Abstract
Lung injuries, predominantly arising from blast exposure, are a clinical problem in a significant minority of current military casualties. This special feature consists of a series of articles on lung injury. This first article examines the mechanism of the response to blast lung (primary blast injury to the lung). Subsequent articles examine the incidence of blast lung, clinical consequences and current concepts of treatment, computer (in silico) modelling of lung injury and finally chemical injuries to the lungs. Blast lung is caused by a shock wave generated by an explosion causing widespread damage in the lungs, leading to intrapulmonary haemorrhage. This, and the ensuing inflammatory response in the lung, leads to a compromise in pulmonary gas exchange and hypoxia that can worsen over several hours. There is also a characteristic cardio-respiratory effect mediated via an autonomic reflex causing apnoea (or rapid shallow breathing), bradycardia and hypotension (the latter possibly also due to the release of nitric oxide). An understanding of this response, and the way it modifies other reflexes, can help the development of new treatment strategies for this condition and for the way it influences the patient's response to concomitant injuries.
Keywords: explosion, blast lung, pulmonary contusion
1. Introduction
Explosive or blast injuries are a current and very significant clinical issue in military medicine, with a significant number of casualties being injured by improvised explosive devices in the current conflicts in Iraq and Afghanistan [1–3]. Unfortunately, blast injuries also impact on civilian medicine, most dramatically when terrorist bombings cause mass casualties [4–11]. Medical investigation and reporting of blast injury precedes the First World War, with a number of case reports dating back to 1768 (cited in [12]). Systematic descriptions are found in observations made on casualties during the First World War, with descriptions such as ‘men subjected to concussion of large shells often developed a condition of shock which was unrelated to obvious trauma since no external wounds were visible’ [13]. More detailed observations giving insight into potential autonomic mechanisms were made during the Second World War with descriptions of casualties displaying bradycardia and hypotension [14].
Observations on casualties will always play a central role in any investigation of a clinical problem, not least because they initiate the definition of the problem and guide a systematic scientific study. However, a detailed investigation of mechanisms often requires the development of models, ranging from physical and computer (in silico), through in vitro to complex in vivo studies. These are necessary for a variety of reasons, including the ability to conduct studies under controlled conditions to exclude confounding variables and to conduct detailed mechanistic studies that are too invasive to perform on human casualties. The loop is finally closed, usually, by further observations in casualties to confirm that mechanisms deduced from models truly represent the ‘real world’. Hence, the most powerful research is an integration of basic science and clinical studies often with iterative steps in laboratory and clinical settings.
This special feature contains a series of papers that examine lung injury, predominantly focusing on blast injury since this is currently a high-profile clinical issue. This paper discusses some of the models used to elucidate the mechanism of the response to thoracic blast. How concerned should we be about blast lung? The next paper by Smith [1] evaluates the most recent clinical data to determine the incidence of blast lung in current casualties. A further paper by Mackenzie & Tunnicliffe [15] describes the clinical consequences of blast exposure in casualties returning to the UK from Afghanistan and discusses current concepts in the management of these casualties. Perhaps the most rapid means of screening new treatment strategies is by using computer-based in silico models, provided the models are faithful representations of the relevant aspects of the real world, and this is the basis of the next paper by Harvey & Hardman [16]. Finally, lest we forget that blast lung is not the only threat faced by military and civilian casualties, the final paper by Jugg et al. [17] provides a brief examination of the consequences and models used to evaluate the treatment of chemical injuries of the lung.
2. Blast injuries
Blast injuries fall into four main categories [18,19]: primary, secondary and tertiary, with miscellaneous additional injuries forming a further (quaternary) group (table 1).
Table 1.
