Abstract
Better prognostic predictors for invasive candidiasis (IC) are needed to tailor and individualize therapeutic decision-making and minimize its high morbidity and mortality. We investigated whether molecular profiling of IgG-antibody response to the whole soluble Candida proteome could reveal a prognostic signature that may serve to devise a clinical-outcome prediction model for IC and contribute to known IC prognostic factors. By serological proteome analysis and data-mining procedures, serum 31-IgG antibody-reactivity patterns were examined in 45 IC patients randomly split into training and test sets. Within the training cohort, unsupervised two-way hierarchical clustering and principal-component analyses segregated IC patients into two antibody-reactivity subgroups with distinct prognoses that were unbiased by traditional IC prognostic factors and other patients-related variables. Supervised discriminant analysis with leave-one-out cross-validation identified a five-IgG antibody-reactivity signature as the most simplified and accurate IC clinical-outcome predictor, from which an IC prognosis score (ICPS) was derived. Its robustness was confirmed in the test set. Multivariate logistic-regression and receiver-operating-characteristic curve analyses demonstrated that the ICPS was able to accurately discriminate IC patients at high risk for death from those at low risk and outperformed conventional IC prognostic factors. Further validation of the five-IgG antibody-reactivity signature on a multiplexed immunoassay supported the serological proteome analysis results. The five IgG antibodies incorporated in the ICPS made biologic sense and were associated either with good-prognosis and protective patterns (those to Met6p, Hsp90p, and Pgk1p, putative Candida virulence factors and antiapoptotic mediators) or with poor-prognosis and risk patterns (those to Ssb1p and Gap1p/Tdh3p, potential Candida proapoptotic mediators). We conclude that the ICPS, with additional refinement in future larger prospective cohorts, could be applicable to reliably predict patient clinical-outcome for individualized therapy of IC. Our data further provide insights into molecular mechanisms that may influence clinical outcome in IC and uncover potential targets for vaccine design and immunotherapy against IC.
Despite recent advances in antifungal therapy, invasive candidiasis (IC)1 remains a leading infectious cause of morbidity and mortality in cancer, postsurgical, and intensive care patients (1–3). Its significant impact on patient clinical outcome, as reflected in its increased attributable mortality (10%–49%), length of hospital stay (3–30 days per patient), and healthcare costs (US $ 6214–92,266 per episode), could however be ameliorated if early and appropriate antifungal therapeutic strategies were administered (1, 4). This precondition highlights the need to search for prognostic features that may reliably predict the clinical outcome in IC patients at presentation to tailor and individualize therapeutic decision-making accordingly and, as a result, to minimize the burden of the invasive infections caused by Candida spp. (commonly Candida albicans (1)).
Several factors have classically been reported to adversely influence the clinical outcome of IC patients (3, 5–7). Nonetheless, the prognostic potential of some of these traditional factors for IC is controversial (8, 9) and overall these have a limited prognostic power. For this reason, alternative laboratory tests based on measurement of Candida d-arabinitol/creatinine ratio, Candida antigen titer, or anti-Candida antibody levels (10–15) have been developed to explore their prognostic usefulness in IC. However, none of them has yet been validated for routine clinical practice. Furthermore, these few biomarkers may lack sensitivity for individual prediction of clinical outcomes in the first stages of infection and/or are not yet sufficiently accurate to attain widespread clinical use. In the light of these limitations, and considering the heterogeneity and intricacy of the host responses and molecular mechanisms underlying IC pathogenesis, it is likely that optimally combined multiple biomarkers may cover a broader range of IC patients and pathogenicity-related issues and more reliably predict IC prognosis in an early stage.
Serological proteome analysis (SERPA) may be a promising tool in this context because this global profiling technique enables the simultaneous assessment of reactivities of antibodies to a large panel of immunogenic proteins (i.e. the immunome of a (micro)organism (16)) in one experimental approach (17–21). This strategy has widely been applied to antibody-reactivity profiling for diagnostic and therapeutic purposes in cancers, autoimmune disorders, allergies, and infectious diseases (including IC (13, 15, 22, 23)) (18, 24–30). Despite that attractive clinical value, little is known, however, about the potential of this immunoproteomic method to identify antibody-reactivity patterns or signatures (18, 31) that may have utility in predicting the prognosis of individual patients with these pathologies. These prognostic signatures might further offer insights into IC pathogenesis and uncover potential targets for molecular therapies against IC. This approach could also profit from bioinformatics to search for hidden trends within generated multidimensional data and derive useful new knowledge (models, algorithms or rules) (32, 33).
Here, we examined the reactivity profiles of serum antibodies to the whole soluble Candida immunome at an early stage of IC by using SERPA and data-mining procedures in order to determine whether these could be indicative of distinct clinical outcomes in IC patients at presentation. We investigated whether these patterns could further reveal a prognostic signature that may serve to create a robust and consistent molecular predictor of clinical outcome for IC applicable to clinical practice and contribute to the traditional prognostic factors for IC. We then developed a multiplexed immunoassay to simultaneously and rapidly measure this simplified molecular fingerprint in each serum specimen and evaluate whether this could be a useful method for individual prediction of clinical outcomes in IC. We also explored whether this prognostic signature could yield biologic insights into molecular mechanisms that confer protection against IC and provide potential molecular targets for the design of novel vaccine- and/or immunotherapy-based strategies to prevent and control IC.
EXPERIMENTAL PROCEDURES
Study Population and Serum Specimens
Serum specimens from 48 adult IC patients belonging to different risk groups were obtained on the day of culture sampling at the Salamanca Clinic Hospital (Spain), a 750-bed tertiary care university-affiliated hospital, between December 1997 and March 2003. All patients were enrolled according to protocols approved by the Ethics Committee of Clinical Research from Salamanca Clinic Hospital, after informed consent was obtained. Patients were defined as having IC if they had clinical signs of infection or sepsis, and the same Candida species in one or more blood cultures and/or in cultures from at least three noncontiguous sites with an inadequate response to broad-spectrum antibiotics. Blood was cultured by the Bactec 9000 method (Becton Dickinson, Baltimore, MD), and specimens from other anatomical sites were plated on Sabouraud glucose agar. Yeast species were identified with the germ-tube formation test and the API-20C AUX system (BioMérieux, France). Three patients who had received antifungal drug prophylaxis before diagnosing IC were excluded from the study to ensure (i) the proper identification of potential prognostic biomarkers as these indicate the likely course of the disease in an “untreated” patient once the disease status has been established (15, 34, 35), and (ii) no overestimation or underestimation of the accuracy of our prediction model in patients who were responsive or nonresponsive, respectively, to the administered antifungal therapy given correlation between anti-Candida antibody levels and antifungal treatment efficacy (22, 36). The 45 specimens used in the present report were taken from an earlier retrospective case-control study (13). The outcome of hospital stay, death (n = 12) or discharge (n = 33), was recorded for each patient within 2 months. Details of baseline characteristics of the patients recruited in this retrospective study were described previously (13) and are shown in Table I on the basis of their clinical outcome.
Table I. Base-line characteristics of the study population in the training, test and entire-data sets according to the clinical outcome of IC patients within two months after presentation.
| Characteristics | Number (%) of IC patients |
|||||
|---|---|---|---|---|---|---|
| Training set (n = 22) |
Test (validation) set (n = 23) |
Entire-data set (n = 45) |
||||
| Good prognosis (n = 16) | Poor prognosis (n = 6)a,b | Good prognosis (n = 17) | Poor prognosis (n = 6)a,b | Good prognosis (n = 33) | Poor prognosis (n = 12)b | |
| Demographic factors | ||||||
| Sex | ||||||
| Male | 7 (43.8) | 5 (83.3) | 12 (70.6) | 4 (66.7) | 19 (57.6) | 9 (75.0) |
| Female | 9 (56.3) | 1 (16.7) | 5 (29.4) | 2 (33.3) | 14 (42.4) | 3 (25.0) |
| Age (years) | ||||||
| ≤49 years | 3 (18.8) | 0 (0.0) | 5 (29.4) | 3 (50.0) | 8 (24.2) | 3 (25.0) |
| 50–69 years | 6 (37.5) | 4 (66.7) | 10 (58.8) | 2 (33.3) | 16 (48.5) | 6 (50.0) |
| ≥70 years | 7 (43.8) | 2 (33.3) | 2 (11.8) | 1 (16.7) | 9 (27.3) | 3 (25.0) |
| Comorbiditiesc | ||||||
| Malignancies | ||||||
| Hematological malignancyd | 6 (37.5) | 1 (16.7) | 7 (41.2) | 2 (33.3) | 13 (39.4) | 3 (25.0) |
| Solid tumore | 5 (31.3) | 3 (50.0) | 2 (11.8) | 1 (16.7) | 7 (21.2) | 4 (33.3) |
| Nonmalignant diseases | ||||||
| Respiratory dysfunctionf | 2 (12.5) | 1 (16.7) | 3 (17.6) | 2 (33.3) | 5 (15.2) | 3 (25.0) |
| Gastrointestinal pathologyg | 1 (6.3) | 1 (16.7) | 4 (23.5) | 0 (0.0) | 5 (15.2) | 1 (8.3) |
| Othersh | 2 (12.5) | 0 (0.0) | 1 (5.9) | 1 (16.7) | 3 (9.1) | 1 (8.3) |
| Risk factors for mortality in ICi | ||||||
| Older age (>65 years) | 9 (56.3) | 3 (50.0) | 7 (41.2) | 2 (33.3) | 16 (48.5) | 5 (41.7) |
| Leukocytosisj | 4 (25.0) | 3 (50.0) | 6 (35.3) | 3 (50.0) | 10 (30.3) | 6 (50.0) |
| Presence or persistence of neutropeniak | 4 (25.0) | 2 (33.3) | 3 (17.6) | 1 (16.7) | 7 (21.2) | 3 (25.0) |
| Underlying malignant condition | 11 (68.8) | 4 (66.7) | 9 (52.9) | 3 (50.0) | 20 (60.6) | 7 (58.3) |
| Immunosuppressive therapyl | 5 (31.3) | 4 (66.7) | 3 (17.6) | 4 (66.7)m | 8 (24.2) | 8 (66.7)n |
| Recent major surgeryo | 3 (18.8) | 3 (50.0) | 5 (29.4) | 2 (33.3) | 8 (24.2) | 5 (41.7) |
| Hematopoietic transplantation | 2 (12.5) | 1 (16.7) | 1 (5.9) | 1 (16.7) | 3 (9.1) | 2 (16.7) |
| Central venous catheters | 6 (37.5) | 2 (33.3) | 5 (29.4) | 2 (33.3) | 11 (33.3) | 4 (33.3) |
| Intensive care unit stay | 2 (12.5) | 3 (50.0) | 4 (23.5) | 4 (66.7) | 6 (18.2) | 7 (58.3)n |
| Multiple trauma | 0 (0.0) | 0 (0.0) | 1 (5.9) | 1 (16.7) | 1 (3.0) | 1 (8.3) |
| Diabetes mellitusp | 2 (12.5) | 1 (16.7) | 1 (5.9) | 1 (16.7) | 3 (9.1) | 2 (16.7) |
| Adult respiratory distress syndrome (ARDS)p | 2 (12.5) | 2 (33.3) | 2 (11.8) | 2 (33.3) | 4 (12.1) | 4 (33.3) |
| Acute renal failure | 1 (6.3) | 1 (16.7) | 1 (5.9) | 1 (16.7) | 2 (6.1) | 2 (16.7) |
| Sepsis or septic shock at presentation | 1 (6.3) | 2 (33.3) | 3 (17.6) | 1 (16.7) | 4 (12.1) | 3 (25.0) |
| Recent use of broad-spectrum antibiotics | 8 (50.0) | 4 (66.7) | 11 (64.7) | 6 (100.0) | 19 (57.6) | 10 (83.3) |
| Pneumonia or other concomitant bacterial infection | 4 (25.0) | 2 (33.3) | 5 (29.4) | 3 (50.0) | 9 (27.3) | 5 (41.7) |
| Causative Candida species | ||||||
| C. albicans | 14 (87.5) | 4 (66.7) | 9 (52.9)q | 4 (66.7) | 23 (69.7) | 8 (66.7) |
| Non-albicans Candida spp. | 2 (12.5) | 2 (33.3) | 8 (47.1)q | 2 (33.3) | 10 (30.3) | 4 (33.3) |
| Inappropriate initial antifungal treatment | 2 (12.5) | 1 (16.7) | 1 (5.9) | 1 (16.7) | 3 (9.1) | 2 (16.7) |
| Hospital wards | ||||||
| Intensive care unit | 2 (12.5) | 3 (50.0) | 4 (23.5) | 4 (66.7) | 6 (18.2) | 7 (58.3)n |
| Hematology-oncology unit | 5 (31.3) | 1 (16.7) | 7 (41.2) | 1 (16.7) | 12 (36.4) | 2 (16.7) |
| General medicine wards | 9 (56.3) | 2 (33.3) | 6 (35.3) | 1 (16.7) | 15 (45.5) | 3 (25.0) |
a Training and test sets were balanced for mortality rate (see Experimental Procedures for further details).
b IC patients who died within two months of Candida isolation.
c Only the primary condition is shown.
d Includes the following diseases: leukemia, lymphoma, myelodysplasia, and multiple myeloma.
e Includes the following diseases: bronchopulmonary neoplasm, pancreas/colon adenocarcinomas, and bladder neoplasm.
f Includes the following diseases: pneumonia, chronic obstructive pulmonary disease, and adult respiratory distress syndrome.
g Includes the following diseases: cholecystitis, angiocholitis, pancreatitis, peritonitis, and hepatitis.
h Include the following diseases: multiple trauma, acute renal insufficiency, and diabetes mellitus.
j Leukocytosis was defined as a white blood cell count above 11,000 cells/mm3.
k Neutropenia was defined as an absolute neutrophil count below 500 cells/mm3.
l Includes systemic corticosteroids (≥7.5 mg prednisone per day or equivalent), immunosuppressive or cytotoxic drugs and/or total-body irradiation. All patients with systemic corticosteroid therapy received 1–3 mg prednisone per kilogram per day for >15 days.
m p < 0.05 for the comparison with the good-prognosis test group.
n p < 0.05 for the comparison with the good-prognosis entire-data group.
o Includes colectomy, duodenotomy, cholecystectomy, pancreatectomy, pulmonary lobotomy, and thoracotomy.
p Diabetes mellitus and ARDS were not the primary condition in most IC patients.
q A slight, but not statistically significant, difference (p = 0.06) was observed for the comparison with the good-prognosis training group.
