Abstract
Over a half of all proteins are glycosylated, and their proper glycosylation is essential for normal function. Unfortunately, because of structural complexity of nonlinear branched glycans and the absence of genetic template for their synthesis, the knowledge about glycans is lagging significantly behind the knowledge about proteins or DNA. Using a recently developed quantitative high throughput glycan analysis method we quantified components of the plasma N-glycome in 99 children with attention-deficit hyperactivity disorder (ADHD), 81 child and 5 adults with autism spectrum disorder, and a total of 340 matching healthy controls. No changes in plasma glycome were found to associate with autism spectrum disorder, but several highly significant associations were observed with ADHD. Further structural analysis of plasma glycans revealed that ADHD is associated with increased antennary fucosylation of biantennary glycans and decreased levels of some complex glycans with three or four antennas. The design of this study prevented any functional conclusions about the observed associations, but specific differences in glycosylation appears to be strongly associated with ADHD and warrants further studies in this direction.
Attention-deficit hyperactivity disorder (ADHD)1 and autism spectrum disorders (ASDs) are both highly heritable multifactorial and clinically heterogeneous childhood-onset neuro-developmental disorders (1, 2). The hallmarks of ADHD are severe inattention, hyperactivity, and impulsivity whereas the characteristic symptoms of ASD include impaired communication and social interaction skills as well as repetitive and restricted behavior and interests (3). Several pieces of evidence point toward some shared heritability and thus genetic factors contributing to the occurrence of both ADHD an ASD (4). Children with ADHD and ASD frequently share symptoms of impaired communication skills, impaired social interactions (4), and develop difficulties in familial, educational, and academic functioning (5, 6). The abnormal functioning of the dopaminergic and serotonergic systems (5, 7–9), among other neurotransmitters, were repeatedly found in ADHD and ASD, and therefore the candidate genes common for both disorders are the dopaminergic transporter gene, dopaminergic receptor type 3 and type 4 genes, serotonin transporter gene, and genes for catechol-o-methyl-transferase and monoamine oxidase type A (4), although genome-wide association studies did not confirm with certainty all these candidates (10). A shared genetic basis is further suggested by the higher frequency of ADHD and ASD traits in children with a defined genetic diagnosis such as 22q11 deletion syndrome, XXYY syndrome, and microdeletions or duplications in the chromosome 15q13.2-q13.3 region (11, 12). However, the molecular agents related to various biological processes and interactions between different risk factors responsible for development of ADHD and ASD remain elusive (6).
Protein N-glycosylation is an ancient metabolic pathway invented before the diversification of Archea, Bacteria, and Eukarya, which still exists in all three domains of life (13, 14). At least 2000 different glycan determinants have been added to the backbone of mammalian polypeptides (15) and the complexity of the glycoproteome is estimated to be several orders of magnitude greater than for the proteome itself (16). N-glycosylation is essential for multicellular life and its complete absence is embryonically lethal (17). Disregulation of glycosylation is associated with a wide range of diseases, including cancer, diabetes, and cardiovascular, congenital, immunological, and infectious disorders (18–20), but because of experimental difficulties in analyzing complex glycan structures, the knowledge about glycan structure and function is still lagging behind the knowledge about other macromolecules (21). However, recent technological advances have allowed reliable, high-throughput quantification of N-glycans (22–24), which now permit large scale studies aimed at understanding of the role of protein glycosylation in complex diseases.
Variations in glycosylation are of great physiological significance because changes in glycans significantly change the structure and function of polypeptide parts of glycoproteins (25). Proper glycosylation of membrane receptors is particularly important as it modulates adaptive properties of the cell and affects communication between cells (26). Altered glycosylation and ensuing differences in function of various receptors could be a predisposing factor in different psychiatric disorders, and indeed some of them were reported to be associated with altered protein glycosylation (27–31). Here we report the first analysis of plasma N-glycans in patients with ADHD and ASD.
EXPERIMENTAL PROCEDURES
Sample Population and Study Design
The study included 99 medication-free children with ADHD (9.00 ± 2.78 years old), recruited sequentially from the Polyclinic Kocijan/Hercigonja, Zagreb, diagnosed using psychiatric interview (APA, 1994), according to the DSM-IV criteria, separate interviews with the children and their parents, psychological interview and psychological tests, physical examination, and the Conners' Rating Scale for Parents-short version (32). Demographical data were uniform for all children: they were Caucasians of urban origin, attended kindergarten or primary school, and were coming from the undisrupted families, with average intellectual abilities. Exclusion criteria were comorbid depression, schizophrenia, oppositional defiant disorder, conduct disorder, borderline personality disorder and somatic disorders, all assessed by psychiatric interview based on DSM-IV criteria (APA 1994), or pharmacotherapy with carbamazepine, clomipramine, lamotrigine or selective serotonin reuptake inhibitors.
Control group included 175 healthy children (10.94 ± 4.01), attending their regular physical exams, served as controls. Somatic or other psychiatric comorbidities were excluded according to DSM-IV criteria and the Child Symptoms Inventory-4 (APA 1994). All parents signed written informed consent.