Classification of blast injuries.
| primary | the effects of the shock wave. The shock wave travels through the body tissues depositing energy (and hence causing damage) especially at gas/liquid interfaces. The lungs are among the organs most likely to suffer this form of injury, where it is called ‘blast lung’. |
| secondary | fragments and debris energized by the explosion collide with the body causing penetrating injuries. |
| tertiary | the body being thrown against obstacles by the mass movement of air (blast wind) causing blunt injuries. |
| quaternary | other injuries including burns and crush from collapsed buildings etc. |
In vivo models using shock waves generated by a range of devices including real explosions, shock tubes and compressed air shock wave ‘generators’ have been used extensively to characterize the response to blast lung injury.
3. The development of blast lung injury
Blast lung is a primary blast injury. The shock wave causes an immediate lung injury that is characterized by rupture of alveolar capillaries, the influx of blood and extravasation of oedema fluid into lung tissue [20,21], giving rise to haemorrhagic foci that can be substantial depending on the level of blast loading. The intrapulmonary haemorrhage and oedema contribute to the initial respiratory compromise in blast lung [22]. The problem is exacerbated because free haemoglobin (Hb) and extravasated blood have been shown to induce free radical reactions that cause oxidative damage [22] and initiate/augment a pro-inflammatory response [21]. Free Hb also causes an accumulation of inflammatory mediators and chemotactic attractants [23], thereby amplifying the problem.
Within 3 h leucocytes can be demonstrated within the haemorrhagic areas, and levels increase for 24 h or more after exposure [22]. This accumulation of leucocytes is associated with increasing levels of myeloperoxidase activity, which in turn is indicative of oxidative events and developing inflammation in the affected areas [22]. Histological and electron microscopic examination reveal prominent perivascular oedema and extensive alveolar haemorrhages without widespread visible damage to endothelial cells during the first 12 h after exposure [22]. Thereafter (12–24 h after exposure), type 1 epithelial cells show evidence of developing damage followed later (24–56 h after exposure) by secondary damage to endothelial cells which become detached from their basement membrane into the capillary lumen [22]. This process is summarized in table 2.
Table 2.
Evolution of blast lung.
| event | apparent clinical problem | time (h) |
|---|---|---|
| shock wave damage | 0 | |
| rupture of alveolar capillaries | ||
| blood into interstitium and alveoli | reduced gas transfer (esp O2) | |
| free Hb and blood | inflammation | |
| free radicals/oxidative stress | ||
| augmented inflammatory response | ||
| more oedema | reduced gas transfer (esp O2) | |
| leucocyte accumulation | more inflammation and reduced gas transfer | 3 |
| more oxidative stress, inflammation and oedema | ||
| epithelial cell damage evident | further impairment | 12–24 |
| endothelial cell damage evident | lung mechanics | 24–56 |
Recent studies examining novel pharmacological means of attenuating the development of blast lung have shown considerable promise. Initial demonstration that resolution of the inflammatory component of blast lung coincided with engagement of adaptive antioxidant and anti-inflammatory mechanisms [24] led to studies using these mechanisms as targets for therapy. Activation of haemoxygenase-1 using haemin was reported to increase survival in rats with blast lung, possibly via an anti-inflammatory mechanism [25], while administration of the antioxidant N-acetylcysteine amide was found to attenuate the development of blast lung and the associated pulmonary inflammatory response [26].
4. Physiological response to primary blast injury
Primary blast injury results in a characteristic cardiorespiratory response that is mediated in large part by the autonomic nervous system. However, it must also be recognized that other mechanisms such as the release of mediators (e.g. nitric oxide (NO)) into the circulation may also play a significant role in the acute response to blast injury.
(a). Cardiorespiratory response to primary blast injury to the thorax
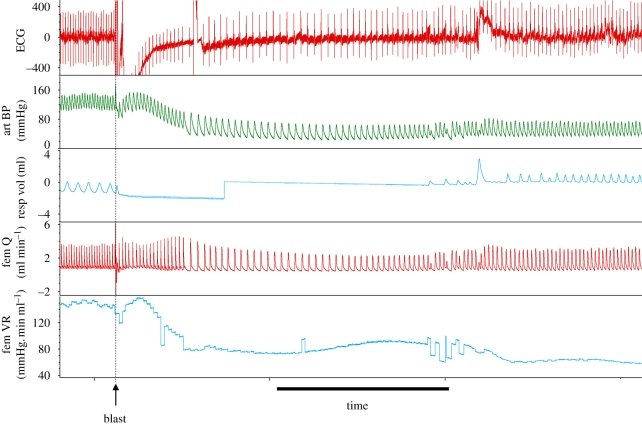
A number of experimental studies and clinical reports have indicated that primary blast injury to the thorax produces bradycardia [14,27–32], prolonged hypotension [14,27,29,30,32] and apnoea followed by rapid shallow breathing [28,29,31,32] (figure 1). This response is thought to be an autonomic reflex.