Collection, processing and storage of serum specimens were uniform to avoid any systematic bias, and performed according to standard procedures. Briefly, blood samples were obtained by venepuncture using BD Vacutainer safety-lok blood collection set (Becton Dickinson) and collected in 10-ml, BD Vacutainer tubes without additives (Becton Dickinson). They were allowed to clot at room temperature for 30 min and centrifuged at 3500 rpm for 10 min at room temperature. The upper phase (serum) of each specimen was then transferred to four 1.5-ml tubes, which were labeled with a code (to protect patient confidentiality) and kept at −80 °C. Serum samples were submitted to the Department of Microbiology II (Complutense University of Madrid, Spain) in dry ice. Upon arrival, one tube of each sample was thawed on ice and divided into smaller aliquots using tubes for one-time use. These were labeled with the same code and stored at −80 °C. Serum aliquots from IC patients were thawed on ice just before analyses and assessed in a blinded fashion within 2 months following sample collection for the SERPA assay or at the present for the multiplexed immunoassay. We followed the proposed guidelines for reporting studies on clinical proteomics (37) and diagnostic accuracy (38).
SERPA Assay
Preparation of Whole Soluble C. albicans Protein Extracts
Whole soluble protein extracts of a clinical C. albicans isolate (strain SC5314) were used as an antigen source and prepared as reported (39, 40). Briefly, yeast cells were grown in YPD medium (1% Difco yeast extract, 2% peptone, and 2% glucose) at 30 °C up to an A600 nm of 1.0, and washed with water. Cells were resuspended in cold lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm dithiotreitol, 0.5 mm phenylmethylsulfonyl fluoride, and 5 μg/ml each of leupeptin, pepstatin, and antipain (Sigma)) and lysed mechanically with an equal volume of glass beads in a fast-prep cell breaker (Q-Biogene, Carlsbad, CA). The clarified supernatant was stored at −80 °C. Protein concentration was measured with the Bradford assay (Bio-Rad, Hercules, CA).
Two-Dimensional Polyacrylamide Gel Electrophoresis (2-DE)
Whole soluble C. albicans protein extracts were separated by 2-DE as described elsewhere (22, 41), using immobilized, nonlinear pH 3–10 gradient strips (18 cm; GE Healthcare, Buckinghamshire, UK) for isoelectric focusing, and 10% SDS-polyacrylamide gels (10% T; 1.6% C) for the second-dimension separation. The 2-DE-separated proteins were then either visualized with silver nitrate (42, 43) or colloidal Coomassie Brilliant Blue (40, 44) or electrotransferred to nitrocellulose membranes (40).
Two-Dimensional Western Blot Analysis
Two-dimensional Western blot assay was carried out essentially as reported (13, 22, 40). Serum anti-Candida IgG antibody levels were indirectly measured in each screened sample by densitometric analysis of the corresponding Western-blotting results using the ImageMaster 2D Platinum software (GE Healthcare) and expressed as arbitrary units relative to the integrated optical density of their related spot area following background subtraction and normalization analyses as described previously (13, 40).
Mass Spectrometry (MS) Analysis
Protein spots of interest that were not identified using our reference two-dimensional map of C. albicans immunogenic proteins (22) (also available on our COMPLUYEAST-2DPAGE database at http://www.expasy.ch/ch2d/2d-index.html (45, 46)) were manually excised from silver or colloidal Coomassie-stained preparative 2-DE gels, and in-gel destained, reduced, alkylated, and digested with trypsin (42, 47). The resulting tryptic digests were analyzed on a matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometer (Voyager-DE STR, PerSeptive Biosystems, Framingham, MA). All MS spectra were calibrated externally with the Sequazyme peptide mass standard kit (PerSeptive Biosystems, Framingham, MA) and internally using trypsin auto-digestion products.
Raw data were processed using the Data Explorer software v.4.5 (Applied Biosystems, Framingham, MA) prior to searching with the Mascot search engine. The parameters used to analyze the data were: signal-to-noise threshold of 20, a minimum area of 100, a resolution of over 10,000, and exclusion of all known contaminant ions (including keratins). Protein identification from processed spectrum data was performed on a local Mascot server v.1.9 using CandidaDB (2003.05.22; 6165 sequences; 2952183 residues) and Candida Genome Database (CGD) (2007.01.31; 6107 sequences; 2981547 residues) under the following search parameters: enzyme, trypsin; fixed modifications, S-carbamidomethylation of Cys; variable modifications, oxidation of Met; mass values, monoisotopic; peptide mass tolerance, ±50 ppm; and number of missed cleavage sites, up to 1. No relative molecular mass or isoelectric point constrictions were used. Identifications were accepted as positive when there were at least five matching peptide masses with 25–50 ppm mass accuracy, at least 20% sequence coverage, and a significant gap between the first and the next best (false-positive) database hits. Protein scores greater than 50 were significant (p < 0.05).
Production of Recombinant C. albicans Proteins
Cloning
The MET6, SSB1, GAP1/TDH3, HSP90 and PGK1 coding sequences flanked with SalI and NotI sites were PCR-amplified from C. albicans SC5314 genomic DNA using the primers reported in Supplemental Table S1. The PCR products were cloned into the pGEM-T vector (Promega, Madison, WI), and the recombinant plasmids were transformed into Escherichia coli DH5α. The inserts were subcloned into the SalI and NotI sites of the E. coli pGEX-6P-3 vector (GE Healthcare) in-frame with the glutathione-S-transferase (GST) coding sequence. The plasmids were then transformed into DH5α cells for propagation and maintenance. Following in-frame insertion confirmation, the constructs were transformed into E. coli BL21 for expression of fusion proteins.
Protein Expression
Single colonies of selected recombinants were grown in LB medium (0.5% Difco yeast extract, 1% tryptone, and 1% NaCl) at 37 °C up to an A600 nm of 0.6–0.7. Protein expression was then induced by adding isopropyl-β-d-thiogalactoside to a final concentration of 1 mm. After growing for another 12 h at 24 °C (for Met6p, Ssb1p and Hsp90p) or 3 h at 37 °C (for Gap1p/Tdh3p and Pgk1p), cells were resuspended in precooled lysis buffer (PBS containing 0.1% lysozyme, 1% Triton X-100 and 1 mm phenylmethylsulfonyl fluoride) and sonicated on ice for 15–60 s. Expression of GST fusion proteins was monitored before and after induction using SDS-PAGE followed by Coomassaie blue staining (44) and Western blotting using anti-GST antibodies (1:5000 dilution, Santa Cruz Biotechnologies, Santa Cruz, CA).
Protein Purification
The GST-tagged recombinant proteins were purified using glutathione Sepharose beads (4B, GE Healthcare) and treated with the Pre-Scission protease (GE Healthcare) to remove the GST moiety according to the manufacturer's instructions.
Protein Characterization
The identity of the purified proteins was confirmed by MS analysis as aforementioned, with slight modifications. Briefly, following trypsin digestion, the resulting peptides were analyzed using the 4800 Plus MALDI-TOF/TOF Analyzer (Applied Biosystems) and the 4000 Series Explorer software v.3.5.3 (Applied Biosystems). MALDI-TOF spectra were internally calibrated with trypsin auto-digestion products. MS peak filtering was performed through the Global Protein Server Explorer software v.3.6 (Applied Biosystems) using the same parameters and thresholds as cited above. Database searches of processed MS data for protein identification were carried out through the global protein server Explorer software on a local Mascot server v.2.1 using CandidaDB and CGD (2003.05.22 and 2007.01.31; 6165 and 6107 sequences; 2981547 and 2952183 residues, respectively) and the same search parameters and acceptance criteria as described above. Preservation of antigenic properties of the recombinant proteins was verified by SDS-PAGE Western-blotting using three IC specimens with different serum levels of IgG antibodies to them as reported previously (23).
Multiplexed Immunoassay for the Best Predictor Variables
Multiplexed Dot-Blot Assay
For each immunoarray, 0.5 μg of each purified recombinant protein, bovine serum albumin (negative control) (Sigma), and C. albicans protein extract (positive control) were spotted on a nitrocellulose membrane (GE Healthcare). Blots were first visualized by transient Ponceau S staining (Serva, Heidelberg, Germany), scanned, and rinsed with TBS. They were then blocked with 5% nonfat dry milk in TBS for 2 h, washed with TBS containing 0.01% Tween-20 (TTBS), and incubated with serum samples at a 1:500 dilution (or at a 1:250–1:1000 dilution whether the initial relative dot volume value was not within the log-linear interval of the calibration curve) in assay buffer (1% nonfat dry milk in TTBS) for 2 h. Following rinsing, immunoblots were incubated with horseradish peroxidase-labeled anti-human IgG antibodies (GE Healthcare) at a 1:3000 dilution in assay buffer for 1 h, and washed again. Immunoblots were revealed using the enhanced chemiluminescence detection system (GE Healthcare). Thereafter, densitometric analyses were performed on them using the Quantity-One software (Bio-Rad). The concentration of each signature IgG antibody in each serum was calculated as the relative volume of its corresponding immunoreactive protein dot following background subtraction and normalization to the loading control (Ponceau-stained blots), and expressed in reference units (RU)/ml relative to threefold serial dilutions of a serum pool from one IC survivor and one IC nonsurvivor (calibrator) with 6000 RU/ml signature IgG antibodies, as arbitrary assigned, given no availability of reference standards. Samples were tested on three independent experiments except where noted otherwise.
Analytical Validation
The limit of detection was calculated as the signature IgG antibody concentration corresponding to a signal 2.5 S.D. above the mean of 24 replicates of the zero calibrator (assay buffer). Imprecision was estimated for three serum samples (with different signature antibody concentrations within the assay range) tested in four replicates per run per day for six nonconsecutive days. Imprecision profiles were determined at different calibrator concentrations assayed on six nonconsecutive days. The limit of quantification was defined as the minimum signature IgG antibody concentration with an interassay imprecision ≤20%. The assay linearity was established in six serum specimens serially diluted from 1:250 to 1:1000 in the assay buffer. Analytical recovery was examined in six serum samples assayed before and after addition of 0.5, 1.5, and 2.5 RU/ml signature IgG antibodies. Recovery was calculated as the ratio of recovered to expected concentrations, and expressed as a percentage.
Gene Ontology (GO) Classification
The GeneCoDis web-based tool v.2 (http://genecodis.dacya.ucm.es) (48) was used to assess whether certain GO categories and their combinations were over-represented among the genes encoding C. albicans proteins recognized by IgG-antibody signatures. GO analyses were performed by comparing the number of genes in a defined input list (related to IgG-antibody signatures) that fall into a GO to the number of genes in the reference list (the entire C. albicans genome at the CGD database) that fall into the same ontology. Over-representation of selected GO categories (biological processes and molecular functions) and their combinations in the input gene lists was determined using the hypergeometric test with the stimulation-based correction.
Statistical Analysis
Training and test sets were randomly generated using the Bernoulli distribution with the specified probability parameter 0.5 following sample prestratification to ensure a similar number of patients with good and poor prognoses in each group. The Kolmogorov-Smirnov test and Shapiro-Wilk W-test were applied to determine whether data conformed to a normal distribution. Categorical variables were evaluated using the Yates' corrected χ2 test or Fisher's test, as appropriate. The pairwise group and multiple comparisons of continuous data were examined using the Mann-Whitney test and the Kruskal-Wallis test, respectively. Paired observations were compared with the Wilcoxon signed-rank test. Correlations were estimated by the Spearman's rank-correlation coefficient. In the multiplexed immunoassay, nonlinear-regression analyses using a four-parameter logistic function were applied for calibration curves. Imprecision profiles were established using quadratic-regression models. Linear-regression analyses were performed on dilution linearity and analytical recovery assays.
Two-way hierarchical clustering analysis (HCA) and principal-component analysis (PCA) were used to group the serum samples according to the similarity of their IgG antibody-reactivity patterns (49, 50). IgG antibody levels were normalized by median-centering antibodies for each sample and then by median-centering of each antibody across all samples. The Mann-Whitney test and analysis of variance were applied on PCA data to assess the homology and homogeneity, respectively, of the antibody-reactivity profiles among groups. Distances among clinical and molecular prognostic indicators for IC were examined by one-way HCA and PCA.
Discriminant analysis was applied to model the relationship between the clinical outcome of IC patients and their anti-Candida IgG antibody patterns as well as between patient outcomes and traditional IC prognostic factors, and to find a linear combination of the minimum number of predictor variables that best separated the compared groups (classification algorithm or discriminant function). The colinearity degree among the predictor variables was estimated with the tolerance level. Correlations between predictor variables were visualized with the within-groups correlation matrix. The Box's M test was used to determine whether covariances across groups were equal. The contribution of each predictor variable to the discriminant model was assessed by canonical correlation analysis using the equality tests of group means, the discriminant function coefficients, and the combined within-groups correlations between discriminant variables and functions. The best predictor variables to incorporate in the discriminant model were selected with the stepwise method based on the F statistic. Correlation between the discriminant scores and the groups was estimated by the canonical correlation coefficient. The proportion of the total variance in the discriminant scores not explained by differences among groups was calculated with the Wilks' λ statistic. The class prediction accuracy and error of the discriminant model were calculated by means of the U-method or leave-one-out cross-validation (LOOCV) within the training set, followed by further validation within the test set.
Receiver-operating-characteristic (ROC) curves were used to examine the discriminative ability of the prediction models. ROC areas were compared with a nonparametric asymptotic method (51). Model cutoff thresholds were defined on the basis of ROC curve analysis on training set data. These discrimination threshold values correspond to the highest combined sensitivity and specificity (i.e. the minimum sum of false-negative plus false-positive test results) for poor or good clinical outcomes when mortality or survival predictors, respectively, for IC were determined. The exact 95% confidence intervals (CIs) for the test operating characteristics of the prognostic predictors were calculated on the basis of a binomial approximation to the normal distribution. The degree of agreement between prediction models was established using the Cohen's kappa statistic. Odds ratios were estimated to determine the association between IgG antibody patterns and clinical outcomes in IC. Values below the 25th percentile of the level distribution of IgG antibodies to Met6p, Hsp90p and Pgk1p in IC survivors or to Ssb1p and Gap1p/Tdh3p in IC nonsurvivors were considered low levels. Multivariate logistic-regression models were performed to control potential confounding factors and to identify independent prognostic predictors for IC. Model goodness-of-fit was assessed using the Hosmer-Lemeshow test. Statistical significance was set at p < 0.05 (two-sided).
RESULTS
Anti-Candida IgG Antibody-Reactivity Profiles of IC
To devise an antibody reactivity-based prognostic predictor for IC, serum samples from 45 IC patients with favorable (n = 33) and fatal (n = 12) clinical outcomes within 2 months following presentation were individually screened by SERPA for IgG antibodies to the whole soluble C. albicans immunome, with the patients randomly, but equally, split into a training set for prediction-model development and into a test set for model validation (Fig. 1). Both sets, balanced for mortality, did not significantly differ in their baseline characteristics (Table I). Protein spots detected in at least three training IC patients were identified by MS analysis or using our reference two-dimensional map (22). In all, 31 C. albicans proteins, 21 of which showed several protein species on the two-dimensional recognition profiles (Fig. 2), from six functional groups were characterized as elicitors of early antibody responses in IC patients (Table II and Supplemental Fig. S1). Similar two-dimensional antibody-reactivity patterns and consistent recognition intensities for this antigen panel (coefficient of variation, 3.8%–13.6%) were obtained with the 45 serum specimens assessed on two independent experiments, indicating good reliability of the SERPA assay.