Sixty families with one or more children with ASD were included. These families were selected from the Autism Family Study by the Leuven Autism Research consortium (LAuRes). All patients have a clinical diagnosis of ASD according to DSM-IV criteria. All diagnoses were made by a child psychiatrist and a multidisciplinary team in the Expert Center for Autism in Leuven. All patients, siblings, and parents have been examined by a clinical geneticist and patients with known underlying genetic conditions were excluded. The criterion for inclusion in this study was normal intelligence of all family members. All family members have a normal karyotype and FMR1 mutations and premutations were excluded. In total the sample includes 251 individuals (81 children and 5 adults with ASD and 165 individuals without ASD).
Blood samples (4 ml) were taken during routine laboratory tests from a forearm vein into a plastic syringe with 1 ml of acid citrate dextrose anticoagulant at 08.00 a.m. following overnight fasting.
Local ethics committees approved all protocols. The study was carried out in accordance with the Helsinki declaration, and the samples were coded in sequential way without recording any personal information about the patients.
Glycan Release and Labeling
Glycan release and labeling was performed as reported previously (33). Plasma proteins were immobilized in a block of SDS-polyacrylamide gel and N-glycans were released by digestion with recombinant N-glycosidase F. This was done in a 96-well microtitre plate to achieve the best throughput of sample preparation. Following extraction, glycans were fluorescently labeled with 2-aminobenzamide.
Hydrophilic Interaction High Performance Liquid Chromatography (HILIC)
Released glycans were subjected to hydrophilic interaction high performance liquid chromatography (HILIC) on a 250 × 4.6 mm i.d. 5-μm particle packed TSK gel Amide 80 column (Tosoh Bioscience, Stuttgart, Germany) at 30 °C with 50 mm formic acid adjusted to pH 4.4 with ammonia solution as solvent A and acetonitrile as solvent B. Sixty-minute runs were performed with fluorescence detector set with excitation and emission wavelengths of 330 and 420 nm, respectively. The system was calibrated using an external standard of hydrolyzed and 2-AB-labeled glucose oligomers from which the retention times for the individual glycans were converted to glucose units.
The chromatograms obtained were all separated in the same manner into 16 chromatographic areas (peaks) for initial profile and into 13 peeks for desialylated glycans, regarding the peak resolutions and similarity of glycan structures present as described before. The amounts of glycan present in each area were expressed as the percent of total integrated chromatogram (amount of total glycan structures and total serum N-glycome).
Weak Anion Exchange High Performance Liquid Chromatography (WAX-HPLC)
Glycans were separated according to the number of sialic acids by weak anion exchange HPLC. The analysis was performed using a Prozyme GlycoSep C 75 mm × 7.5 mm column (Prozyme, Leandro, CA) at 30 °C with 20% (v/v) acetonitrile in water as solvent A and 0.1 m acetic acid adjusted to pH 7.0 with ammonia solution in 20% (v/v) acetonitrile as solvent B. Compounds were retained on the column according to their charge density, the higher charged compounds being retained the longest. A fetuin N-glycan standard was used for calibration.
Sialidase Digestion
Aliquots of the 2AB-labeled glycan samples were dried down in 96-well PCR plates. To these, the following was added: 1 μl of 250 mm sodium phosphate incubation buffer (pH 6.0), 0.5 μl (0.0025 U) of ABS, Arthrobacter ureafaciens sialidase (releases α2–3,6,8 sialic acid, Prozyme) and H2O to make up to 5 μl. This was incubated overnight (16–18 h) at 37 °C and then passed through AcroPrep™ 96 Filter Plates, 350 μl well, 10K (Pall Corporation, Port Washington, NY) before applying to the HPLC.
Fucosidase Digestion
To examine differences in levels of structures present in peak DG7 between ADHD samples and controls, aliquots of the 2AB-labeled glycans were mixed together to make an ADHD glycan pool and a control glycan pool. These pooled samples were dried down and concentrated pool samples were applied on HILIC column. Peaks DG7 were collected from both glycan pools and dried down in PCR tubes before desired digestion. To these, the following was added: 1 μl of 250 mm sodium acetate incubation buffer (pH 5.0), 1 μl (0.003 mU) of AMF, Almond meal fucosidase (releases α1–3,4 fucose, Prozyme) and H2O to make up to 5 μl. This was incubated overnight (16–18 h) at 37 °C and then passed through AcroPrep™ 96 Filter Plates, 350 μl well, 10K (Pall Corporation, Port Washington, NY) before applying to the HPLC.
Glycan Structural Features
Levels of glycans sharing the same structural features were approximated by adding the structures having the same characteristic, from either HILIC, weak anion exchange, or following sialidase treatment integrated glycan profiles (individual glycan structures present in each glycan group that were reported previously are shown in Supplemental Table 1). Glycans were quantified from weak anion exchange profiles according to the level of sialylation (monosialylated, disialylated, trisialylated, and tetrasialylated). Glycan features were defined as: Core fucosylated glycans (FUC-C) = DG6/(DG5+DG6)*100; Antennary fucosylated glycans (FUC-A) = DG7/(DG5+DG7)*100; Biantennary glycans = DG1+DG2+DG3+DG4+DG5+DG6+DG7; Monosialylated biantennary glycans = (GP7+GP8)/(DG5+DG6+DG7)*100; Disialylated biantennary glycans BADS = (GP9+GP10+GP11)/(DG5+DG6+DG7)*100; Triantennary glycans = DG8+DG9+DG10; Tetraantennary glycans = DG11+DG12+DG13; Nongalactosylated glycans (G0) = DG1+DG2; Monogalactosylated glycans (G1) = DG3+DG4; Digalactosylated glycans (G2) = DG5+DG6+DG7; Trigalactosylated glycans (G3) = GP12+GP13+GP14; Tetragalactosylated glycans (G4) = GP15+GP16, Biantennary nongalactosylated glycan (A2) = (GP1+DG1)/2.
