]
- 32.Bickell W. H., Wall M. J., Jr, Pepe P. E., Martin R. R., Ginger V. F., Allen M. K., Mattox K. L. 1994. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N. Engl. J. Med. 331, 1105–1109 10.1056/NEJM199410273311701 (doi:10.1056/NEJM199410273311701) [DOI] [PubMed] [Google Scholar]
- 33.2001. Battlefield advanced trauma life support (BATLS). J. R. Army Med. Corps 147, 187–194 [DOI] [PubMed] [Google Scholar]
- 34.Damon E. G., Yelverton J. T., Luft U. C., Mitchell K., Jones R. K. 1971. Acute effects of air blast on pulmonary function in dogs and sheep. Aerospace Med. 42, 1–9 [PubMed] [Google Scholar]
- 35.Guy R. J., Kirkman E., Watkins P. E., Cooper G. J. 1998. Physiologic responses to primary blast. J. Trauma 45, 983–987 10.1097/00005373-199812000-00001 (doi:10.1097/00005373-199812000-00001) [DOI] [PubMed] [Google Scholar]
- 36.Harban F. M. J., Kirkman E., Kenward C. E., Watkins P. E. 2001. Primary thoracic blast injury causes acute reduction in cardiac function in the anaesthetised pig. J. Physiol.-Lond. 533, 81P [Google Scholar]
- 37.Ohnishi M., Kirkman E., Guy R. J., Watkins P. E. 2001. Reflex nature of the cardiorespiratory response to primary thoracic blast injury in the anaesthetised rat. Exp. Physiol. 86, 357–364 10.1113/eph8602145 (doi:10.1113/eph8602145) [DOI] [PubMed] [Google Scholar]
- 38.Zunic G., Romic P., Vueljic M., Jovanikic O. 2005. Very early increase in nitric oxide formation and oxidative cell damage associated with the reduction of tissue oxygenation is a trait of blast casualties. Vojnosanit. Pregl. 62, 273–280 [DOI] [PubMed] [Google Scholar]
- 39.Garner J. P., Watts S., Parry C., Bird J., Kirkman E. 2009. Development of a large animal model for investigating resuscitation after blast and hemorrhage. World J. Surg. 33, 2194–2202 10.1007/s00268-009-0105-4 (doi:10.1007/s00268-009-0105-4) [DOI] [PubMed] [Google Scholar]
- 40.Brohi K., Cohen M. J., Ganter M. T., Matthay M. A., Mackersie R. C., Pittet J. F. 2007. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann. Surg. 245, 812–818 10.1097/01.sla.0000256862.79374.31 (doi:10.1097/01.sla.0000256862.79374.31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee C. C., Marill K. A., Carter W. A., Crupi R. S. 2001. A current concept of trauma-induced multiorgan failure. Ann. Emerg. Med. 38, 170–176 10.1067/mem.2001.114313 (doi:10.1067/mem.2001.114313) [DOI] [PubMed] [Google Scholar]
- 42.Kirkman E., Watts S., Garner J., Parry C., Bird J., Hesketh A. 2006. Strategies for the resuscitation of combined blast and haemorrhage casualties. Dstl/TR19030 Version 1.0. [Google Scholar]
- 43.Cole D. J., et al. 1999. A pilot study evaluating the efficacy of a fully acetylated poly-N-acetyl glucosamine membrane formulation as a topical hemostatic agent. Surgery 126, 510–517 [PubMed] [Google Scholar]
- 44.Bickell W. H., Bruttig S. P., Millnamow G. A., O'Benar J., Wade C. E. 1991. The detrimental effects of intravenous crystalloid after aortotomy in swine. Surgery 110, 529–536 [PubMed] [Google Scholar]
- 45.Dronen S. C., Stern S. A., Wang X., Stanley M. A. 1993. Comparison of the response of near-fatal acute hemorrhage models with and without a vascular injury to rapid volume expansion. Am. J. Emerg. Med. 11, 331–335 [DOI] [PubMed] [Google Scholar]
- 46.Sondeen J. L., Coppes V. G., Holcomb J. B. 2003. Blood pressure at which rebleeding occurs after resuscitation in swine with aortic injury. J. Trauma 54(Suppl. 5), S110–S117 [DOI] [PubMed] [Google Scholar]
- 47.Moore E. E., Cogbill T. H., Jurkovich G. J., Shackford S. R., Malangoni M. A., Champion H. R. 1995. Organ injury scaling—spleen and liver [1994 Revision]. J. Trauma 38, 323–324 10.1097/00005373-199503000-00001 (doi:10.1097/00005373-199503000-00001) [DOI] [PubMed] [Google Scholar]
- 48.Holcomb J. B., et al. 1999. Effect of dry fibrin sealant dressings versus gauze packing on blood loss in grade V liver injuries in resuscitated swine. J. Trauma 46, 49–57 10.1097/00005373-199901000-00009 (doi:10.1097/00005373-199901000-00009) [DOI] [PubMed] [Google Scholar]