Figure 1.
Typical effects of a single blast exposure to the thorax of an anaesthetized male Wistar rat on the electrocardiogram (ECG), systemic arterial blood pressure (art BP), respiratory tidal volume (resp vol, inspiration upwards), femoral arterial blood flow (fem Q) and femoral vascular resistance (fem VR). A single blast wave was applied to the ventral thorax at the point indicated (blast). Scale bar, 10 s.
A detailed study of the immediate response to primary blast injury to the thorax has shown that the cardiovascular and respiratory responses are not instantaneous; the bradycardia had a latency of onset of approximately 4 s, while blood pressure began to fall approximately 2 s after blast [33]. This latency is consistent with the response being reflex in nature rather than being the consequence of direct effects, e.g. on the heart or central nervous system. More recent studies have shown that the response also includes a reduction in vascular resistance, at least in the skeletal muscle (figure 1).
The bradycardia and apnoea seen after blast are both mediated by a vagal reflex [33–36]. The aetiology of the hypotension seen after primary blast injury is complex. The fall in blood pressure appears to be due to a fall in peripheral resistance and cardiac output, the latter because of a myocardial impairment that can last many hours after blast injury [37]. Although the autonomic nervous system plays some part in the hypotension, it is not solely responsible. Recent findings have suggested that primary blast injury causes a rapid release of the potent vasodilator NO from the pulmonary circulation [38–40]. It is thought that such a brisk overproduction of NO could lead to a systemic response that includes vasodilatation ([41]; J. L. Atkins 2008, WRAIR, personal communication).
In summary, blast lung is a progressive condition characterized by the development of pulmonary inflammation and oedema following initial intrapulmonary haemorrhage as a consequence of damage by the blast shock wave. The combined influence of pulmonary haemorrhage and oedema is to reduce pulmonary gas transfer and lead initially to hypoxia and, with worsening blast lung, hypercarbia. Thoracic, but not abdominal [32], blast produces a triad of bradycardia, hypotension and apnoea. The bradycardia and apnoea are mediated entirely by a vagal reflex, the most likely candidate being the pulmonary afferent C-fibre reflex. The effects of the hypoxia and altered cardiovascular reflexes can have profound effects on the ability of the casualty to respond to concomitant or further events such as haemorrhage and resuscitation [42].
Acknowledgements
© Crown copyright 2011. Published with the permission of the Defence Science and Technology Laboratory on behalf of the Controller of HMSO.
Footnotes
One contribution of 20 to a Theme Issue ‘Military medicine in the 21st century: pushing the boundaries of combat casualty care’.