Fig. 1.
Overview of the strategy used for the development and validation of an IC prognostic prediction model based on molecular fingerprints of anti-Candida IgG antibodies in serum. The present approach combines the strengths of unsupervised and supervised methods. nT represents the training data without the jth case, and nV the test data (the jth case).
Fig. 2.
The whole soluble Candida immunome in IC. A, Silver-stained 2-DE map of the whole soluble C. albicans proteome. Protein names refer to those in Table II. Asterisks show the proteins that were identified by MS analyses in the present work. B, Representative two-dimensional reactivity profiles of serum IgG antibodies to the whole soluble Candida immunome in IC patients of the training set who had good (left) and poor (right) clinical outcomes within two months. Immunorecognized protein species are labeled (Table II).
Table II. Serum anti-Candida IgG antibody signature associated with IC and seroprevalence of these antibodies according to the clinical outcome in IC patients within the training set.
| Main Candida immunogenic proteins (Candida immunome)a |
Serum IgG antibodies to the specified Candida immunogenic protein |
||||||
|---|---|---|---|---|---|---|---|
| Nameb | Function or homologyb | Candida DB accession number | Mrc | pIc | % Difference (95% CI) in seroprevalence (good versus poor prognosis)d | Predictive strengthe | Prognosis signaturef |
| I. Cell rescue, defense and virulence | |||||||
| a. Stress response | |||||||
| Hsp90p | 90kDa-Heat shock protein | CA4959 | 82 | 4.48 | 38 (11 to 64) | 2.05 | Good |
| Ssa4p/Hsp70pg | Heat shock protein of the Hsp70p family | CA1230 | 70 | 4.71 | −50 (−77 to −23) | 2.96 | Poor |
| Ssb1p | Heat shock protein of the Hsp70p family | CA3534 | 66 | 4.86–5.03 | −58 (−102 to −15) | 1.94 | Poor |
| Ssc1p | Heat shock protein of the Hsp70p family | CA4474 | 68 | 4.81–4.96 | −25 (−72 to 22) | 0.55 | Poor |
| Sse1p/Msi3p | Heat shock protein of the Hsp70p family and Hsp90p co-chaperone | CA1911 | 80 | 5.13–5.18 | 38 (11 to 64) | 2.05 | Good |
| Ipf17186p/(Hsp31p)h | Unknown function; ortholog Saccharomyces cerevisiae 31-kDa heat shock protein | CA0828 | 26 | 4.32–4.40 | 10 (−41 to 62) | 0.17 | Good |
| b. Detoxification and stress response | |||||||
| Grp2pg | Reductase; similar to plant dihydroflavonol-4-reductases | CA2644 | 37 | 5.95–6.31 | 27 (−19 to 73) | 0.65 | Good |
| Ipf2431p/Tsa1pg | Similar to S. cerevisiae thiol-specific antioxidant protein | CA5714 | 20 | 4.72 | 2 (−38 to 42) | 0.04 | Good |
| II. Carbon compound and carbohydrate metabolism | |||||||
| a. Glycolysis/gluconeogenesis pathway | |||||||
| Pgi1p | Glucose-6-phosphate isomerase | CA3559 | 58 | 6.33 | −19 (−68 to 31) | 0.36 | Poor |
| Fba1p | Fructose biphosphate aldolase | CA5180 | 39–40 | 5.85–6.00 | 27 (−19 to 73) | 0.65 | Good |
| Tpi1p | Triose phosphate isomerase | CA5950 | 26–27 | 5.59–5.85 | −8 (−55 to 38) | 0.15 | Poor |
| Gap1p/Tdh3pi | Glyceraldehyde-3-phosphate dehydrogenase | CA5892 | 35 | 6.67–7.38 | −48 (−91 to −5) | 1.49 | Poor |
| Pgk1p | Phosphoglycerate kinase | CA1691 | 44–46 | 5.40–6.12 | 17 (−26 to 60) | 0.44 | Good |
| Gpm1p | Phosphoglycerate mutase | CA4671 | 27–28 | 5.73–5.96 | 31 (6 to 57) | 1.71 | Good |
| Eno1p | Enolase | CA3874 | 48–49 | 5.25–5.65 | 0j | n.a.j | Good |
| Cdc19p | Pyruvate kinase | CA3483 | 62 | 6.64–6.89 | −40 (−86 to 7) | 1.07 | Poor |
| b. Fermentation pathway | |||||||
| Pdc11p | Pyruvate decarboxylase | CA2474 | 60–63 | 5.00–5.22 | 38 (−3 to 78) | 1.18 | Good |
| Adh1p | Alcohol dehydrogenase | CA4765 | 44–45 | 5.56–5.83 | −35 (−84 to 13) | 0.84 | Poor |
| c. Glyoxylate and tricarboxylic acid (TCA) cycles | |||||||
| Aco1p | Aconitate hidratase | CA3546 | 84–85 | 5.82–5.90 | 25 (1 to 49) | 1.39 | Good |
| Mdh1p | Mitochondrial malate dehydrogenase | CA5164 | 35 | 5.56 | 21 (−25 to 67) | 0.47 | Good |
| d. Pentose phosphate pathwayi | |||||||
| Tkl1p | Transketolase | CA3924 | 73 | 5.54–5.59 | −15 (−58 to 29) | 0.31 | Poor |
| III. Amino acid metabolism | |||||||
| Met6p | Methionine synthase | CA0653 | 84–85 | 5.27–5.55 | 77 (47 to 107) | 4.57 | Good |
| Shm2p | Serine hydroxymethyl-transferase | CA0895 | 53 | 7.29 | 2 (−42 to 46) | 0.04 | Poor |
| Leu1p | 3-Isopropylmalate dehydratase | CA5842 | 91 | 5.63 | 19 (−3 to 40) | 1.08 | Good |
| IV. Nucleotide, nucleoside and nucleobase metabolism | |||||||
| Ade17p | 5-Aminoimidazole-4-carboxamide ribotide transformylase | CA4513 | 67 | 6.28–6.70 | −31 (−76 to 13) | 0.80 | Poor |
| Imh3p | Inosine-5'-monophosphate dehydrogenase | CA1246 | 57 | 6.67 | −38 (−95 to 20) | 0.78 | Poor |
| V. Cofactor, prosthetic group and vitamin metabolism | |||||||
| Hem13p | Coproporphyrinogen III oxidase | CA0517 | 36 | 5.83 | −27 (−81 to 27) | 0.58 | Poor |
| Ach1p | Acetyl-coenzyme-A hydrolase | CA0345 | 66 | 7.00 | 8 (−35 to 52) | 0.16 | Good |
| VI. Protein synthesis | |||||||
| a. Elongation factors | |||||||
| Eft3p/Cef3p | Translation elongation factor 3 | CA3081 | 117 | 5.39–5.45 | 25 (1 to 49) | 1.39 | Good |
| Eft2p | Translation elongation factor 2 | CA2810 | 93 | 6.55–6.72 | 2 (−42 to 46) | 0.04 | Poor |
| b. Ribosomal enzymes | |||||||
| Bel1p/Asc1p | 40S small subunit | CA4588 | 31 | 6.07 | −4 (−40 to 32) | 0.09 | Poor |
| ribosomal protein | CA4589 | ||||||
a Immunogenic proteins were identified either by using our published reference two-dimensional map of (MS-characterized) C. albicans proteins specifically recognized by serum IgG antibodies from IC patients (22) (also available on our electronic COMPLUYEAST-2DPAGE database (45, 46)) or by using MALDI-TOF MS analyses (see Ssa4p/Hsp70p, Grp2p, Ipf2431p/Tsa1p, and Supplemental Fig. S1).
b Protein names and functions/homologies according to CandidaDB and CGD (Candida Genome Database) web resources. When protein names differ in both databases, the first and second names correspond to those from CandidaDB and CGD, respectively, except where noted otherwise (see Ipf17186p/Hsp31p). Proteins are clustered into six functional groups in keeping with the comprehensive yeast genome database (CYGD) at MIPS (http://mips.gsf.de/proj/yeast/CYGD/db/).
c Experimental Mr and pI values (calculated by using the ImageMaster 2D Platinum software).
d Represents the percentage difference in the seroprevalence of the specified IgG antibody in IC patients who had a good prognosis minus that in IC patients who had a poor prognosis. A positive value thus signifies that its seroprevalence is higher in IC patients who had a favorable clinical outcome than in those who had a fatal clinical outcome, and vice versa.
e Corresponds to the statistical weight of the percentage difference in the seroprevalence of each specified IgG antibody in good- vs. poor-prognosis groups, and was computed by using the negative natural log of the p value.
f Overall, a good correlation was found between the seroprevalence of each specified IgG antibody and its reactivity signature (as determined by unsupervised HCA). See Fig. 3A for further details.
g Protein identified by using MALDI-TOF MS analysis. See Supplemental Fig. S1 for further information on Mascot score, matched/unmatched peptides and protein coverage, among others.
h Protein name in parenthesis is according to SGD (S. cerevisiae Genome Database) web resource, and corresponds to the S. cerevisiae ortholog.
i Gap1p/Tdh3p is also involved in pentose phosphate pathway.
j All IC patients (with good and poor prognoses) were seropositive for anti-Eno1p IgG antibodies. n.a. denotes not applicable.
Within the training set, the overall reactivity profiles of IgG antibodies to these 31 Candida proteins were examined by unsupervised clustering analyses to determine whether there was an evident relationship among samples and underlying antibody-reactivity patterns (Fig. 3). Intriguingly, unsupervised two-way HCA revealed separate highly correlated antibody-reactivity profiles (antibody-reactivity signatures) that, without any prior statistical test to select prognosis-associated antibodies, segregated the 22 training specimens into two major clusters corresponding to patients with distinct clinical outcomes (p < 0.001; Fig. 3A). To elucidate the potential effects of traditional IC prognostic factors and other baseline characteristics on these antibody-reactivity subgroups, their distribution was next explored among samples. No significant associations were found between these variables and antibody-reactivity patterns (Fig. 3B), suggesting that these subgroups basically reflect differences in patient outcomes not outlined by these variables.
Fig. 3.
Unsupervised learning (or clustering) analyses on SERPA-based anti-Candida IgG antibody-reactivity profiling data obtained from IC patients in the training set. A, Unsupervised two-way HCA of global anti-Candida IgG antibody-reactivity patterns in IC. Heat map and dendrograms depict clustering of serum specimens (columns) and IgG antibodies (rows) from IC patients in the training set according to overall similarities in SERPA-based antibody-reactivity profiles and reactivities across the sample population, respectively. Red or green oblongs correspond to IgG antibody-reactivity levels above or below, respectively, the median value (black oblongs). Protein names refer to those in Table II. D, IC patients who died; S, IC patients who survived. B, Sample dendrogram from the hierarchical cluster with the demographic and clinical information for each IC patient. Color code of the dendrogram is the same as in panel A (on the basis of the patient outcome). Matrix is color-coded as indicated in the scales for each demographic or clinical parameter at the bottom. p values for the association between the two major sample subgroups and these variables are shown to the right of the matrix. n.a. denotes not applicable because no training IC patients (with good and poor prognoses) had multiple trauma. C, Unsupervised PCA of global anti-Candida IgG antibody-reactivity profiles in IC within a three-dimension vector space. Each circle denotes the IgG antibody-reactivity pattern of a single sample. Specimens are color-coded as indicated. Note that color codes are the same as in panels A and B. Asterisks and daggers indicate the degree of homology and homogeneity, respectively, of these antibody-reactivity patterns among the specified study groups. Only clustering data based on clinical outcomes (left panel) and three known IC prognostic factors (center and right panels) of IC patients are depicted. ARDS, adult respiratory distress syndrome.
Unsupervised PCA was then performed on these antibody-reactivity profiles in order to confirm HCA results and support the robustness of these two classes. PCA demonstrated that these antibody-reactivity patterns were discrete and heterogeneous (p ≤ 0.001) between the clinical-outcome subgroups but similar and homogeneous (p > 0.05) between the different subsets defined by classical IC prognostic factors (Fig. 3C).
Creation of an IgG Antibody Reactivity-Based Prognostic Predictor for IC
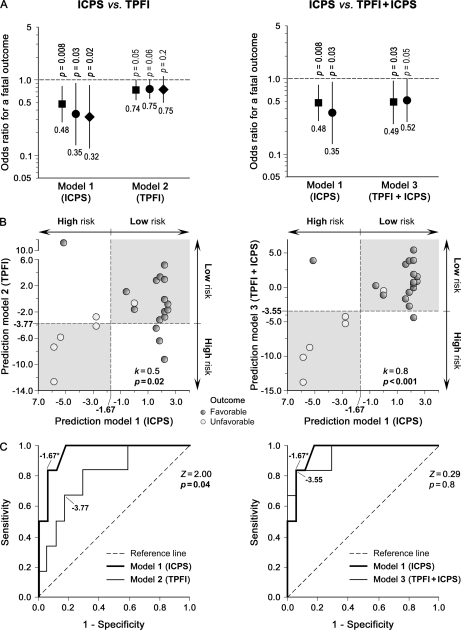
Having shown the presence of anti-Candida IgG antibody-reactivity signatures in serum at an early stage of IC that significantly correlated with the prognosis of IC patients by unsupervised methods, we next sought to develop a molecular predictor of clinical outcome for IC based on these antibody-reactivity profiles. To select the most relevant and critical panel of IgG antibodies for the prognostic signature and create a practical scoring system, the reactivity levels of the 31 IgG antibodies (predictor variables) in the training set were modeled as categorical variables (dichotomized as detectable versus undetectable, given good correlation between their seroprevalence and reactivity signatures; Table II) by supervised discriminant analyses (Fig. 1). A stepwise multivariate discriminant model containing five IgG antibodies (those to Met6p, Ssb1p, Gap1p/Tdh3p, Hsp90p, and Pgk1p; Fig. 4A) was identified, after ruling colinearity out among them, as the best prognostic predictor for IC (canonical correlation, 0.95; p < 0.001). This serum five-IgG antibody signature accounted for 91% of total variance in the reactivity profiles between survivors and nonsurvivors (Wilks' λ, 0.09; χ2, 41.63; p < 0.001).
Fig. 4.