Statistical Analysis
Associations between ADHD and N-glycans were analyzed using a standard linear fixed effects model (i.e. linear regression) with N-glycans as the criterion and ADHD as the predictor. Additionally, sex and age were used as covariates. The analysis was performed using the lm() and anova() functions of the statistical analysis system R (Version 2.7.2) (34).
Associations between ASD and N-glycans were calculated using a linear mixed effects model with N-glycans as the criterion and ASD as the predictor (fixed effect). Additionally, sex and age were used as covariates (both fixed effects). The pedigree structure (i.e. father, mother, child) was modeled as a random effect. We used a linear mixed effects model because a standard linear fixed effects model is not adequate for this data structure and will give progressive results, i.e. to low p values. The sample is clustered in families, and therefore each individual of two related individuals contains less “information” than a sample consisting of two unrelated individuals. The analysis was performed using the statistical analysis program SOLAR (http://solar.sfbrgenetics.org/) (35).
Multiple tests on associations between mental disorders and plasma N-glycans were performed. To account for false positive results because of α inflation, we performed a Bonferroni adjustment per disorder. Therefore, we used an α error threshold of 0.05/46 = 0.001. Because we are aware of the fact that this method is conservative if applied to correlated traits such as plasma N-glycans, as the method is based on the assumption of independent traits, we have taken this into account in the discussion.
RESULTS
Using a recently developed method for high throughput quantitative analysis of glycans we analyzed the plasma glycome in 99 medication-free children with ADHD (9.00 ± 2.78 years old) and 175 healthy control children (10.94 ± 4.01 years old). Three HPLC analyses were performed for each sample resulting in 33 directly quantified groups of glycans. The exact composition of the each glycan group is shown in Supplemental Table 1. Further 13 glycosylation features were derived from the measured values as described in the Materials and Methods section, resulting in the total number of 46 analyzed glycan variables. Basic descriptive parameters for these 46 glycan features are shown in Table I.
Table I. Descriptive parameters of plasma glycome in children with ADHD and controls. Glycan structures present in each glycan peaks (GP1–16, DG1–13) are shown in Supplementary Table I, and the way other glycans traits were calculated is described in the Materials and Methods section. Associations between ADHD and N-glycans were analyzed using a standard linear fixed effects model (i.e. linear regression) with N-glycans as the criterion and ADHD as the predictor. Additionally, sex and age were used as covariates. Associations with statistical significance below the pre-set level of p < 0.001 are shown in bold.
| ADHD |
Controls |
Linear regression with ADHD as a predictor |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | Percentiles |
N | Median | Percentiles |
|||||
| 25th | 75th | 25th | 75th | |||||||
| Age | 99 | 9.00 | 7.00 | 11.00 | 173 | 11.00 | 6.00 | 15.00 | ||
| Glycan feature | P | β | ||||||||
| GP1 | 96 | 0.07 | 0.05 | 0.09 | 175 | 0.06 | 0.04 | 0.08 | 4.04E-01 | 0.12 |
| GP2 | 96 | 3.39 | 2.71 | 4.02 | 175 | 3.17 | 2.57 | 3.79 | 7.15E-01 | −0.05 |
| GP3 | 96 | 1.66 | 1.52 | 1.86 | 175 | 1.65 | 1.47 | 1.84 | 9.69E-01 | 0.01 |
| GP4 | 96 | 5.65 | 4.84 | 6.53 | 175 | 5.70 | 4.84 | 6.22 | 9.92E-01 | 0.00 |
| GP5 | 96 | 2.21 | 2.01 | 2.41 | 175 | 2.12 | 1.92 | 2.39 | 7.91E-03 | 0.37 |
| GP6 | 96 | 4.47 | 3.86 | 5.05 | 175 | 4.28 | 3.83 | 4.80 | 3.80E-02 | 0.30 |