References
- 1.Smith J. E. 2011. The epidemiology of blast lung injury during recent military conflicts: a retrospective database review of cases presenting to deployed military hospitals, 2003–2009. Phil. Trans. R. Soc. B 366, 291–294 10.1098/rstb.2010.0251 (doi:10.1098/rstb.2010.0251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Champion H. R., Holcomb J. B., Young L. A. 2009. Injuries from explosions: physics, biophysics, pathology, and required research focus. J. Trauma 66, 1468–1477 10.1097/TA.0b013e3181a27e7f (doi:10.1097/TA.0b013e3181a27e7f) [DOI] [PubMed] [Google Scholar]
- 3.Ritenour A. E., Blackbourne L. H., Kelly J. F., Mclaughlin D. F., Pearse L. A., Holcomb J. B., Wade C. E. 2010. Incidence of primary blast injury in US military overseas contingency operations: a retrospective study. Ann. Surg. 251, 1140–1144 10.1097/SLA.0b013e3181e01270 (doi:10.1097/SLA.0b013e3181e01270) [DOI] [PubMed] [Google Scholar]
- 4.Ad-El D. D., Eldad A., Mintz Y., Berlatzky Y., Elami A., Rivkind A. I., Almogy G., Tzur T. 2006. Suicide bombing injuries: the Jerusalem experience of exceptional tissue damage posing a new challenge for the reconstructive surgeon. Plast. Reconstr. Surg. 118, 383–387 10.1097/01.prs.0000227736.91811.c7 (doi:10.1097/01.prs.0000227736.91811.c7) [DOI] [PubMed] [Google Scholar]
- 5.Avidan V., Hersch M., Armon Y., Spira R., Aharoni D., Reissman P., Schecter W. P. 2005. Blast lung injury: clinical manifestations, treatment, and outcome. Am. J. Surg. 190, 927–931 10.1016/j.amisurg.2005.08.022 (doi:10.1016/j.amisurg.2005.08.022) [DOI] [PubMed] [Google Scholar]
- 6.De Ceballos J. P., Turegano-Fuentes F., Perez-Diaz D., Sanz-Sanchez M., Martin-Llorente C., Guerrero-Sanz J. E. 2005. 11 March 2004: the terrorist bomb explosions in Madrid, Spain—an analysis of the logistics, injuries sustained and clinical management of casualties treated at the closest hospital. Crit. Care 9, 104–111 10.1186/cc2995 (doi:10.1186/cc2995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holden P. J. 2005. The London attacks–a chronicle: improvising in an emergency. N. Engl. J. Med. 353, 541–543 10.1056/NEJMp058179 (doi:10.1056/NEJMp058179) [DOI] [PubMed] [Google Scholar]
- 8.Jaffe D. H., Peleg K. 2010. Terror explosive injuries: a comparison of children, adolescents, and adults. Ann. Surg. 251, 138–143 10.1097/SLA.0b013e3181b5d7ab (doi:10.1097/SLA.0b013e3181b5d7ab) [DOI] [PubMed] [Google Scholar]
- 9.Mayo A., Kluger Y. 2006. Terrorist bombing. World J. Emerg. Surg. 1, 33. 10.1186/1749-7922-1-33 (doi:10.1186/1749-7922-1-33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pizov R., Oppenheim A., Matot I., Weiss Y. G., Eidelman L. A., Rivkind A., Sprung C. L. 1997. Lung blast injury following a bomb explosion on a civilian bus. Anesthesiology 87, A236. 10.1097/00000542-199709001-00236 (doi:10.1097/00000542-199709001-00236) [DOI] [Google Scholar]
- 11.Ryan J., Montgomery H. 2005. The London attacks–preparedness: terrorism and the medical response. N. Engl. J Med. 353, 543–545 10.1056/NEJMp058177 (doi:10.1056/NEJMp058177) [DOI] [PubMed] [Google Scholar]
- 12.Clemedson C. J. 1949. An experimental study on air blast. Acta Physiol. Scand. 18(Suppl. LXI), 7–200 [Google Scholar]
- 13.Hooker D. R. 1924. Physiological effects of air concussion. Am. J. Physiol. 67, 219 [Google Scholar]
- 14.Barrow D. W., Rhoads H. Y. 1944. Blast concussion injury. JAMA 125, 900–902 [Google Scholar]