Development of an anti-Candida IgG antibody reactivity-based prognostic predictor for IC in the training set. A, Representative two-dimensional immunoreactivity patterns of the five predictor variables (signature IgG antibodies) selected to develop the IC prognostic prediction model. See Fig. 2 for their relative position on 2-DE gels and two-dimensional immunoblots. Protein names refer to those in Table II. B, Contribution of these five predictor variables to the multivariate model before its creation. The p values for the equality tests of group means (above bars) were not greater than 0.1 (probability value to leave the model), indicating that these five predictor variables could substantially contribute to the discriminant model. Predictor variables with smaller Wilks' λ values show better discriminating abilities. C, Combined within-groups correlations of predictor variables with the standardized canonical discriminant functions. The centroids (the multidimensional center points) in the discriminant function were 1.83 (positive value) for the good-prognosis group and –4.87 (negative value) for the poor-prognosis group. A positive weight value in the pooled within-groups correlations indicates that seroprevalence of the specified predictor variable is higher in IC survivors than in IC non-survivors, and vice versa (shaded area). D, Box-and-whisker plots of the ICPS in the training IC patients on the basis of their clinical outcome. The boxes represent the interquartile ranges (25th to 75th percentiles), the horizontal thick lines portray the medians, the black squares denote the means, the whiskers extend to 1.5 times the interquartile range, and the asterisks depict the extreme values. The dot diagrams show the distribution of ICPS values in individual IC patients. The dashed line indicates the cutoff ICPS value for a fatal outcome (shaded rectangle) as defined by ROC curve analysis.
Among the predictor variables included in the model, IgG antibodies to Met6p showed the highest discriminating ability, followed in order by those to Ssb1p, Gap1p/Tdh3p, Hsp90p, and Pgk1p (Figs. 4B and 4C). Combined within-group correlations of predictor variables with the standardized canonical discriminant functions highlighted that elevated seroprevalence of IgG antibodies to Met6p, Hsp90p, and Pgk1p was associated with good prognosis whereas high frequency of those to Ssb1p and Gap1p/Tdh3p correlated with poor prognosis (Fig. 4C). Supervised univariate logistic-regression analyses demonstrated that these five IgG antibodies associated with good- or poor-prognosis signatures were also related to protective or risk patterns, respectively; i.e. those to Met6p, Pgk1p, and Hsp90p were protective antibodies whereas those to Ssb1p and Gap1p/Tdh3p were risk (nonprotective) antibodies (Table III).
Table III. Two-month risk of a fatal clinical outcome in IC patients of the training set according to the seroprevalence and circulating levels of the predictor variables (signature antibodies) that were included in the multivariate discriminant model.
| Predictor variables | No. (%) of IC patients who had a poor/good outcome | Crude OR (95% CI) for death a | p value | Coefficient β | Effect of increased seroprevalence and/or serum level on survival | Factor |
|---|---|---|---|---|---|---|
| Anti-Met6p IgG antibodies | Favorable | Protection | ||||
| Seroprevalence | 0.004 | –4.32 | ||||
| Seronegative | 5/1 (83/6) | 1.00 | ||||
| Seropositive | 1/15 (17/94) | 0.01 (0.001–0.25) | ||||
| Serum levelsb | –c | −11.61 | ||||
| Low | 6/4 (100/25) | 1.00 | ||||
| High | 0/12 (0/75) | 0.00 (–)c | ||||
| Anti-Ssb1p IgG antibodies | Unfavorable | Risk | ||||
| Seroprevalence | 0.03 | 2.71 | ||||
| Seronegative | 1/12 (17/75) | 1.00 | ||||
| Seropositive | 5/4 (83/25) | 15.00 (1.32–169.84) | ||||
| Serum levelsb | 0.008 | 3.55 | ||||
| Low | 1/14 (17/87) | 1.00 | ||||
| High | 5/2 (83/12) | 34.99 (2.58–475.09) | ||||
| Anti-Gap1p/Tdh3p IgG antibodies | Unfavorable | Risk | ||||
| Seroprevalence | 0.04 | 2.16 | ||||
| Seronegative | 2/13 (33/81) | 1.00 | ||||
| Seropositive | 4/3 (67/19) | 8.67 (1.05–71.57) | ||||
| Serum levelsb | 0.04 | 2.16 | ||||
| Low | 2/13 (33/81) | 1.00 | ||||
| High | 4/3 (67/19) | 8.67 (1.05–71.57) | ||||
| Anti-Hsp90p IgG antibodies | Favorable | Protection | ||||
| Seroprevalence | –d | −8.69 | ||||
| Seronegative | 6/10 (100/62) | 1.00 | ||||
| Seropositive | 0/6 (0/37) | 0.00 (–)d | ||||
| Serum levelsb | –d | −8.69 | ||||
| Low | 6/10 (100/62) | 1.00 | ||||
| High | 0/6 (0/37) | 0.00 (–)d | ||||
| Anti-Pgk1p IgG antibodies | Favorable | Protection | ||||
| Seroprevalence | –e | −9.37 | ||||
| Seronegative | 1/0 (17/0) | 1.00 | ||||
| Seropositive | 5/16 (83/100) | 0.00 (–)e | ||||
| Serum levelsb | 0.04f | −2.16e | ||||
| Low | 4/3 (67/19) | 1.00 | ||||
| High | 2/13 (33/81) | 0.11 (0.01–0.95)f |
a IC patients who were seronegative for or had low serum levels of the specified predictor variable (signature IgG antibody) served as the reference group in the corresponding univariate logistic-regression model.
b Serum IgG antibody levels were stratified according to the 25th percentile of the distribution of values in IC survivors (for IgG antibodies to Met6p, Hsp90p, and Pgk1p) or IC nonsurvivors (for IgG antibodies to Ssb1p and Gap1p/Tdh3p), and odds ratios were estimated for death in the IC patients who had serum IgG antibody levels equal to or above this threshold (defined as high serum levels) compared with those who had IgG antibody levels below this threshold (defined as low serum levels).
c Because no IC patients who had high serum levels of anti-Met6p IgG antibodies had a poor prognosis within two months after presentation, the estimated crude OR for a fatal clinical outcome is 0.00. The corresponding 95% CI and p values could not be determined (dashes).
d As no IC patients who were seropositive for or had high serum levels of anti-Hsp90p IgG antibodies had a poor prognosis within 2 months after presentation, the estimated crude ORs for a fatal clinical outcome are 0.00. The corresponding 95% CI and p values could not be determined (dashes).
e Because no IC patients who were seronegative for anti-Pgk1p IgG antibodies had a good prognosis within 2 months after presentation, the estimated crude OR for a fatal clinical outcome is 0.00. The 95% CI and p value could not be determined (dashes).
f IC patients with high anti-Pgk1p IgG antibody levels had a significantly reduced risk of a fatal clinical outcome in the ensuing two-month period (β = −2.16; OR = 0.11; 95% CI = 0.01–0.95; p = 0.04). It suggests that high levels, rather than a positive status (since five out of six poor-outcome patients in the training set were seropositive), of this signature antibody confer protection from a fatal clinical outcome in IC.
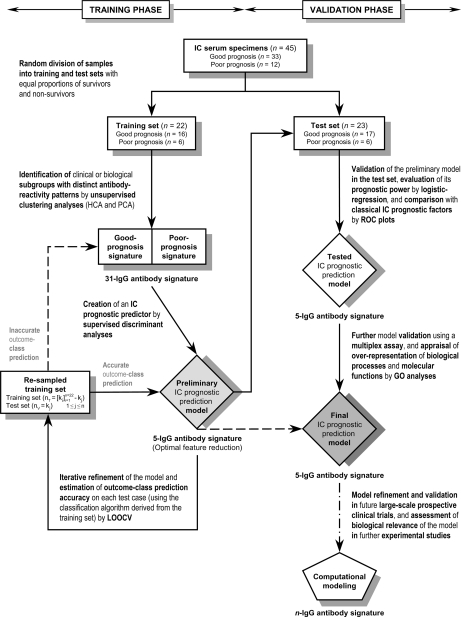
On the basis of the relative contributions of each of the five IgG antibodies in the discriminant model, we built a classification algorithm (IC prognosis score or ICPS) that provided each IC patient the likelihood of having a fatal clinical outcome in the ensuing two-month period. The median ICPS was significantly higher among IC patients who had a benign clinical course than among those who had an unfavorable outcome (2.22 versus –5.60; p < 0.001; Fig. 4D). An ICPS ≤–1.67 (defined by ROC plots) among the training IC patients was strongly predictive of a poor prognosis within 2 months (p < 0.001). The prognostic accuracy of this five-IgG antibody signature for IC was excellent (c-statistic, 1.00). It correctly classified 100% (22/22) of the training patients according to their clinical outcomes using this ICPS cutoff.
Validation of the Prognostic Predictor for IC
In an attempt to minimize the possible overestimation of the prognostic value of this molecular predictor (because of overoptimistic trend of classifications based on the cases used to create the prediction model (52)), we next tested its ability to accurately predict the prognosis of an unknown sample (not involved in the selection of predictor variables) using LOOCV within the training cohort (Fig. 1). For this class prediction analysis, one of the 22 training specimens was removed and a new prognostic predictor for IC was constructed, as described above, on the 21 remaining samples and then used to predict the clinical outcome of the left-out sample. Once this process was cycled through all of these specimens one by one, we found that the molecular fingerprint of the five IgG antibodies used in the original model displayed the highest recurrence over left-out iterations. This serum signature was able to correctly predict the true clinical outcome of 21 (95%; 95% CI, 87%–100%) out of the 22 iteratively left-out samples (classification error rate, 5%; 95% CI, 3%–7%; Figs. 5A and 5B, left), with 83% sensitivity (1 out of 6 nonsurvivors was misclassified) and 100% specificity (all survivors were correctly classified) for early identifying a fatal clinical outcome in the training set using an ICPS threshold of –1.67 (Table IV). Strikingly, the ICPS provided good LOOCV discriminatory power in this patient cohort (ROC area, 0.97; 95% CI, 0.90–1.00; p = 0.001; Fig. 5C, left).
Fig. 5.
Cross-validation and further validation of the prognostic performance of the ICPS. A, Prediction strengths of the ICPS for the specimens in LOOCV on the training set (left) and in further validation on the test set (right). Horizontal lines represent medians, and vertical lines interquartile ranges. The dashed line depicts the cutoff ICPS value for a fatal outcome (shaded rectangle) as defined by ROC curve analysis from the original training set (Fig. 4D). B, Estimated classification probabilities of a fatal clinical outcome within 2 months for each sample in the LOOCV training (left) and test (right) cohorts according to the ICPS. The sunflower diagrams show overlapped cases. Each petal or sunflower line represents an individual case (IC patient). The actual clinical-outcome is color-coded as indicated (black for a good prognosis, and white for a poor prognosis). The horizontal dashed line indicates the probability threshold (an arbitrary cutoff value of 0.5) chosen to discriminate between predicted good- and poor-prognosis patients. The vertical dashed line depicts the cutoff ICPS value for a fatal outcome (shaded rectangle). Errors in LOOCV and further validation of the IC prognostic prediction model using the five-antibody signature are shown. C, ROC curves of the ICPS for the prediction of an unfavorable (thick line) or favorable (thin line) clinical outcome within 2 months in IC patients of the LOOCV training (left) and test (right) sets. The cutoff ICPS value to distinguish IC patients with good and poor prognoses as estimated by ROC plots in the original training set is labeled in the curves of both cohorts. See Table IV for details.
Table IV. Test operating characteristics of the ICPS in the LOOCV training, test and entire-data sets.
| Test operating characteristics of the ICPSa | Mortality predictor for IC (≤ −1.67)a,b |
Survival predictor for IC (>−1.67)a,b |
||||
|---|---|---|---|---|---|---|
| LOOCV training set (n = 22) | Test set (n = 23) | Entire-data setc (n = 45) | LOOCV training set (n = 22) | Test set (n = 23) | Entire-data setc (n = 45) | |
| Percentage (95% CI) | ||||||
| Sensitivity | 83 (68–99) | 83 (68–99) | 83 (72–94) | 100 | 94 (84–100) | 97 (92–100) |
| Specificity | 100 | 94 (84–100) | 97 (92–100) | 83 (68–99) | 83 (68–99) | 83 (72–94) |
| Positive predictive value | 100 | 83 (68–99) | 91 (82–99) | 94 (84–100) | 94 (84–100) | 94 (87–100) |
| Negative predictive value | 94 (84–100) | 94 (84–100) | 94 (87–100) | 100 | 83 (68–99) | 91 (82–99) |
| Accuracy | 95 (84–100) | 91 (80–100) | 93 (86–100) | 95 (84–100) | 91 (80–100) | 93 (86–100) |
| Classification error | 5 (3–7) | 9 (7–10) | 7 (5–8) | 5 (3–7) | 9 (7–10) | 7 (5–8) |
| Ratio (95% CI) | ||||||
| Positive likelihood ratio | ∞ | 14.17 (0.00–28.42) | 27.50 (14.45–40.55) | 6.00 (3.95–8.05) | 5.65 (3.62–7.67) | 5.82 (4.38–7.26) |
| Negative likelihood ratio | 0.17 (0.01–0.32) | 0.18 (0.02–0.33) | 0.17 (0.06—0.28) | 0.00 | 0.07 (0.05–0.09) | 0.04 (0.02–0.05) |
| Prognostic odds ratio | ∞d | 80.00 (63.65–96.35)d | 160.00 (52.89–267.11)d | ∞e | 80.00 (63.65–96.35)e | 160.00 (52.89–267.11)e |
| Mean±S.E. (95% CI) | ||||||
| ROC area | 0.97 ± 0.04 (0.90–1.00)f | 0.96 ± 0.04 (0.88–1.00)f | 0.96 ± 0.02 (0.91–1.00)g | 0.97 ± 0.04 (0.90–1.00)f | 0.96 ± 0.04 (0.88–1.00)f | 0.96 ± 0.02 (0.91–1.00)g |
a The ICPS is a simplified clinical-outcome prediction model for IC based on serum five-IgG antibody-reactivity signatures in IC patients at presentation (see Fig. 4). The test operating characteristics summarized in the table are for the ICPS modeled as a mortality or survival predictor for IC in order to identify IC patients who had a poor or good clinical outcome, respectively, in the ensuing 2-month period after presentation.
b Model cutoffs were defined on the basis of ROC curve analysis on training set data (see Fig. 4D). These discrimination threshold values correspond to the highest combined sensitivity and specificity (i.e., the minimum sum of false-negative plus false-positive test results) for poor or good clinical outcomes when mortality or survival predictors, respectively, for IC were determined.
c To minimize the likely overestimation of the prognostic value of the ICPS, data derived both from LOOCV training set rather than original training set and from the test set were considered in the entire data set (see Fig. 5C).
d The odds for a positive test result in IC patients who died within 2 months relative to the odds of a positive result in those who had a favorable clinical outcome.
e The odds for a positive test result in IC patients who had a favorable clinical outcome relative to the odds of a positive result in those who died within 2 months.
f p = 0.001 as compared with an area of 0.5 for a nonuseful test. A value ranging from 0.7 to 0.8 indicates reasonable discrimination, and a value more than 0.8 represents good discrimination (51).
g p < 0.001 as compared with an area of 0.5 for a nonuseful test. See footnote f.