| GP7 | 96 | 8.97 | 8.48 | 9.57 | 175 | 8.82 | 8.34 | 9.60 | 1.76E-01 | 0.19 |
| GP8 | 96 | 10.32 | 9.72 | 10.84 | 175 | 10.29 | 9.57 | 10.98 | 3.98E-01 | −0.12 |
| GP9 | 96 | 39.80 | 37.74 | 41.85 | 175 | 39.28 | 37.83 | 41.58 | 3.33E-01 | 0.13 |
| GP10 | 96 | 6.06 | 5.54 | 6.69 | 175 | 6.48 | 5.90 | 7.01 | 3.18E-01 | −0.14 |
| GP11 | 96 | 1.45 | 1.33 | 1.56 | 175 | 1.29 | 1.08 | 1.41 | 1.22E-13 | 0.99 |
| GP12 | 96 | 1.70 | 1.48 | 2.01 | 175 | 1.77 | 1.53 | 2.17 | 2.84E-06 | −0.53 |
| GP13 | 96 | 6.02 | 5.06 | 6.95 | 175 | 5.98 | 5.03 | 6.72 | 6.05E-01 | −0.07 |
| GP14 | 96 | 5.27 | 4.53 | 6.24 | 175 | 6.04 | 5.21 | 6.87 | 2.44E-03 | −0.42 |
| GP15 | 96 | 0.84 | 0.69 | 1.03 | 175 | 0.76 | 0.59 | 0.95 | 7.94E-01 | 0.03 |
| GP16 | 96 | 1.49 | 1.29 | 1.75 | 175 | 1.51 | 1.25 | 1.89 | 2.36E-03 | −0.38 |
| DG1 | 96 | 0.08 | 0.07 | 0.11 | 175 | 0.09 | 0.07 | 0.11 | 3.08E-01 | −0.14 |
| DG2 | 96 | 3.67 | 2.94 | 4.20 | 175 | 3.36 | 2.76 | 3.96 | 8.58E-01 | 0.02 |
| DG3 | 96 | 2.32 | 2.11 | 2.58 | 175 | 2.29 | 2.07 | 2.53 | 4.62E-01 | 0.10 |
| DG4 | 96 | 6.73 | 5.84 | 7.71 | 175 | 6.72 | 5.86 | 7.37 | 9.25E-01 | 0.01 |
| DG5 | 96 | 50.54 | 48.60 | 52.95 | 175 | 50.68 | 48.22 | 53.20 | 1.66E-01 | 0.19 |
| DG6 | 96 | 14.54 | 12.70 | 16.03 | 175 | 14.73 | 13.28 | 16.52 | 2.41E-01 | −0.17 |
| DG7 | 96 | 1.54 | 1.31 | 1.87 | 175 | 1.39 | 1.16 | 1.65 | 1.02E-04 | 0.53 |
| DG8 | 96 | 11.65 | 10.25 | 13.08 | 175 | 11.69 | 10.53 | 13.08 | 2.80E-01 | −0.15 |
| DG9 | 96 | 2.80 | 2.32 | 3.64 | 175 | 3.33 | 2.58 | 4.13 | 1.29E-02 | −0.35 |
| DG10 | 96 | 0.98 | 0.89 | 1.06 | 175 | 0.93 | 0.85 | 1.02 | 1.63E-02 | 0.34 |
| DG11 | 96 | 2.83 | 2.49 | 3.43 | 175 | 2.72 | 2.32 | 3.30 | 7.92E-01 | −0.03 |
| DG12 | 96 | 0.80 | 0.61 | 1.01 | 175 | 0.83 | 0.65 | 1.03 | 1.90E-01 | −0.19 |
| DG13 | 96 | 0.72 | 0.54 | 0.80 | 175 | 0.63 | 0.50 | 0.75 | 2.40E-01 | 0.16 |
| Monosialo | 88 | 21.68 | 20.40 | 22.76 | 175 | 21.59 | 20.06 | 23.41 | 7.31E-01 | 0.05 |
| Disialo | 88 | 60.77 | 59.22 | 61.92 | 175 | 59.54 | 58.27 | 61.26 | 3.45E-02 | 0.27 |
| Trisialo | 88 | 15.07 | 13.42 | 16.71 | 175 | 15.48 | 14.07 | 17.00 | 6.95E-02 | −0.25 |
| Tetrasialo | 88 | 2.72 | 2.34 | 3.13 | 175 | 2.79 | 2.34 | 3.51 | 1.24E-03 | −0.40 |
| FUC-C | 96 | 22.38 | 19.14 | 24.59 | 175 | 22.41 | 20.15 | 24.98 | 2.01E-01 | −0.18 |
| FUC-A | 96 | 2.98 | 2.49 | 3.48 | 175 | 2.69 | 2.30 | 3.18 | 9.56E-04 | 0.46 |
| BAMS | 96 | 28.75 | 27.62 | 30.09 | 175 | 28.80 | 27.40 | 30.27 | 7.95E-01 | −0.04 |
| BADS | 96 | 70.63 | 68.65 | 72.71 | 175 | 70.46 | 68.65 | 72.48 | 5.18E-01 | 0.09 |
| BA | 96 | 80.00 | 78.19 | 81.71 | 175 | 79.60 | 77.91 | 81.37 | 4.07E-02 | 0.27 |
| TRIA | 96 | 15.46 | 14.25 | 16.91 | 175 | 16.12 | 14.93 | 17.21 | 1.52E-02 | −0.33 |
| TA | 96 | 4.37 | 3.99 | 5.05 | 175 | 4.23 | 3.59 | 5.01 | 5.93E-01 | −0.07 |
| G0 | 96 | 3.75 | 3.00 | 4.28 | 175 | 3.43 | 2.86 | 4.07 | 8.89E-01 | 0.02 |
| G1 | 96 | 9.09 | 8.20 | 10.01 | 175 | 9.00 | 8.19 | 9.73 | 8.59E-01 | 0.03 |
| G2 | 96 | 67.11 | 65.22 | 68.60 | 175 | 67.16 | 65.20 | 68.74 | 8.71E-02 | 0.22 |
| G3 | 96 | 13.17 | 11.58 | 14.70 | 175 | 13.86 | 12.54 | 15.31 | 1.21E-03 | −0.42 |
| G4 | 96 | 2.38 | 1.97 | 2.73 | 175 | 2.25 | 1.91 | 2.83 | 4.18E-02 | −0.25 |
| A2 | 96 | 0.08 | 0.06 | 0.11 | 175 | 0.07 | 0.06 | 0.10 | 8.73E-01 | 0.02 |
Some glycans significantly change with age (36), thus associations between ADHD and N-glycans were analyzed using a standard linear fixed effects model with N-glycans as the criterion, ADHD as the predictor, and sex and age as covariates. Following correction for multiple testing (0.05/46 = 0.001) four associations of glycan levels were found to be significant, whereas further five were suggestively close to statistical significance (Table I).