- 15.Mackenzie I. M. J., Tunnicliffe B. 2011. Blast injuries to the lung: epidemiology and management. Phil. Trans. R. Soc. B 366, 295–299 10.1098/rstb.2010.0252 (doi:10.1098/rstb.2010.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey D. J. R., Hardman J. G. 2011. Computational modelling of lung injury: is there potential for benefit? Phil. Trans. R. Soc. B 366, 300–305 10.1098/rstb.2010.0250 (doi:10.1098/rstb.2010.0250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jugg B. J. A., Smith A. J., Rudall S. J., Rice P. 2011. The injured lung: clinical issues and experimental models. Phil. Trans. R. Soc. B 366, 306–309 10.1098/rstb.2010.0235 (doi:10.1098/rstb.2010.0235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maynard R. L., Cooper G. J., Scott R. 1989. Mechanism of injury in bomb blasts and explosions. In Trauma (ed. Westaby S.), London, UK: Heinemann [Google Scholar]
- 19.Zuckerman S. 1941. Discussion on the problem of blast injuries. Proc. R. Soc. Med. 34, 171–188 [PMC free article] [PubMed] [Google Scholar]
- 20.Brown R. F., Cooper G. J., Maynard R. L. 1993. The ultrastructure of rat lung following acute primary blast injury. Int. J. Exp. Pathol. 74, 151–162 [PMC free article] [PubMed] [Google Scholar]
- 21.Gorbunov N. V., Elsayed N. M., Kisin E. R., Kozlov A. V., Kagan V. E. 1997. Air blast-induced pulmonary oxidative stress: interplay among hemoglobin, antioxidants, and lipid peroxidation. Am. J. Physiol 272, L320–L334 [DOI] [PubMed] [Google Scholar]
- 22.Gorbunov N. V., Asher L. V., Ayyagari V., Atkins J. L. 2006. Inflammatory leukocytes and iron turnover in experimental hemorrhagic lung trauma. Exp. Mol. Pathol. 80, 11–25 10.1016/j.yexmp.2005.06.007 (doi:10.1016/j.yexmp.2005.06.007) [DOI] [PubMed] [Google Scholar]
- 23.Gorbunov N. V., Mcfaul S. J., Januszkiewicz A., Atkins J. L. 2005. Pro-inflammatory alterations and status of blood plasma iron in a model of blast-induced lung trauma. Int. J. Immunopathol. Pharmacol. 18, 547–556 [DOI] [PubMed] [Google Scholar]
- 24.Chavko M., Prusaczyk W. K., Mccarron R. M. 2006. Lung injury and recovery after exposure to blast overpressure. J. Trauma 61, 933–942 10.1097/01.ta.0000233742.75450.47 (doi:10.1097/01.ta.0000233742.75450.47) [DOI] [PubMed] [Google Scholar]
- 25.Chavko M., Prusaczyk W. K., Mccarron R. M. 2008. Protection against blast-induced mortality in rats by hemin. J. Trauma 65, 1140–1145 10.1097/TA.0b013e3181870a8c (doi:10.1097/TA.0b013e3181870a8c) [DOI] [PubMed] [Google Scholar]
- 26.Chavko M., Adeeb S., Ahlers S. T., Mccarron R. M. 2009. Attenuation of pulmonary inflammation after exposure to blast overpressure by N-acetylcysteine amide. Shock 32, 325–331 10.1097/SHK.0b013e31819c38f1 (doi:10.1097/SHK.0b013e31819c38f1) [DOI] [PubMed] [Google Scholar]
- 27.Cernak I., Savic J., Malicevic Z., Zunic G., Radosevic P., Ivanovic I., Davidovic L. 1996. Involvement of the central nervous system in the general response to pulmonary blast injury. J. Trauma 40(Suppl. 3), S100–S104 10.1097/00005373-199603001-00023 (doi:10.1097/00005373-199603001-00023) [DOI] [PubMed] [Google Scholar]
- 28.Clark S. L., Ward J. W. 1943. The effects of rapid compression waves on animals submerged in water. Surgical Gynaecology and Obstetrics 77, 403–412 [Google Scholar]
- 29.Clemedson C. J. 1949. An experimental study of air blast injuries. Acta Physiol. Scand. 18, 1–20018152937 [Google Scholar]