We then assessed the robustness and consistency of this clinical-outcome prediction model for IC in the test set (n = 23), in which none of the samples had been used for its creation (Fig. 1). Interestingly, the ICPS accurately classified five out of six IC nonsurvivors and 16 out of 17 IC survivors (Figs. 5A and 5B, right), resulting in 91% (95% CI, 80%–100%) true predictive accuracy, 83% (95% CI, 68%–99%) sensitivity and 94% (95% CI, 84%–100%) specificity for poor-prognosis detection using a cutoff of –1.67 (Table IV). ROC plots of the ICPS showed that the clinical outcome could be predicted in this cohort with good true discriminatory ability (ROC area, 0.96; 95% CI, 0.88–1.00; p = 0.001; Fig. 5C, right).
With the purpose of strengthening the clinical performance of our ICPS, random selections of predictor variables (of comparable sizes) were also evaluated for class prediction accuracy of the test set using models built as outlined above. Not surprisingly, molecular fingerprints of these random combinations yielded poor true predictive accuracies to classify survivors and nonsurvivors in the test set (c-statistic, 0.51–0.67; p = 0.2–0.9).
Prognostic Power of the ICPS
We next investigated the ICPS ability to predict the risk of a fatal outcome in IC patients within 2 months following presentation (Fig. 1). Univariate logistic-regression models indicated that each unit increase in the ICPS was associated with a 52% (95% CI, 17%–72%) decrease in the 2-month death risk in the test set (p = 0.008; Fig. 6A). These differences were more prominent when the test cohort was dichotomized according to the ICPS cutoff defined in the training set. IC patients with an ICPS > –1.67 (low-risk group) had a 99% (95% CI, 76%–100%) less mortality risk than those with an ICPS ≤–1.67 (high-risk group) (p = 0.004). Adjustment for known prognostic factors for IC and other baseline variables (potential confounders) did not significantly weaken the strong prognostic value of the ICPS in the test set (multivariate-adjusted OR, 0.04–0.57; p < 0.05). After validating its prognostic power, the IPCS was applied to the entire-data set (n = 45) in an effort to more reliably examine the effect size. Similar risk patterns were evidenced when all 45 patients were considered together. The Hosmer-Lemeshow test demonstrated good fit to data of all these multivariate models (p = 0.7–1.0), confirming their validity.
Fig. 6.
Prognostic power of the ICPS. A, Two-month mortality risk in IC patients according to the ICPS in the test and entire-data cohorts. The ICPS was modeled both as a continuous variable (where odds ratios were estimated for each unit increase) and as a categorical variable (dichotomized on the basis of the threshold defined by ROC analysis in the training set; Fig. 4C) in logistic-regression models. The squares indicate unadjusted odds ratios. The circles denote odds ratios adjusted for traditional IC prognostic factors, sex, age, underlying condition and hospital ward (Table I). Horizontal lines represent 95% CIs. For the ORs and p values shown, the reference category (OR = 1.00) was the lowest unit increase or an ICPS value equal to or below the threshold defined in the training set (≤ −1.67) for models that included the ICPS as a continuous or categorical variable, respectively. B, PCA of clinical and molecular indicators of IC prognosis in the test set within a two-dimension vector space. The percentages of total variance explained using the first two principal components are displayed on the corresponding axes. C, HCA of clinical and molecular clinical-outcome predictors for IC in the test cohort. The dendrogram branch length reflects the degree of relatedness among predictor variables: short branches cluster variables with high similarity whereas longer branches group those with lower correlation.
To further visualize the relationship between established IC prognostic factors and the ICPS, clustering analyses were then conducted in the test set. PCA revealed that conventional prognostic factors for IC clustered into two groups, based on their association with the immune system status of IC patients, and away from the ICPS (Fig. 6B). HCA substantiated these results, showing no connection of the ICPS with specific branches (Fig. 6C).
Comparison of the ICPS and Traditional IC Prognostic Factors
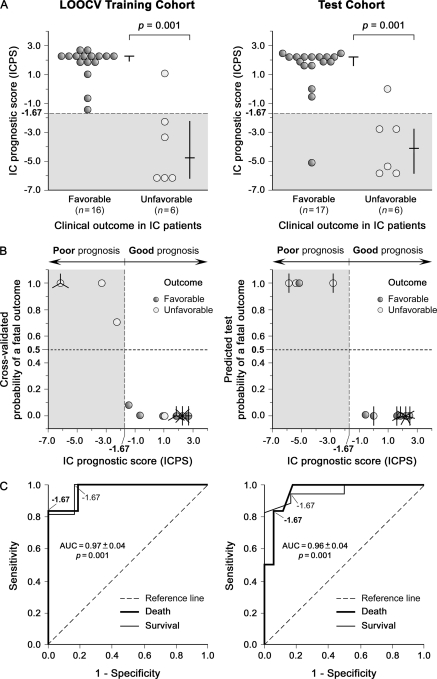
To determine whether the ICPS added prognostic information beyond that provided by classical IC prognostic factors, we next created further multivariate models based on them (Table I) without and with the ICPS (referred to as traditional prognostic factors-index (TPFI) and TPFI+ICPS, respectively) in the same way as detailed above, and compared our ICPS with these models in the test cohort (Fig. 1). The ICPS was a stronger IC prognostic predictor than the TPFI (OR, 0.48 versus 0.74; p = 0.008 versus 0.05; Fig. 7A). Clinical concordance in outcome prediction for individual IC patients proved moderate-to-good between ICPS and TPFI/TPFI+ICPS (k = 0.5–0.8; p < 0.02; Fig. 7B). Although the TPFI (ROC area, 0.79; 95% CI, 0.59–0.99; p = 0.04) and the ICPS used both alone (ROC area, 0.96; 95% CI, 0.88–1.03; p = 0.001) and in combination (ROC area, 0.94; 95% CI, 0.84–1.04; p = 0.002) were able to discriminate between survivors and nonsurvivors (Fig. 7C), differences in their ROC areas revealed that the ICPS was more accurate in predicting clinical outcomes in IC than the TPFI (Z = 2.00; p = 0.04; Fig. 7C, left) or individual classical IC prognostic factors (Z > 1.96; p < 0.05), and that the combined model (TPFI+ICPS) did not contribute to a better discrimination beyond the ICPS (Z = 0.29; p = 0.8; Fig. 7C, right).
Fig. 7.
Comparison of prognostic abilities of the ICPS and models based on traditional IC prognostic factors without and with the ICPS (TPFI and TPFI+ICPS, respectively) on the test set. A, Forest plots of odds ratios for a fatal clinical outcome in testing IC patients within two months following presentation according to the ICPS, TPFI and TPFI+ICPS. The squares represent unadjusted odds ratios. The circles indicate odds ratios adjusted for sex, age, underlying condition, and hospital ward (Table I). The diamonds correspond to odds ratios adjusted for each other (model 2 or 1). Horizontal lines denote 95% CIs. The numbers below each forest plot depict the corresponding odds ratios for a fatal clinical outcome. B, Two-dimensional representation of prediction strengths of ICPS versus TPFI (left) and TPFI+ICPS (right) in the test set. The shaded rectangles depict clinical concordance in the (good/poor) outcome predictions for individual IC patients between the compared prediction models. The actual clinical-outcome is color-coded as indicated (black for a good prognosis, and white for a poor prognosis). The vertical dashed lines indicate the cutoff ICPS value for a poor prognosis, and horizontal dashed lines denote the cutoff TPFI (left) and TPFI+ICPS (right) values for a fatal outcome as defined by ROC plots. C, ROC curves of the ICPS versus TPFI (left) and TPFI+ICPS (right) for the prediction of an unfavorable clinical outcome within two months in testing IC patients. The cutoff values of the three prediction models to discriminate IC patients with good and poor prognoses as defined by ROC plots in the initial training set are labeled in their corresponding curves.
Development of a Multiplexed Immunoassay for the Five Signature IgG Antibodies
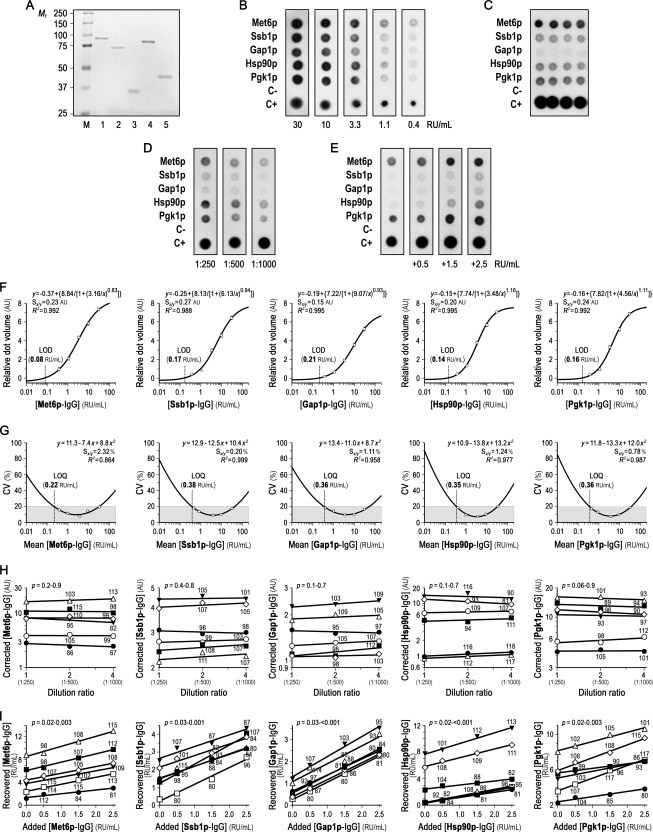
To further validate the prediction model derived from SERPA, we next developed a multiplexed dot-blot assay for simultaneous and rapid measurement of the five signature IgG antibodies in each serum specimen (Fig. 1). First, Met6p, Ssb1p, Gap1p/Tdh3p, Hsp90p, and Pgk1p were expressed in E. coli as GST-fusion proteins, purified by affinity chromatography, and treated with a protease to remove the GST moiety (Fig. 8A). Their identity was then verified by MS analysis (Supplemental Fig. S2), and retention of their antigenic properties was confirmed by Western-blotting using three IC samples with different serum levels of the five signature IgG antibodies.
Fig. 8.
Analytical performance of the multiplexed immunoassay for simultaneous and rapid measurement of the five signature IgG antibodies in each serum sample. A, Recombinant C. albicans proteins used as diagnostic reagents in the multiplexed immunoassay. Purified and Pre-Scission protease-treated recombinant Met6p (lane 1), Ssb1p (lane 2), Gap1p/Tdh3p (lane 3), Hsp90p (lane 4), and Pgk1p (lane 5) were separated on a 10% SDS-PAGE gel and silver-stained. Lane M, molecular mass standards. B, Representative immunorecognition patterns of threefold serial dilutions of the calibrator. In addition to the five recombinant C. albicans protein dots, each multiplexed immunoblot included negative (C-; bovine serum albumin) and positive (C+; C. albicans protein extract) control dots to monitor experimental performance. Numbers below each panel depict signature IgG antibody concentration. C, Typical multiplexed dot-blot array showing intra-assay imprecision of one serum with different signature IgG antibody concentrations in four replicates. See Supplemental Table S2 for further details. D, Representative multiplexed immunoblots depicting dilution effect (1:250–1:1000) in the assay buffer for one serum with different signature IgG antibody concentrations. E, Typical multiplexed dot-blots illustrating analytical recovery of known amounts of calibrator (0.5, 1.5, and 2.5 RU/ml signature IgG antibodies) added exogenously to one serum with different signature IgG antibody concentrations. F, Calibration curves for each signature IgG antibody. Threefold serial dilutions of a serum pool from one IC survivor and one IC nonsurvivor with an arbitrary value of 6000 RU/ml signature IgG antibodies were used as a calibrator, because no reference standards are available. The dose-response curves were fit to a four-parameter logistic-regression model. Each point represents the mean relative dot volume (for the relative concentration of the calibrator) of six different analytical assays, and bars designate S.D. G, Imprecision profiles for each signature IgG antibody. Inter-assay CVs at different concentrations of the calibrator were fit to a quadratic-regression model, and are given as the mean of different six determinations. The shaded rectangles show the portion of the curves with an optimal inter-assay imprecision. H, Dilution linearity for each signature IgG antibody. Six serum specimens with different signature IgG antibody concentrations were twofold serially diluted (from 1:250 to 1:1000) in the assay buffer. Dilution ratio and recovery are referred to the less diluted sample (1:250). I, Analytical recovery for each signature IgG antibody. Known amounts of calibrators (0.5, 1.5, and 2.5 RU/ml signature IgG antibodies) were added exogenously to six serum samples with different signature IgG antibody concentrations. In panels H and I, p values are for slope deviation from zero, each symbol designates an individual serum sample, and the numbers above or below each symbol depict the recovery percentage of signature IgG antibodies at that relative (H) dilution ratio or (I) concentration of calibrator added to the tested specimen. For clarity, the scales on the y-axes of panels H and I are different. Square brackets in the axis legends denote concentration; AU, arbitrary units; RU, reference units; CV, coefficient of variation; LOD, limit of detection; LOQ, limit of quantification; Sy/x, standard deviation about the regression line; R2, goodness-of-fit statistic.
After empirically optimizing the best conditions for the multiplexed immunoassay, we evaluated its analytical performance (Figs. 8B–8E). Calibration curves for each signature IgG antibody resulted in a good log-linear response within working range, in the interval of 0.5–20 RU/ml (R2 = 0.988–0.995; Fig. 8F). Limit of detection ranged from 0.08 (Met6p-IgG) to 0.21 (Gap1p/Tdh3p-IgG) RU/ml (Fig. 8F), and limit of quantification ranged from 0.22 (Met6p-IgG) to 0.38 (Ssb1p-IgG) RU/ml (Fig. 8G). The multiplexed immunoassay was reproducible in the entire range of analyzed concentrations (Fig. 8G) and, overall, it showed good intra- and interassay imprecision for each signature IgG antibody (Supplemental Table S2). The assay achieved good dilution linearity, with a median dilution recovery of 103% (range, 81%–118%; Fig. 8H). Analytical recovery of exogenously added signature IgG antibodies was almost complete (median, 97%; range, 80%–117%; Fig. 8I). Dilution/analytical recovered and expected concentrations of each signature antibody correlated well (r = 0.996 for dilution linearity, and 0.982 for analytical recovery; p < 0.001 for both analyses) and did not significantly differ (p = 0.6 and 0.7 for linearity and recovery studies, respectively). Accordingly, sample dilution did not systematically affect the multiplexed immunoassay nor did potential interfering substances present in the sample matrix (Figs. 8H, 8I).