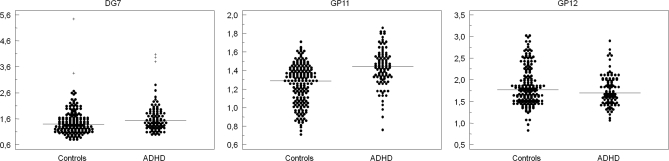
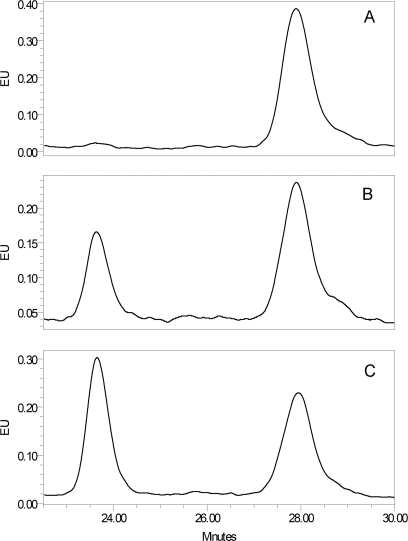
The most prominent changes in plasma glycans in ADHD were the increases in glycan groups GP11 and DG7 and a decrease in GP12 (Fig. 1). Examples of control and ADHD chromatograms are presented in the Supplemental Fig. 1. Although GP11 partly overlapped with GP10 and could not have been collected following chromatographic separation, the DG7 group was clearly separated from other peaks which enabled us to collect it for more detailed structural analysis. Main glycan structures present in GP7 are M7 (oligomannose glycan composed of two core N-acetylglucosamine and seven mannose residues) and A2FG2 (complex biantennary glycan with two core N-acetylglucosamine, three mannose, two antennary N-acetylglucosamine, one antennary fucose, and two antennary galactose residues). To determine which of the two structures is associated with ADHD, we pooled desialylated labeled glycans from 16 ADHD and 16 control samples, separated glycans by HILIC chromatography and collected eluates corresponding to DG7 peaks. These two eluates (ADHD and control) were then treated with an enzyme (α-1–3,4 almond meal fucosidase), which removes antennary fucose. Defucosylated ADHD and control glycan pools were then reanalyzed by HPLC to separate A2G2 (A2FG2 following the removal of fucose) from M7. Results presented in Fig. 2 clearly demonstrate that although M7 is the major component of DG7 in controls, A2FG2 is the major component of DG7 in ADHD samples, and that it is the increase in A2FG2 that associates DG7 with ADHD. Confirmation for this observation was provided by treating the same pair of samples with the enzyme α(1–2,3,6) Jack bean mannosidase that specifically cleaves mannose residues. This experiment (data not shown) showed that whereas in control samples the major glycan structure in DG7 is M7, in ADHD A2FG2 is increased and becomes the main glycan structure in DG7. This conclusion was further supported by digestion of the complete plasma glycome with α(1–2,3,6) Jack bean mannosidase, which confirmed that there are no changes in the proportion of oligomannose structures (including M7) in ADHD (Supplemental Fig. 2).
Fig. 1.
Levels of glycan groups DG7, GP11 and GP12 in ADHD and controls. Plasma glycome of ADHD patients and controls was separated into 13 desialylated chromatographic peeks (DG series) and 16 sialylated chromatographic peeks (GP series) and expressed as percentages of the total plasma glycome. The majority of peaks did not differ between groups, but statistically significant differences were observed for DG7, GP11 and GP12. Each individual is represented as a black dot and median level is marked with a horizontal line.
Fig. 2.
Structural analysis of glycans associated with ADHD. A, Glycan peak DG7 isolated by HPLC from the control glycan pool. This peak consists of two structures: A2FG2 and M7, which both elute at 26 min. B, Analysis of DG7 from control glycan pool following digestion with α(1–3,4) fucosidase that converts A2FG2 to A2G2. The new peak eluted at 23.75 min, contrary to M7 structure, which remained on the original of DG7 peak. C, Analysis of DG7 from ADHD glycan pool following digestion with α(1–3,4) fucosidase. Comparison of panels B and C clearly reveals that in ADHD there is an increase of the relative ratio of A2FG2 compared with M7.