- 49.Klemcke H. G., et al. 2005. Effect of recombinant FVIIa in hypothermic, coagulopathic pigs with liver injuries. J. Trauma 59, 155–161 10.1097/01.TA.0000174557.89804.A2 (doi:10.1097/01.TA.0000174557.89804.A2) [DOI] [PubMed] [Google Scholar]
- 50.Pusateri A. E., Delgado A. V., Dick E. J., Jr, Martinez R. S., Holcomb J. B., Ryan K. L. 2004. Application of a granular mineral-based hemostatic agent (QuikClot) to reduce blood loss after grade V liver injury in swine. J. Trauma 57, 555–562 10.1097/01.TA.0000136155.97758.CD (doi:10.1097/01.TA.0000136155.97758.CD) [DOI] [PubMed] [Google Scholar]
- 51.Martinowitz U., et al. 2001. Intravenous rFVIIa administered for hemorrhage control in hypothermic coagulopathic swine with grade V liver injuries. J. Trauma 50, 721–729 10.1097/00005373-200104000-00021 (doi:10.1097/00005373-200104000-00021) [DOI] [PubMed] [Google Scholar]
- 52.Jeroukhimov I., et al. 2002. Early injection of high-dose recombinant factor VIIa decreases blood loss and prolongs time from injury to death in experimental liver injury. J. Trauma 53, 1053–1057 10.1097/00005373-200212000-00004 (doi:10.1097/00005373-200212000-00004) [DOI] [PubMed] [Google Scholar]
- 53.Sawdon M., Ohnishi M., Watkins P. E., Kirkman E. 2002. The effects of primary thoracic blast injury and morphine on the response to haemorrhage in the anaesthetised rat. Exp. Physiol. 87, 683–689 10.1113/eph8702432 (doi:10.1113/eph8702432) [DOI] [PubMed] [Google Scholar]
- 54.Yang H., Tracey K. J. 2010. Targeting HMGB1 in inflammation. Biochim. Biophys. Acta 1799, 149–156 10.1016/j.bbagrm.2009.11.019 (doi:10.1016/j.bbagrm.2009.11.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen L., Lorand L. 1983. Contribution of fibrin stabilization to clot strength. Supplementation of factor XIII-deficient plasma with the purified zymogen. J. Clin. Invest. 71, 1336–1341 10.1172/JCI110885 (doi:10.1172/JCI110885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dubick M. A., Atkins J. L. 2003. Small-volume fluid resuscitation for the far-forward combat environment: current concepts. J. Trauma 54, S43–S45 [DOI] [PubMed] [Google Scholar]
- 57.Alam H. B., Sun L., Ruff P., Austin B., Burris D., Rhee P. 2000. E- and P-selectin expression depends on the resuscitation fluid used in hemorrhaged rats. J. Surg. Res. 94, 145–152 10.1006/jsre.2000.6011 (doi:10.1006/jsre.2000.6011) [DOI] [PubMed] [Google Scholar]
- 58.Bahrami S., Zimmermann K., Szelenyi Z., Hamar J., Scheiflinger F., Redl H., Junger W. G. 2006. Small-volume fluid resuscitation with hypertonic saline prevents inflammation but not mortality in a rat model of hemorrhagic shock. Shock 25, 283–289 10.1097/01.shk.0000208808.03148.ea (doi:10.1097/01.shk.0000208808.03148.ea) [DOI] [PubMed] [Google Scholar]
- 59.Bulger E. M., Cuschieri J., Warner K., Maier R. V. 2007. Hypertonic resuscitation modulates the inflammatory response in patients with traumatic hemorrhagic shock. Ann. Surg. 245, 635–641 10.1097/01.sla.0000251367.44890.ae (doi:10.1097/01.sla.0000251367.44890.ae) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rizoli S. B., Rhind S. G., Shek P. N., Inaba K., Filips D., Tien H., Brenneman F., Rotstein O. 2006. The immunomodulatory effects of hypertonic saline resuscitation in patients sustaining traumatic hemorrhagic shock: a randomized, controlled, double-blinded trial. Ann. Surg. 243, 47–57 10.1097/01.sla.0000193608.93127.b1 (doi:10.1097/01.sla.0000193608.93127.b1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White H., Cook D., Venkatesh B. 2008. The role of hypertonic saline in neurotrauma. Eur. J. Anaesthesiol. Suppl. 42, 104–109 [DOI] [PubMed] [Google Scholar]
- 62.Zakaria E. R., Tsakadze N. L., Garrison R. N. 2006. Hypertonic saline resuscitation improves intestinal microcirculation in a rat model of hemorrhagic shock. Surgery 140, 579–588 10.1016/j.surg.2006.05.015 (doi:10.1016/j.surg.2006.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leach R. M., Davidson A. C. 2009. Use of emergency oxygen in adults. Br. Med. J. 338, a2790. 10.1136/bmj.a2790 (doi:10.1136/bmj.a2790) [DOI] [PubMed] [Google Scholar]