Prognostic Performance of the Multiplexed Immunoassay in IC
Having proven that our multiplexed immunoassay fulfilled the requirements of detection limit, functional sensitivity, imprecision, linearity and analytical recovery, we next assessed its prognostic performance in the test cohort (Fig. 9A). Unsupervised PCA separated testing IC survivors and nonsurvivors in two distinct groups on the basis of their five-IgG antibody-reactivity profiles (Fig. 9B). As expected, not only did unsupervised HCA confirm these results (Fig. 9C) but it also revealed that the Met6p-Hsp90p-Pgk1p-IgG and Ssb1p-Gap1p/Tdh3p-IgG antibody-reactivity signatures were synergistic in predicting clinical outcomes in IC patients (Fig. 9D). Remarkably, no significant differences in known IC prognostic indicators and other baseline characteristics were identified between these antibody-reactivity subgroups.
Fig. 9.
Prognostic performance of the multiplexed immunoassay on the test cohort. A, Representative multiplexed dot-blots obtained using serum specimens both from seven and four IC patients with good and poor clinical outcomes, respectively, within 2 months following presentation and from one non-IC patient (negative control; with no detectable serum levels to the five signature IgG antibodies in a two-dimensional blot). In addition to the five recombinant C. albicans protein dots, each immunoarray included negative (C-; bovine serum albumin) and positive (C+; C. albicans protein extract) control dots to monitor experimental performance. Numbers above each pattern indicate its corresponding IC-MAPS value (see panels D–G). B, Unsupervised PCA of the five-IgG antibody-reactivity profiles in testing IC patients within a three-dimension vector space. The percentages of variance explained using the first three principal components are shown on the corresponding axes. Each circle denotes the 5-IgG antibody-reactivity pattern of a single serum sample. Samples are color-coded as depicted. The color-shaded areas represent clustering of specimens. The asterisk and dagger indicate the degree of homology and homogeneity, respectively, of these antibody-reactivity patterns between the specified groups. C, Unsupervised two-way HCA of the five-IgG antibody-reactivity profiles (rows) and serum specimens (columns) from testing IC patients. Red or green oblongs correspond to IgG antibody-reactivity levels above or below, respectively, the median value (black oblongs). Protein names refer to those in Table II. D, IC patients who died; S, IC patients who survived. D, Good/poor prognosis signature averages and the IC-MAPS (in LOOCV) for each testing IC patient. Sample dendrogram is the same as in panel C. Matrixes are color-coded as shown in the corresponding scales. Good-prognosis signature IgG antibodies were those to Met6p, Hsp90p and Pgk1p, whereas poor-prognosis signature IgG antibodies were those to Ssb1p and Gap1p/Tdh3p (see panel C). E, Prediction strength of the IC-MAPS for testing IC specimens in LOOCV. Horizontal lines depict medians, and vertical lines interquartile ranges. The dashed line represents the cutoff IC-MAPS value for a fatal outcome (shaded rectangle) as determined by ROC plots (see panel G). F, Estimated classification probabilities of a fatal clinical outcome within two months for each testing IC sample in LOOCV according to the IC-MAPS. The actual clinical-outcome is color-coded as depicted. The horizontal dashed line portrays the probability threshold (an arbitrary cutoff value of 0.5) chosen to discriminate between predicted good and poor clinical-outcome patients. The vertical dashed line indicates the cutoff IC-MAPS value for a fatal outcome (shaded rectangle) as defined by ROC analysis (panel G). G, ROC curves of the IC-MAPS (in LOOCV) and individual signature IgG antibodies for the prediction of an unfavorable clinical outcome within 2 months following presentation in testing IC patients. The prognostic threshold for the IC-MAPS is labeled. Dashed curves indicate that direction of the corresponding models (signature Ssb1p and Gap1p/Tdh3p IgG antibodies) for clinical-outcome prediction in IC is distinct from the remaining univariate or multivariate models.
These profiles were then modeled as described above. Supervised stepwise multivariate discriminant analyses with LOOCV identified the five signature IgG antibodies as the most simplified and accurate molecular fingerprint for clinical-outcome prediction in IC, from which a similar algorithm (IC multiplex antibody prognosis score or IC-MAPS) was derived. Low IC-MAPS values were associated with an adverse clinical outcome in IC patients (Figs. 9D–9F). Using a threshold of –0.97, the IC-MAPS properly classified 21 out of the 23 IC patients from the test set (Figs. 9E and 9F), resulting in the same accuracy (91%; 95% CI, 80%–100%) as the ICPS, 100% sensitivity, and 88% (95% CI, 75%–100%) specificity for poor-prognosis detection in IC. The IC-MAPS (ROC area, 0.98; 95% CI, 0.93–1.00; p = 0.001) showed a higher ability to discriminate IC survivors from non-IC survivors than individual signature IgG antibodies (Z = 2.04–2.30; p = 0.04–0.02; Fig. 9G), and it was not gained confidence when less than five signature IgG antibodies were tested. Its combination with the TPFI or individual classical IC prognostic factors did not lead to additionally improved clinical-outcome prediction for IC either.
Biologic Insights into the ICPS and Global Prognostic Antibody Signature for IC
In an endeavor to gain further insights into the biologic significance of the ICPS and molecular basis underlying the distinct clinical courses in IC, GO analyses were then performed (Fig. 1). Significant enrichments for host-interaction, protein-binding, steroid hormone signaling pathway, response to stress and glycolysis/gluconeogenesis ontologies (for ICPS and global prognostic antibody signature) and entry into host, ATP binding and lyase activity ontologies (for global prognostic antibody signature) were identified in the good-prognosis signatures, whereas apoptosis and oxygen/reactive-oxygen-species metabolic process ontologies (for ICPS and global prognostic antibody signature) were over-represented in the poor-prognosis signatures.
DISCUSSION
Differences in Anti-Candida IgG Antibody-Reactivity Profiles among IC Patients at Presentation Reflect Distinct Clinical Outcomes
We found that anti-Candida IgG antibody-reactivity patterns of IC patients at presentation were distinct according to their likely clinical outcomes within 2 months. This molecular heterogeneity may be ascribed to the existence of certain anti-Candida antibodies that can mediate protection against IC and consequently determine the IC fate (53–55). Accordingly, protective and nonprotective antibody responses, which appear to differ in epitope specificity, isotype, and/or titer (54, 55), could show separate effects to IC and delineate discrete molecular fingerprints corresponding to distinct patient outcomes. This may explain why the good-prognosis signature was characterized by higher levels of known protective antibodies, such as those to Hsp90p, Eno1p, Met6p, or Fba1p (13, 15, 56–62), whereas the poor-prognosis signature was associated with undetectable/lower levels of them (possibly insufficient to induce protection (55)) and higher levels of nonprotective antibodies, like those to Gap1p/Tdh3p (62, 63) or Ssa4p, Ssb1p or Ssc1p (cell wall-exposed, Hsp70p family members (42, 64)). The latter is gathered from the nonprotective effect of anti-surface Hsp70p antibodies (65) and their high homology degree (see below). Intriguingly, the presence of the 31 identified antigens at the Candida surface (42, 64) (probably solubilized in the whole soluble proteome given their loose association (64, 66, 67)) provides further support for our finding that generation of antibodies to some of them could be of benefit to the host and, feasibly, to future prophylactic/therapeutic purposes for IC.
The ICPS Derived from the Molecular Fingerprints of Five Anti-Candida IgG Antibodies May Reliably Predict the Clinical Outcome in Individual IC Patients at Presentation
Although our 31-IgG antibody-reactivity signature was able to discriminate between distinct clinical-outcome subgroups, this may be too complex and costly to apply as a clinical test in routine general practice. To reduce its dimension, we developed a classification algorithm that optimally combined the minimum number of predictor variables with the best accuracy. Because this type of nonbiological and mathematical multivariate classifiers is often associated with overfitting and potential bias or chance (52), we used cross-validation techniques to evaluate its internal consistency within a training set followed by further validation within an external test set. The similarity in clinical performances between the LOOCV training and test sets, poor predictive accuracies of molecular fingerprints of random combinations, and confirmation of SERPA-derived predictions by the multiplexed immunoassay validate our five-IgG antibody-reactivity signature as a reliable IC prognostic predictor. These findings are not only in line with an earlier report showing the SERPA utility for identifying individual IC prognostic candidates (anti-wall Eno1p or anti-Bgl2p IgG antibodies) (13) but also extend this potential to uncover a panel of optimally combined biomarkers that may cover a broader range of IC patients given host-response heterogeneity as well as novel therapeutic options against IC (see below). To our knowledge, this study is the first attempt to develop and validate a molecular predictor for IC prognosis.
We chose a mutiplexed dot-blot assay for model validation because this is a simple, rapid, accurate and cost-effective method that can simultaneously measure several biomarkers in each sample. For small-scale validation studies, this format thus provides an attractive alternative both to the traditional ELISA, limited by its ability to quantify only a single biomaker in each sample, and to new high-throughput multiplexed technologies (such as multiplexed microplate or fluorescent beads-based immunoassays), which entail an elevated investment in equipment and disposable supplies (68). However, future large-scale validation studies will certainly require the latter given that these are easier-to-automate and will enable better data quantification. Clearly, our antibody-reactivity signature will have to be appropriately adjusted, optimized and further validated in these larger studies as these may, for example, lead to changes in the prognosis threshold value and/or relative contributions of the predictor variables in the multivariate model.
The ICPS Is Unbiased by and Outperforms Traditional IC Prognostic Factors
Because diverse patients-related variables represent another major source of potential bias or chance in these studies (38, 52), known IC prognostic factors and other baseline characteristics were assessed as potential confounders. Multivariate logistic-regression models pointed out that the ICPS prognostic ability is at least unbiased by them. Clustering analyses demonstrated this signature identifies distinct features of IC patients that influence their clinical outcomes. And, ROC plots showed that our ICPS provides improvements in IC prognostic performance over univariate and multivariate models containing these factors, and that its combination with them does not result in additionally enhanced clinical-outcome prediction for IC. Although these findings are promising, it is worth emphasizing that the sample size was small and TPFI model and accuracy could differ in other clinical settings (3, 5). Hence, these should be validated in future larger multicenter prospective studies including these or further IC prognostic factors to elucidate whether our ICPS might replace or complement them in clinical practice.
The ICPS May Be Useful for 2-Month Mortality Risk Level Estimation and More Appropriate Initial Therapeutic Decision-Making in Individual IC Patients
Another remarkable finding was the ICPS ability to properly discriminate IC patients at high-risk for death from those at low-risk. This could, if confirmed prospectively in a larger patient population, be valuable to adequately guide initial therapeutic decision-making in the future (1). This ICPS might therefore aid physicians in identifying IC patients at high-risk for a likely fatal outcome who may benefit from the best and most aggressive therapeutic method and closer clinical follow-up. Conversely, this molecular signature might also help them to select low-risk patients who may need less intensive clinical follow-up and/or be referred for healthcare strategies that aim to minimize antifungal therapy-related costs and (nephro)toxicities.
Antibody-Reactivity Profiling May Provide Biologic Insights into Molecular Mechanisms that Influence the Clinical Outcome in IC to Guide Future Hypothesis-Driven Studies
Beyond the practical impact of providing a prediction model for IC prognosis, SERPA-based profiling may also reveal dysregulated biological processes and/or molecular functions during distinct clinical outcomes in IC that could otherwise not be revealed. The noticeable enrichment for antibodies to pathogenicity-related proteins (involved in host protein binding, steroid hormone signaling pathway, stress response, and host tissue invasion (69–75)) in the good-prognosis signature may reflect important defense mechanisms responsible for Candida clearance. Neutralization of glycolysis and gluconeogenesis, ATP-binding, and lyase-activity proteins, prominent molecular features of the favorable clinical-outcome group, could represent other pathways that may also contribute to an overall improved prognosis. In fact, the breakdown of glycolysis and gluconeogenesis-related proteins has been associated with rapid or slow ATP depletion and subsequent cell necrosis or apoptosis, respectively (76). Mutations that eliminate nucleotide-exchange-factor activity of certain ATP-binding proteins have proved lethal (77). Although obvious link has been hitherto unreported between lyase activity and IC pathogenesis, this interaction may promote new research fields. All these processes/functions neutralized by the good-prognosis signature should be targeted as novel therapeutic options for IC in future studies. In contrast, apoptosis and reactive-oxygen-species metabolism neutralization were uncovered in the poor-prognosis signature as argued below.
The Biologic Relevance of the Antibodies Included in the ICPS to IC Prognosis Confirms Our Five-IgG Antibody Signature as a Credible Clinical-Outcome Predictor for IC
The Good Prognosis-Associated, Protective Antibodies in the ICPS are Directed to Putative Candida Virulence Factors and Antiapoptotic Mediators
Candida Met6p is essential for growth and viability, and is involved in virulence and morphogenesis (74, 78). Hsp90p is a putative Candida virulence factor (73, 79) that may interfere with proper human protein folding, functioning and/or degradation and activate human endothelial nitric oxide synthase (altering cardiovascular hemodynamics) (58, 80). Met6p and Hsp90p levels are increased in response to the human steroid hormone 17-β-estradiol (73, 74), which adds to Candida growth, toxin production, and hypha formation rates (81). Candida surface-associated Pgk1p may bind human plasminogen and mediate its cleavage into plasmin, enabling Candida to invade and degrade tissues (69, 71). These three proteins might further act as antiapoptotic modulators (82–84). Accordingly, production of antibodies that coordinately neutralize these putative virulence factors and antiapoptotic mediators may provide an effective protective barrier against IC with potential synergistic effects and influence infection outcome for human benefit. This is consistent with the protective effect and immunotherapeutic potential of antibodies to Met6p or Hsp90p against IC (15, 56–58, 61, 62) and with the potential of a nonconserved Pgkp epitope and a Δpgk mutant as vaccine candidates for other infections (85, 86). Our findings may therefore serve as a rationale for the design of an innovative vaccine- or immunotherapy-based formulation containing Met6p, Hsp90p, and Pgk1p (or specific epitopes of these) or their related antibodies to prevent/control IC.
The Poor Prognosis-Associated, Risk Antibodies in the ICPS are Directed to Potential Candida Proapoptotic Mediators
Although the mechanisms by which antibodies to Gap1p/Tdh3p and Ssb1p may negatively affect the clinical outcome of IC patients are unclear, earlier studies strengthen our results (62, 63, 65, 87). Passive transfer and immunization assays in animal models have shown that anti-Gap1p/Tdh3p antibodies are neither protective nor can they stimulate protective responses against IC (62, 63). Likewise, not only does immunization with a surface-exposed Hsp70p induce nonprotective responses against IC, but it may also play an apparent role in accelerating the animal's death (65). Clues as to these risk (nonprotective) patterns may come from the roles of Gap1p/Tdh3p and Hsp70p as proapoptotic mediators (88–90). Antibodies to Candida surface-associated Gap1p/Tdh3p (enzymatically active (91)) might either neutralize its activity and reduce the concentration of its intracellular form (a major messenger mediating apoptotic cell death (88, 92)) in compensatory response (oversecretion), or block it at the cell surface, where Gap1p/Tdh3p might act as an extracellular proapoptotic sensor. Both ways would lead to an increase in Candida survival and a poor prognosis in IC patients. Conversely, surface-associated Hsp70p can mediate apoptosis in tumor cells through specific binding and uptake of granzyme B released by natural killer cells, whose lytic activity can be inhibited by anti-surface Hsp70p antibodies (90, 93). Similarly, Candida surface-associated Ssa1p and Ssa2p (Hsp70p family members) mediate binding and uptake of human β-defensins 2/3 and histatin 5 required for their fungistatic and fungicidal activity against Candida (94, 95). Both mechanisms could explain why anti-Ssb1p antibodies (which might analogously neutralize these or other innate immune system components) may not be of benefit to the host. Accordingly, our results might offer a basis for development of anti-idiotype antibodies, i.e. antibodies that block the antigen-combining (idiotypic) region of these risk antibodies, as novel IC therapeutics in the future.