The same type of glycan analyses were also performed on 81 autistic children and their 168 healthy siblings and parents. Basic descriptive parameters for the 46 measured glycan features are presented in Table II. Because this study sample was clustered in families, a linear mixed effects model was used to calculate statistical significance of the observed differences. No significant associations between glycan levels and ASD were observed.
Table II. Descriptive parameters of plasma glycome in children with ASD and controls. Glycan structures present in each glycan peaks (GP1–16, DG1–13) are shown in Supplementary Table I, and the way other glycans traits were calculated is described in the Materials and Methods section. Associations between ASD and N-glycans were calculated using a linear mixed effects model with N-glycans as the criterion and ASD as the predictor (fixed effect). Additionally, sex and age were used as covariates (both fixed effects).
| ASD |
Controls |
Linear regression with ASD as predictor | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median | Percentiles |
N | Median | Percentiles |
||||
| 25th | 75th | Valid | 25th | 75th | |||||
| Age | 78 | 11.00 | 9.00 | 14.00 | 48 | 12.50 | 9.25 | 15.75 | |
| Glycan feature | p | ||||||||
| GP1 | 78 | 0.06 | 0.05 | 0.07 | 48 | 0.05 | 0.04 | 0.07 | 5.03E-01 |
| GP2 | 78 | 2.93 | 2.53 | 3.43 | 48 | 2.55 | 2.09 | 3.19 | 3.76E-01 |
| GP3 | 78 | 1.66 | 1.55 | 1.84 | 48 | 1.61 | 1.40 | 1.82 | 6.39E-02 |
| GP4 | 78 | 5.50 | 4.89 | 5.95 | 48 | 5.21 | 4.55 | 6.03 | 7.81E-01 |
| GP5 | 78 | 2.34 | 2.20 | 2.49 | 48 | 2.29 | 2.13 | 2.52 | 6.90E-02 |
| GP6 | 78 | 4.16 | 3.77 | 4.48 | 48 | 4.27 | 3.74 | 4.80 | 7.22E-01 |
| GP7 | 78 | 9.65 | 9.14 | 10.35 | 48 | 9.57 | 9.03 | 10.36 | 6.87E-01 |
| GP8 | 78 | 10.53 | 9.85 | 11.08 | 48 | 10.78 | 10.01 | 11.24 | 8.88E-01 |
| GP9 | 78 | 40.28 | 38.37 | 41.58 | 48 | 40.38 | 38.25 | 41.64 | 9.87E-01 |
| GP10 | 78 | 6.62 | 5.81 | 7.25 | 48 | 6.58 | 6.04 | 7.02 | 5.86E-01 |
| GP11 | 78 | 1.62 | 1.48 | 1.76 | 48 | 1.59 | 1.41 | 1.74 | 1.00E+00 |
| GP12 | 78 | 1.56 | 1.42 | 1.66 | 48 | 1.56 | 1.42 | 1.71 | 8.71E-01 |
| GP13 | 78 | 5.52 | 4.97 | 6.14 | 48 | 5.76 | 5.01 | 6.62 | 6.22E-01 |
| GP14 | 78 | 5.38 | 4.63 | 6.47 | 48 | 5.56 | 4.93 | 6.08 | 6.79E-01 |
| GP15 | 78 | 0.76 | 0.60 | 0.87 | 48 | 0.77 | 0.65 | 0.91 | 6.05E-01 |
| GP16 | 78 | 1.30 | 1.16 | 1.47 | 48 | 1.27 | 1.12 | 1.49 | 5.41E-01 |
| DG1 | 78 | 0.07 | 0.05 | 0.10 | 48 | 0.06 | 0.04 | 0.09 | 9.72E-01 |
| DG2 | 78 | 3.53 | 3.08 | 4.20 | 48 | 3.25 | 2.76 | 3.69 | 7.49E-01 |
| DG3 | 78 | 2.50 | 2.28 | 2.71 | 48 | 2.44 | 2.09 | 2.69 | 1.06E-01 |
| DG4 | 78 | 6.48 | 5.80 | 6.99 | 48 | 6.27 | 5.21 | 6.91 | 7.64E-01 |
| DG5 | 78 | 51.71 | 50.20 | 53.22 | 48 | 51.52 | 50.04 | 53.05 | 9.60E-01 |
| DG6 | 78 | 14.80 | 13.75 | 16.06 | 48 | 14.88 | 13.84 | 15.95 | 7.76E-01 |
| DG7 | 78 | 1.50 | 1.37 | 1.71 | 48 | 1.54 | 1.37 | 1.73 | 3.38E-01 |
| DG8 | 78 | 10.74 | 9.93 | 11.95 | 48 | 11.09 | 10.12 | 12.33 | 7.17E-01 |
| DG9 | 78 | 3.03 | 2.37 | 3.94 | 48 | 3.32 | 2.69 | 3.75 | 3.84E-01 |
| DG10 | 78 | 0.98 | 0.90 | 1.06 | 48 | 0.98 | 0.92 | 1.03 | 4.64E-01 |
| DG11 | 78 | 2.65 | 2.29 | 2.93 | 48 | 2.59 | 2.36 | 2.98 | 9.46E-01 |
| DG12 | 78 | 0.76 | 0.61 | 0.96 | 48 | 0.79 | 0.69 | 0.96 | 4.89E-01 |
| DG13 | 78 | 0.75 | 0.62 | 0.91 | 48 | 0.77 | 0.66 | 0.93 | 9.63E-01 |
| Monosialo | 78 | 22.15 | 21.04 | 23.79 | 48 | 22.29 | 20.82 | 23.60 | 6.27E-01 |
| Disialo | 78 | 60.14 | 58.94 | 61.34 | 48 | 59.94 | 58.74 | 61.11 | 6.05E-01 |
| Trisialo | 78 | 13.55 | 12.64 | 15.06 | 48 | 13.72 | 12.66 | 16.07 | 6.78E-01 |
| Tetrasialo | 78 | 3.47 | 3.10 | 4.00 | 48 | 3.52 | 3.05 | 3.89 | 1.67E-01 |
| FUC-C | 78 | 22.27 | 20.45 | 23.94 | 48 | 22.56 | 20.82 | 24.05 | 8.52E-01 |
| FUC-A | 78 | 2.84 | 2.56 | 3.28 | 48 | 2.88 | 2.64 | 3.31 | 3.38E-01 |
| BAMS | 78 | 29.73 | 28.20 | 31.21 | 48 | 29.76 | 28.50 | 31.04 | 6.80E-01 |
| BADS | 78 | 70.78 | 69.21 | 72.46 | 48 | 71.41 | 68.68 | 72.38 | 5.99E-01 |
| BA | 78 | 80.60 | 79.29 | 82.17 | 48 | 80.73 | 78.23 | 81.73 | 4.32E-01 |