- 30.Irwin R. J., Lerner M. R., Bealer J. F., Brackett D. J., Tuggle D. W. 1997. Cardiopulmonary physiology of primary blast injury. J. Trauma 43, 650–655 10.1097/00005373-199710000-00015 (doi:10.1097/00005373-199710000-00015) [DOI] [PubMed] [Google Scholar]
- 31.Jaffin J. H., et al. 1987. A laboratory model for studying blast overpressure injury. J. Trauma 27, 349–356 10.1097/00005373-198704000-00002 (doi:10.1097/00005373-198704000-00002) [DOI] [PubMed] [Google Scholar]
- 32.Guy R. J., Kirkman E., Watkins P. E., Cooper G. J. 1998. Physiologic responses to primary blast. J. Trauma Injury Infect. Crit. Care 45, 983–987 10.1097/00005373-199812000-00001 (doi:10.1097/00005373-199812000-00001) [DOI] [PubMed] [Google Scholar]
- 33.Ohnishi M., Kirkman E., Guy R. J., Watkins P. E. 2001. Reflex nature of the cardiorespiratory response to primary thoracic blast injury in the anaesthetised rat. Exp. Physiol. 86, 357–364 10.1113/eph8602145 (doi:10.1113/eph8602145) [DOI] [PubMed] [Google Scholar]
- 34.Irwin R. J., Lerner M. R., Bealer J. F., Mantor P. C., Brackett D. J., Tuggle D. W. 1999. Shock after blast wave injury is caused by a vagally mediated reflex. J. Trauma 47, 105–110 10.1097/00005373-199907000-00023 (doi:10.1097/00005373-199907000-00023) [DOI] [PubMed] [Google Scholar]
- 35.Krohn P. L., Whitteridge D., Zuckerman S. 1942. Physiological effects of blast. Lancet I, 252–258 10.1016/S0140-6736(00)57842-8 (doi:10.1016/S0140-6736(00)57842-8) [DOI] [Google Scholar]
- 36.Ohnishi M., Kirkman E., Watkins P. 1998. Effects of atropine on the bradycardia associated with primary thoracic blast injury in the anaesthetized rat. Br. J. Pharmacol. 123, U60 [Google Scholar]
- 37.Harban F. M. J., Kirkman E., Kenward C. E., Watkins P. E. 2001. Primary thoracic blast injury causes acute reduction in cardiac function in the anaesthetised pig. J. Physiol. Lond. 533, P81 [Google Scholar]
- 38.Gorbunov N. V., Das D. K., Goswami S. K., Gurusamy N., Atkins J. L. 2006. Nitric oxide (NO), redox signalling, and pulmonary inflammation in a model of polytrauma. Proc. XIII Congr. Soc. Free Radical Research International, Davos, Switzerland, 15–19 August 2006, pp. 2–4 [Google Scholar]
- 39.Zunic G., Pavlovic R., Malicevic Z., Savic V., Cernak I. 2000. Pulmonary blast injury increases nitric oxide production, disturbs arginine metabolism, and alters the plasma free amino acid pool in rabbits during the early posttraumatic period. Nitric Oxide 4, 123–128 10.1006/niox.2000.0276 (doi:10.1006/niox.2000.0276) [DOI] [PubMed] [Google Scholar]
- 40.Zunic G., Romic P., Vueljic M., Jovanikic O. 2005. Very early increase in nitric oxide formation and oxidative cell damage associated with the reduction of tissue oxygenation is a trait of blast casualties. Vojnosanit. Pregl. 62, 273–280 [DOI] [PubMed] [Google Scholar]
- 41.Kirkman E., Watts S., Sapsford W., Sawdon M. 2008. Effects of blast injury on the autonomic nervous system and the response to resuscitation. In Explosion and blast-related injuries (eds Elsayed N. M., Atkins J. L.), pp. 105–142 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 42.Kirkman E., Watts S., Cooper G. 2011. Blast injury research models. Phil. Trans. R. Soc. B 366, 144–159 10.1098/rstb.2010.0240 (doi:10.1098/rstb.2010.0240) [DOI] [PMC free article] [PubMed] [Google Scholar]