The Whole Five-IgG Antibody Set Appears to Have the Key Information for IC Prognosis
High antibody levels to Ssb1p, Gap1p/Tdh3p and Pgk1p and low anti-Met6p antibody levels at early IC stages were associated with poor prognoses (15, 87). Likewise, a synthetic vaccine combining β-mannan and Pgk1p-derived peptide epitopes slightly enhanced IC susceptibility (62). This correlation between adverse outcomes and anti-Met6p or anti-Pgk1p antibody levels may result from the possibilities that these antibodies may not be present in adequate epitope specificity, isotype, and/or titer to induce protection (54, 55), and/or that whole set of reactivity profile of our five-IgG antibodies could have the key information for clinical-outcome prediction in IC. The latter is reinforced by the ICPS calculated for these published data (15, 87), which was predictive of a poor prognosis (–2.78).
Future Challenges
Our study has revealed that prognostic antibody-reactivity signatures are present in IC patients at presentation and that these may be useful to formulate a molecular predictor of clinical outcome for IC and provide potential targets for upcoming molecular therapies against IC. However, before its routine implementation in clinical practice, our IC prognostic predictor must be refined and validated in future larger longitudinal prospective cohort studies, which are now ongoing, with adjustments for known IC prognostic factors and time-to-event analyses using easier-to-automate mutiplexed clinical platforms. If confirmed, the resulting signature might open the way for individual prediction of clinical outcomes in IC.
Acknowledgments
We are indebted to Dr. A. Jiménez (from the Department of Internal Medicine, Clinic Hospital from Salamanca, Spain) for supplying human serum samples, and to the patients recruited for this study. We appreciate their participation in it. We also thank M.D. Gutiérrez, M. Posada, and M.L. Hernáez (from Proteomics Facility, Complutense University and Scientific Park of Madrid (UCM-PCM), a member of the National Institute for Proteomics, ProteoRed, Spain) for their technical assistance in protein identification.
Footnotes
* This work was supported by the Ministry of Science and Innovation, Health Institute Carlos III cofinanced by European Development Regional Fund “A way to achieve Europe” ERDF, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008); the Interministry Commission of Science and Technology (BIO-2003-00030, BIO-2006-01989 and BIO-2009-07654); the Community of Madrid (S-SAL-0246-2006 DEREMICROBIANA-CM); the Complutense University of Madrid and Community of Madrid (920685); the Merck, Sharp and Dohme (MSD) Special Chair in Genomics and Proteomics; and Ramón Areces Foundation, Spain. C. Nombela is the director of the MSD Special Chair in Genomics and Proteomics in Spain.
 This article contains supplemental material Tables I and II and Figs. 1 and 2.
This article contains supplemental material Tables I and II and Figs. 1 and 2.
1 The abbreviations used are:
- IC
- invasive candidiasis
- 2-DE
- two-dimensional gel electrophoresis
- CI
- confidence interval
- Gap1p/Tdh3p
- glyceraldehyde-3-phosphate dehydrogenase
- GO
- gene ontology
- GST
- glutathione-S-transferase
- HCA
- hierarchical clustering analysis
- Hsp70p
- 70kDa-heat shock protein
- Hsp90p
- 90kDa-heat shock protein
- IC-MAPS
- IC multiplex antibody prognosis score
- ICPS
- IC prognosis score
- LOOCV
- leave-one-out cross-validation
- MALDI
- matrix-assisted laser desorption/ionization
- Met6p
- methionine synthase
- OR
- odds ratio
- PCA
- principal component analysis
- Pgk1p
- phosphoglycerate kinase
- ROC
- receiver-operating-characteristic
- RU
- reference units
- Ssb1p
- heat shock protein of the Hsp70 family
- SERPA
- serological proteome analysis
- TOF
- time-of-flight
- TPFI
- traditional prognostic factors-index.
REFERENCES
- 1. Pfaller M. A., Diekema D. J. (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20, 133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pappas P. G. (2006) Invasive candidiasis. Infect. Dis. Clin. North Am. 20, 485–506 [DOI] [PubMed] [Google Scholar]
- 3. Eggimann P., Garbino J., Pittet D. (2003) Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 3, 685–702 [DOI] [PubMed] [Google Scholar]
- 4. Falagas M. E., Apostolou K. E., Pappas V. D. (2006) Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur. J. Clin. Microbiol. Infect. Dis. 25, 419–425 [DOI] [PubMed] [Google Scholar]
- 5. Uzun O., Anaissie E. J. (2000) Predictors of outcome in cancer patients with candidemia. Ann. Oncol. 11, 1517–1521 [DOI] [PubMed] [Google Scholar]
- 6. Uzun O., Ascioglu S., Anaissie E. J., Rex J. H. (2001) Risk factors and predictors of outcome in patients with cancer and breakthrough candidemia. Clin. Infect. Dis. 32, 1713–1717 [DOI] [PubMed] [Google Scholar]
- 7. Almirante B., Rodríguez D., Cuenca-Estrella M., Almela M., Sanchez F., Ayats J., Alonso-Tarres C., Rodriguez-Tudela J. L., Pahissa A. (2006) Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 44, 1681–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng Y. R., Lin L. C., Young T. G., Liu C. E., Chen C. H., Tsay R. W. (2006) Risk factors for candidemia-related mortality at a medical center in central Taiwan. J. Microbiol. Immunol. Infect. 39, 155–161 [PubMed] [Google Scholar]
- 9. Nucci M., Anaissie E. (2002) Should vascular catheters be removed from all patients with candidemia? An evidence-based review. Clin. Infect. Dis. 34, 591–599 [DOI] [PubMed] [Google Scholar]
- 10. Yeo S. F., Huie S., Sofair A. N., Campbell S., Durante A., Wong B. (2006) Measurement of serum D-arabinitol/creatinine ratios for initial diagnosis and for predicting outcome in an unselected, population-based sample of patients with Candida fungemia. J. Clin. Microbiol. 44, 3894–3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosemurgy A. S., 2nd, Zervos E. E., Sweeney J. F., Djeu J. Y. (1997) Candida antigen titre dilution and death after injury. Br. J. Surg. 84, 771–774 [PubMed] [Google Scholar]
- 12. Matthews R., Burnie J., Smith D., Clark I., Midgley J., Conolly M., Gazzard B. (1988) Candida and AIDS: evidence for protective antibody. Lancet 2, 263–266 [DOI] [PubMed] [Google Scholar]
- 13. Pitarch A., Jiménez A., Nombela C., Gil C. (2006) Decoding serological response to Candida cell wall immunome into novel diagnostic, prognostic, and therapeutic candidates for systemic candidiasis by proteomic and bioinformatic analyses. Mol. Cell Proteomics 5, 79–96 [DOI] [PubMed] [Google Scholar]
- 14. Pitarch A., Nombela C., Gil C. (2006) Contributions of proteomics to diagnosis, treatment, and prevention of candidiasis. Methods Biochem. Anal. 49, 331–361 [DOI] [PubMed] [Google Scholar]
- 15. Pitarch A., Nombela C., Gil C. (2008) The Candida immunome as a mine for clinical biomarker development for invasive candidiasis: From biomarker discovery to assay validation. In Pathogenic Fungi: Insights in Molecular Biology. (San-Blas G., Calderone R. eds), pp. 103–142, Caister Academic Press, Wymondham, UK [Google Scholar]
- 16. Wilson R. A., Curwen R. S., Braschi S., Hall S. L., Coulson P. S., Ashton P. D. (2004) From genomes to vaccines via the proteome. Mem. Inst. Oswaldo Cruz 99, 45–50 [DOI] [PubMed] [Google Scholar]
- 17. Tjalsma H., Schaeps R. M. J., Swinkels D. W. (2008) Immunoproteomics: From biomarker discovery to diagnostic applications. Proteomics Clin. Appl. 2, 167–180 [DOI] [PubMed] [Google Scholar]
- 18. Caron M., Choquet-Kastylevsky G., Joubert-Caron R. (2007) Cancer immunomics using autoantibody signatures for biomarker discovery. Mol. Cell Proteomics 6, 1115–1122 [DOI] [PubMed] [Google Scholar]
- 19. Rauch J., Gires O. (2008) SEREX, Proteomex, AMIDA, and beyond: Serological screening technologies for target identification. Proteomics Clin. Appl. 2, 355–371 [DOI] [PubMed] [Google Scholar]
- 20. Pitarch A., Sánchez M., Nombela C., Gil C. (2003) Analysis of the Candida albicans proteome I. Strategies and applications. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 787, 101–128 [DOI] [PubMed] [Google Scholar]
- 21. Thomas D. P., Pitarch A., Monteoliva L., Gil C., Lopez-Ribot J. L. (2006) Proteomics to study Candida albicans biology and pathogenicity. Infect. Disord. Drug Targets 6, 335–341 [DOI] [PubMed] [Google Scholar]
- 22. Pitarch A., Abian J., Carrascal M., Sánchez M., Nombela C., Gil C. (2004) Proteomics-based identification of novel Candida albicans antigens for diagnosis of systemic candidiasis in patients with underlying hematological malignancies. Proteomics 4, 3084–3106 [DOI] [PubMed] [Google Scholar]
- 23. Pitarch A., Jiménez A., Nombela C., Gil C. (2008) Serological proteome analysis to identify systemic candidiasis patients in the intensive care unit: Analytical, diagnostic and prognostic validation of anti-Candida enolase antibodies on quantitative clinical platforms. Proteomics Clin. Appl. 2, 596–618 [DOI] [PubMed] [Google Scholar]
- 24. Le Naour F., Brichory F., Misek D. E., Bréchot C., Hanash S. M., Beretta L. (2002) A distinct repertoire of autoantibodies in hepatocellular carcinoma identified by proteomic analysis. Mol. Cell. Proteomics 1, 197–203 [DOI] [PubMed] [Google Scholar]
- 25. Fathman C. G., Soares L., Chan S. M., Utz P. J. (2005) An array of possibilities for the study of autoimmunity. Nature 435, 605–611 [DOI] [PubMed] [Google Scholar]
- 26. Nabeta M., Abe Y., Kagawa L., Haraguchi R., Kito K., Ueda N., Sugita A., Yokoyama M., Kusanagi Y., Ito M. (2009) Identification of anti-α-enolase autoantibody as a novel serum marker for endometriosis. Proteomics Clin. Appl. 3, 1201–1210 [DOI] [PubMed] [Google Scholar]
- 27. Nakamura M., Kuramasu A., Nakashima I., Fujihara K., Itoyama Y. (2007) Candidate antigens specifically detected by cerebrospinal fluid-IgG in oligoclonal IgG bands-positive multiple sclerosis patients. Proteomics Clin. Appl. 1, 681–687 [DOI] [PubMed] [Google Scholar]
- 28. Chiu L. L., Lee K. L., Lin Y. F., Chu C. Y., Su S. N., Chow L. P. (2008) Secretome analysis of novel IgE-binding proteins from Penicillium citrinum. Proteomics Clin. Appl. 2, 33–45 [DOI] [PubMed] [Google Scholar]
- 29. Alvarez-Garcia G., Pitarch A., Zaballos A., Fernández-García A., Gil C., Gómez-Bautista M., Aguado-Martínez A., Ortega-Mora L. M. (2007) The NcGRA7 gene encodes the immunodominant 17 kDa antigen of Neospora caninum. Parasitology 134, 41–50 [DOI] [PubMed] [Google Scholar]
- 30. Dea-Ayuela M. A., Ubeira F. M., Pitarch A., Gil C., Martinez-Fernández A. R., Bolás F. (2001) A comparison of antigenic peptides in muscle larvae of several Trichinella species by two-dimensional western-blot analysis with monoclonal antibodies. Parasite 8, S117–S119 [DOI] [PubMed] [Google Scholar]
- 31. Wang X., Yu J., Sreekumar A., Varambally S., Shen R., Giacherio D., Mehra R., Montie J. E., Pienta K. J., Sanda M. G., Kantoff P. W., Rubin M. A., Wei J. T., Ghosh D., Chinnaiyan A. M. (2005) Autoantibody signatures in prostate cancer. N. Engl. J. Med. 353, 1224–1235 [DOI] [PubMed] [Google Scholar]
- 32. van't Veer L. J., Bernards R. (2008) Enabling personalized cancer medicine through analysis of gene-expression patterns. Nature 452, 564–570 [DOI] [PubMed] [Google Scholar]
- 33. Haoudi A., Bensmail H. (2006) Bioinformatics and data mining in proteomics. Expert. Rev. Proteomics 3, 333–343 [DOI] [PubMed] [Google Scholar]
- 34. Zolg J. W., Langen H. (2004) How industry is approaching the search for new diagnostic markers and biomarkers. Mol. Cell Proteomics 3, 345–354 [DOI] [PubMed] [Google Scholar]
- 35. Schrohl A. S., Holten-Andersen M., Sweep F., Schmitt M., Harbeck N., Foekens J., Brünner N. (2003) Tumor markers: from laboratory to clinical utility. Mol. Cell Proteomics 2, 378–387 [DOI] [PubMed] [Google Scholar]
- 36. García-Ruiz J. C., del Carmen Arilla M., Regúlez P., Quindós G., Alvarez A., Pontón J. (1997) Detection of antibodies to Candida albicans germ tubes for diagnosis and therapeutic monitoring of invasive candidiasis in patients with hematologic malignancies. J. Clin. Microbiol. 35, 3284–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mischak H., Apweiler R., Banks R. E., Conaway M., Coon J., Dominiczak A., Ehrich J. H. H., Fliser D., Girolami M., Hermjakob H., Hochstrasser D., Jankowski J., Julian B. A., Kolch W., Massy Z. A., Neusuess C., Novak J., Peter K., Rossing K., Schanstra J., Semmes O. J., Theodorescu D., Thongboonkerd V., Weissinger E. M., Van Eyk J. E., Yamamoto T. (2007) Clinical proteomics: A need to define the field and to begin to set adequate standards. Proteomics Clin. Appl. 1, 148–156 [DOI] [PubMed] [Google Scholar]
- 38. Bruns D. E., Huth E. J., Magid E., Young D. S. (2000) Toward a checklist for reporting of studies of diagnostic accuracy of medical tests. Clin. Chem. 46, 893–895 [PubMed] [Google Scholar]
- 39. Pitarch A., Pardo M., Jiménez A., Pla J., Gil C., Sánchez M., Nombela C. (1999) Two-dimensional gel electrophoresis as analytical tool for identifying Candida albicans immunogenic proteins. Electrophoresis 20, 1001–1010 [DOI] [PubMed] [Google Scholar]
- 40. Pitarch A., Nombela C., Gil C. (2009) Proteomic profiling of serologic response to Candida albicans during host-commensal and host-pathogen interactions. Methods Mol. Biol. 470, 369–411 [DOI] [PubMed] [Google Scholar]