| TRIA | 78 | 15.18 | 13.93 | 16.28 | 48 | 15.17 | 14.40 | 16.96 | 3.02E-01 |
| TA | 78 | 4.16 | 3.72 | 4.65 | 48 | 4.27 | 3.85 | 4.66 | 9.95E-01 |
| A2 | 78 | 0.07 | 0.05 | 0.09 | 48 | 0.06 | 0.04 | 0.08 | 8.13E-01 |
DISCUSSION
We have performed the first analysis of human plasma N-glycome in patients with ADHD and ASD. Although we have found no differences between children with ASD and their healthy siblings, several significant associations between plasma glycans and ADHD were identified (Table I). By performing exoglycosidase sequencing, we have convincingly demonstrated that the observed association between ADHD and increased DG7 (and consequently also increased FUC-A, which is calculated from DG7) derived from the increase in relative levels of biantennary glycans with antennary fucose (A2FG2). In addition, ADHD was associated with a regular pattern of changes in GP11, GP12, GP14, GP16, tetrasialo-glycans, and trigalactosylated glycans. Two of these changes (GP11 and GP12) were statistically highly significant, whereas the remaining four were only slightly below the preset significance level of p < 0.001 (p values between 0.0012 and 0.0024). Because the Bonferroni correction is highly conservative, these results suggest that the levels of GP14, GP16, tetrasialo glycans, and trigalactosylated glycans are also altered in ADHD. All these changes were consistent with the association between ADHD and the decrease in branching of plasma glycans. The only group of glycans that was found to be increased in ADHD was GP11 (which is composed of mostly biantennary glycans), whereas all glycan groups that were decreased in ADHD consisted of tri- and tetraantennary glycans.
Glycan branching is very important for the regulation of adaptive properties of the cell membrane (26) and it was reported that the increased branching promotes order in the cell membrane and prolongs membrane receptor half-life (37). In addition, the exact structure of glycans attached to individual membrane receptors significantly affects their functional properties (25), thus it is easily imaginable that differences in glycosylation efficiency could result in altered neural signaling and represent a predisposition for the development of ADHD. It is important to note that we have analyzed levels of individual glycans in plasma. Each glycan can originate from several different proteins, and because glycosylation is cell- and protein-specific, relatively small difference in plasma levels could have important local functional consequences.
Recently we completed the first comprehensive population study of human plasma N-glycome, which revealed variability that by far exceeds the variability of proteins and DNA (33). However, within a single individual the composition of plasma glycome is remarkably stable (38) and environmental factors have rather limited impact on the majority of glycans (36). Specific altered glyco-phenotypes that can be associated with specific pathologies were also identified to exist in a population (39). Here we have described a new glyco-phenotype composed of increased A2FG2 and decreased tri- and tetraantennary glycans that associates with ADHD, whereas plasma N-glycome was not found to be significantly altered in ASD. We would speculate that the decrease in glycan branching could be one of the predisposing factors for ADHD because it was already reported to be associated with the activity of membrane receptors (26). If this proves to be correct, this would open a new direction for the development of potential therapeutics for ADHD.
Footnotes
* This work was supported by the Croatian Ministry of Science, Education and Sport grants #309-0061194-2023 (to G. L.), #098-0982522-2455 (to N. P.) and #216-1080315-0302 (to I. R.); by European Commission EUROPHARM and EUROGLYCOARRAYS grants, and by a grant from GOA 2006/12 Leuven Belgium and a grant from Cure Autism Now (USA). H. P. is postdoctoral researcher of the Fund for Scientific Research-Flanders FWO, Belgium.