- 41. Valdes I., Pitarch A., Gil C., Bermúdez A., Llorente M., Nombela C., Méndez E. (2000) Novel procedure for the identification of proteins by mass fingerprinting combining two-dimensional electrophoresis with fluorescent SYPRO red staining. J. Mass Spectrom. 35, 672–682 [DOI] [PubMed] [Google Scholar]
- 42. Pitarch A., Sánchez M., Nombela C., Gil C. (2002) Sequential fractionation and two-dimensional gel analysis unravels the complexity of the dimorphic fungus Candida albicans cell wall proteome. Mol. Cell Proteomics 1, 967–982 [DOI] [PubMed] [Google Scholar]
- 43. Shevchenko A., Wilm M., Vorm O., Mann M. (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 44. Pardo M., Ward M., Pitarch A., Sánchez M., Nombela C., Blackstock W., Gil C. (2000) Cross-species identification of novel Candida albicans immunogenic proteins by combination of two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Electrophoresis 21, 2651–2659 [DOI] [PubMed] [Google Scholar]
- 45. Pitarch A., Sánchez M., Nombela C., Gil C. (2003) Analysis of the Candida albicans proteome II. Protein information technology on the Net (update 2002). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 787, 129–148 [DOI] [PubMed] [Google Scholar]
- 46. Pitarch A., Molero G., Moteoliva L., Thomas D. P., López-Ribot J. L., Nombela C., Gil C. (2007) Proteomics in Candida species. In Candida: Comparative and Functional Genomics (d'Enfert C., Hube B. eds), pp. 169–194, Caister Academic Press, Norfolk, UK [Google Scholar]
- 47. Pitarch A., Nombela C., Gil C. (2009) Identification of the Candida albicans immunome during systemic infection by mass spectrometry. Methods Mol. Biol. 470, 187–235 [DOI] [PubMed] [Google Scholar]
- 48. Carmona-Saez P., Chagoyen M., Tirado F., Carazo J. M., Pascual-Montano A. (2007) GENECODIS: a web-based tool for finding significant concurrent annotations in gene lists. Genome Biol. 8, R3.1–R3.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eisen M. B., Spellman P. T., Brown P. O., Botstein D. (1998) Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U.S.A. 95, 14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kluger Y., Tuck D. P., Chang J. T., Nakayama Y., Poddar R., Kohya N., Lian Z., Ben Nasr A., Halaban H. R., Krause D. S., Zhang X., Newburger P. E., Weissman S. M. (2004) Lineage specificity of gene expression patterns. Proc. Natl. Acad. Sci. U.S.A. 101, 6508–6513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hanley J. A., McNeil B. J. (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148, 839–843 [DOI] [PubMed] [Google Scholar]
- 52. Ransohoff D. F. (2005) Lessons from controversy: ovarian cancer screening and serum proteomics. J. Natl. Cancer Inst. 97, 315–319 [DOI] [PubMed] [Google Scholar]
- 53. Casadevall A. (1995) Antibody immunity and invasive fungal infections. Infect. Immun. 63, 4211–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Polonelli L., Casadevall A., Han Y., Bernardis F., Kirkland T. N., Matthews R. C., Adriani D., Boccanera M., Burnie J. P., Cassone A., Conti S., Cutler J. E., Frazzi R., Gregory C., Hodgetts S., Illidge C., Magliani W., Rigg G., Santoni G. (2000) The efficacy of acquired humoral and cellular immunity in the prevention and therapy of experimental fungal infections. Med. Mycol. 38, 281–292 [PubMed] [Google Scholar]
- 55. Cutler J. E. (2005) Defining criteria for anti-mannan antibodies to protect against candidiasis. Curr. Mol. Med. 5, 383–392 [DOI] [PubMed] [Google Scholar]
- 56. Raska M., Belakova J., Wudattu N. K., Kafkova L., Ruzickova K., Sebestova M., Kolar Z., Weigl E. (2005) Comparison of protective effect of protein and DNA vaccines hsp90 in murine model of systemic candidiasis. Folia Microbiol. 50, 77–82 [DOI] [PubMed] [Google Scholar]
- 57. Wang G., Sun M., Fang J., Yang Q., Tong H., Wang L. (2006) Protective immune responses against systemic candidiasis mediated by phage-displayed specific epitope of Candida albicans heat shock protein 90 in C57BL/6J mice. Vaccine 24, 6065–6073 [DOI] [PubMed] [Google Scholar]
- 58. Pachl J., Svoboda P., Jacobs F., Vandewoude K., van der Hoven B., Spronk P., Masterson G., Malbrain M., Aoun M., Garbino J., Takala J., Drgona L., Burnie J., Matthews R. (2006) A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin. Infect. Dis. 42, 1404–1413 [DOI] [PubMed] [Google Scholar]
- 59. Montagnoli C., Sandini S., Bacci A., Romani L., La Valle R. (2004) Immunogenicity and protective effect of recombinant enolase of Candida albicans in a murine model of systemic candidiasis. Med. Mycol. 42, 319–324 [DOI] [PubMed] [Google Scholar]
- 60. van Deventer H. J., Goessens W. H., van Vliet A. J., Verbrugh H. A. (1996) Anti-enolase antibodies partially protective against systemic candidiasis in mice. Clin. Microbiol. Infect. 2, 36–43 [DOI] [PubMed] [Google Scholar]
- 61. Pitarch A., Nombela C., Gil C. (2007) Reliability of antibodies to Candida methionine synthase for diagnosis, prognosis and risk stratification in systemic candidiasis: A generic strategy for the prototype development phase of proteomic markers. Proteomics Clin. Appl. 1, 1221–1242 [DOI] [PubMed] [Google Scholar]
- 62. Xin H., Dziadek S., Bundle D. R., Cutler J. E. (2008) Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc. Natl. Acad. Sci. U.S.A. 105, 13526–13531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gil M. L., Dagan S., Eren R., Gozalbo D. (2006) Evaluation of the usefulness of anti-glyceraldehyde-3-phosphate dehydrogenase antibodies as a treatment for invasive candidiasis in a murine model. Antonie Van Leeuwenhoek 89, 345–350 [DOI] [PubMed] [Google Scholar]
- 64. Chaffin W. L. (2008) Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 72, 495–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bromuro C., La Valle R., Sandini S., Urbani F., Ausiello C. M., Morelli L., Fé, d'Ostiani C., Romani L., Cassone A. (1998) A 70-kilodalton recombinant heat shock protein of Candida albicans is highly immunogenic and enhances systemic murine candidiasis. Infect. Immun. 66, 2154–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pitarch A., Nombela C., Gil C. (2008) Cell wall fractionation for yeast and fungal proteomics. Methods Mol. Biol. 425, 217–239 [DOI] [PubMed] [Google Scholar]
- 67. Pitarch A., Nombela C., Gil C. (2008) Collection of proteins secreted from yeast protoplasts in active cell wall regeneration. Methods Mol. Biol. 425, 241–263 [DOI] [PubMed] [Google Scholar]
- 68. Leng S. X., McElhaney J. E., Walston J. D., Xie D., Fedarko N. S., Kuchel G. A. (2008) ELISA and multiplex technologies for cytokine measurement in inflamation and aging research. J. Gerontol. A Biol. Sci. Med. Sci. 63, 879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Crowe J. D., Sievwright I. K., Auld G. C., Moore N. R., Gow N. A., Booth N. A. (2003) Candida albicans binds human plasminogen: identification of eight plasminogen-binding proteins. Mol. Microbiol. 47, 1637–1651 [DOI] [PubMed] [Google Scholar]
- 70. Jong A. Y., Chen S. H., Stins M. F., Kim K. S., Tuan T. L., Huang S. H. (2003) Binding of Candida albicans enolase to plasmin(ogen) results in enhanced invasion of human brain microvascular endothelial cells. J. Med. Microbiol. 52, 615–622 [DOI] [PubMed] [Google Scholar]
- 71. Pitarch A., Nombela C., Gil C. (2006) Candida albicans biology and pathogenicity: insights from proteomics. Methods Biochem. Anal. 49, 285–330 [PubMed] [Google Scholar]
- 72. Poltermann S., Kunert A., von der Heide M., Eck R., Hartmann A., Zipfel P. F. (2007) Gpm1p is a factor H-, FHL-1-, and plasminogen-binding surface protein of Candida albicans. J. Biol. Chem. 282, 37537–37544 [DOI] [PubMed] [Google Scholar]
- 73. Zhang X., Essmann M., Burt E. T., Larsen B. (2000) Estrogen effects on Candida albicans: a potential virulence-regulating mechanism. J. Infect. Dis. 181, 1441–1446 [DOI] [PubMed] [Google Scholar]
- 74. Burt E. T., O'Connor C., Larsen B. (1999) Isolation and identification of a 92-kDa stress induced protein from Candida albicans. Mycopathologia 147, 13–20 [DOI] [PubMed] [Google Scholar]
- 75. Urban C., Xiong X., Sohn K., Schröppel K., Brunner H., Rupp S. (2005) The moonlighting protein Tsa1p is implicated in oxidative stress response and in cell wall biogenesis in Candida albicans. Mol. Microbiol. 57, 1318–1341 [DOI] [PubMed] [Google Scholar]
- 76. Kim J. S., Ahn K. J., Kim J. A., Kim H. M., Lee J. D., Lee J. M., Kim S. J., Park J. H. (2008) Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells: ROS-mediated cell death by 3-BrPA. J. Bioenerg. Biomembr. 40, 607–618 [DOI] [PubMed] [Google Scholar]
- 77. Polier S., Dragovic Z., Hartl F. U., Bracher A. (2008) Structural basis for the cooperation of Hsp70 and Hsp110 chaperone in protein folding. Cell 133, 1068–1079 [DOI] [PubMed] [Google Scholar]
- 78. Suliman H. S., Appling D. R., Robertus J. D. (2007) The gene for cobalamin-independent methionine synthase is essential in Candida albicans: a potential antifungal target. Arch. Biochem. Biophys. 467, 218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hodgetts S., Matthews R., Morrissey G., Mitsutake K., Piper P., Burnie J. (1996) Over-expression of Saccharomyces cerevisiae hsp90 enhances the virulence of this yeast in mice. FEMS Immunol. Med. Microbiol. 16, 229–234 [DOI] [PubMed] [Google Scholar]
- 80. García-Cardeña G., Fan R., Shah V., Sorrentino R., Cirino G., Papapetropoulos A., Sessa W. C. (1998) Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392, 821–824 [DOI] [PubMed] [Google Scholar]
- 81. White S., Larsen B. (1997) Candida albicans morphogenesis is influenced by estrogen. Cell Mol. Life Sci. 53, 744–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tang C., Zhang Z., Xu B., Li M., Liu J., Cui J. (2008) Two newly synthesized 5-methyltetrahydrofolate-like compounds inhibit methionine synthase activity accompanied by cell cycle arrest in G1/S phase and apoptosis in vitro. Anticancer Drugs 19, 697–704 [DOI] [PubMed] [Google Scholar]
- 83. Schmitt E., Gehrmann M., Brunet M., Multhoff G., Garrido C. (2007) Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J. Leukocyte Biol. 81, 15–27 [DOI] [PubMed] [Google Scholar]
- 84. Mazzoni C., Torella M., Petrera A., Palermo V., Falcone C. (2009) PGK1, the gene encoding the glycolytic enzyme phosphoglycerate kinase, acts as a multicopy suppressor of apoptotic phenotypes in Saccharomyces cerevisiae. Yeast 26, 31–37 [DOI] [PubMed] [Google Scholar]
- 85. Lee K. W., Thakur A., Karim A. M., LoVerde P. T. (1995) Immune response to Schistosoma mansoni phosphoglycerate kinase during natural and experimental infection: identification of a schistosome-specific B-cell epitope. Infect. Immun. 63, 4307–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Trant C. G., Lacerda T. L., Carvalho N. B., Azevedo V., Rosinha G. M., Salcedo S. P., Gorvel J. P., Oliveira S. C. (2010) Brucella abortus phosphoglycerate kinase (pgk) mutant is highly attenuated and induces superior protection than vaccine strain 19 in immune compromised and immune competent mice. Infect. Immun. 78, 2283–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pitarch A., Díez-Orejas R., Molero G., Pardo M., Sánchez M., Gil C., Nombela C. (2001) Analysis of the serologic response to systemic Candida albicans infection in a murine model. Proteomics 1, 550–559 [DOI] [PubMed] [Google Scholar]
- 88. Almeida B., Buttner S., Ohlmeier S., Silva A., Mesquita A., Sampaio-Marques B., Osório N. S., Kollau A., Mayer B., Leão C., Laranjinha J., Rodrigues F., Madeo F., Ludovico P. (2007) NO-mediated apoptosis in yeast. J. Cell Sci. 120, 3279–3288 [DOI] [PubMed] [Google Scholar]
- 89. Magherini F., Tani C., Gamberi T., Caselli A., Bianchi L., Bini L., Modesti A. (2007) Protein expression profiles in Saccharomyces cerevisiae during apoptosis induced by H2O2. Proteomics 7, 1434–1445 [DOI] [PubMed] [Google Scholar]
- 90. Gross C., Koelch W., DeMaio A., Arispe N., Multhoff G. (2003) Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J. Biol. Chem. 278, 41173–41181 [DOI] [PubMed] [Google Scholar]
- 91. Gil-Navarro I., Gil M. L., Casanova M., O'Connor J., Martinez J. P., Gozalbo D. (1997) The glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase of Candida albicans is a surface antigen. J. Bacteriol. 179, 4992–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hara M. R., Snyder S. H. (2006) Nitric oxide-GAPDH-Siah: a novel cell death cascade. Cell Mol. Neurobiol. 26, 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gastpar R., Gehrmann M., Bausero M. A., Asea A., Gross C., Schroeder J. A., Multhoff G. (2005) Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 65, 5238–5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vylkova S., Li X. S., Berner J. C., Edgerton M. (2006) Distinct antifungal mechanisms: β-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob. Agents Ch. 50, 324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li X. S., Sun J. N., Okamoto-Shibayama K., Edgerton M. (2006) Candida albicans cell wall Ssa proteins bind and facilitate import of salivary histatin 5 required for toxicity. J. Biol. Chem. 281, 22453–22463 [DOI] [PubMed] [Google Scholar]