 This article contains supplemental Figs. 1–2 and Table I.
This article contains supplemental Figs. 1–2 and Table I.
Conflict of interest: None declared.
1 The abbreviations used are:
- ADHD
- attention-deficit hyperactivity disorder
- ASD
- autism spectrum disorder
- HILIC
- high performance liquid chromatography
- HPLC
- high pressure liquid chromatography.
REFERENCES
- 1. Faraone S. V., Khan S. A. (2006) Candidate gene studies of attention-deficit/hyperactivity disorder. J. Clin. Psychiatry 67 Suppl. 8, 13–20 [PubMed] [Google Scholar]
- 2. Levy S. E., Mandell D. S., Schultz R. T. (2009) Autism. Lancet. 374, 1627–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th edn, Text Revision, American Psychiatric Association, Washington, DC [Google Scholar]
- 4. Rommelse N. N., Franke B., Geurts H. M., Hartman C. A., Buitelaar J. K. (2010) Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur. Child Adolesc. Psychiatry. 19, 281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biederman J. (2005) Attention-deficit/hyperactivity disorder: a selective overview. Biol. Psychiatry. 57, 1215–1220 [DOI] [PubMed] [Google Scholar]
- 6. Wall D. P., Esteban F. J., Deluca T. F., Huyck M., Monaghan T., Velez de Mendizabal N., Goñi J., Kohane I. S. (2009) Comparative analysis of neurological disorders focuses genome-wide search for autism genes. Genomics 93, 120–129 [DOI] [PubMed] [Google Scholar]
- 7. Nedic G., Pivac N., Hercigonja D. K., Jovancevic M., Curkovic K. D., Muck-Seler D. (2010) Platelet monoamine oxidase activity in children with attention-deficit/hyperactivity disorder. Psychiatry Res. 175, 252–255 [DOI] [PubMed] [Google Scholar]
- 8. Hercigonja Novkovic V., Rudan V., Pivac N., Nedic G., Muck-Seler D. (2009) Platelet serotonin concentration in children with attention-deficit/hyperactivity disorder. Neuropsychobiology 59, 17–22 [DOI] [PubMed] [Google Scholar]
- 9. Sharp S. I., McQuillin A., Gurling H. M. (2009) Genetics of attention-deficit hyperactivity disorder (ADHD). Neuropharmacology 57, 590–600 [DOI] [PubMed] [Google Scholar]
- 10. Franke B., Neale B. M., Faraone S. V. (2009) Genome-wide association studies in ADHD. Hum. Genet. 126, 13–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tartaglia N., Davis S., Hench A., Nimishakavi S., Beauregard R., Reynolds A., Fenton L., Albrecht L., Ross J., Visootsak J., Hansen R., Hagerman R. (2008) A new look at XXYY syndrome: medical and psychological features. Am. J. Med. Genet. A 146A, 1509–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller D. T., Shen Y., Weiss L. A., Korn J., Anselm I., Bridgemohan C., Cox G. F., Dickinson H., Gentile J., Harris D. J., Hegde V., Hundley R., Khwaja O., Kothare S., Luedke C., Nasir R., Poduri A., Prasad K., Raffalli P., Reinhard A., Smith S. E., Sobeih M. M., Soul J. S., Stoler J., Takeoka M., Tan W. H., Thakuria J., Wolff R., Yusupov R., Gusella J. F., Daly M. J., Wu B. L. (2009) Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. J. Med. Genet. 46, 242–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weerapana E., Imperiali B. (2006) Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology 16, 91R–101R [DOI] [PubMed] [Google Scholar]
- 14. Calo D., Kaminski L., Eichler J. (2010) Protein glycosylation in Archaea: Sweet and Extreme. Glycobiology 20, 1065–1076 [DOI] [PubMed] [Google Scholar]
- 15. Cummings R. D. (2009) The repertoire of glycan determinants in the human glycome. Mol. Biosyst. 5, 1087–1104 [DOI] [PubMed] [Google Scholar]
- 16. Lee R. T., Lauc G., Lee Y. C. (2005) Glycoproteomics: protein modifications for versatile functions. EMBO Rep. 6, 1018–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marek K. W., Vijay I. K., Marth J. D. (1999) A recessive deletion in the GlcNAc-1-phosphotransferase gene results in peri-implantation embryonic lethality. Glycobiology 9, 1263–1271 [DOI] [PubMed] [Google Scholar]
- 18. Crocker P. R., Paulson J. C., Varki A. (2007) Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 [DOI] [PubMed] [Google Scholar]
- 19. Marth J. D., Grewal P. K. (2008) Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohtsubo K., Marth J. D. (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867 [DOI] [PubMed] [Google Scholar]
- 21. Lauc G. (2006) Sweet secret of the multicellular life. Biochim. Biophys. Acta. 1760, 525–526 [DOI] [PubMed] [Google Scholar]
- 22. Royle L., Campbell M. P., Radcliffe C. M., White D. M., Harvey D. J., Abrahams J. L., Kim Y. G., Henry G. W., Shadick N. A., Weinblatt M. E., Lee D. M., Rudd P. M., Dwek R. A. (2008) HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal. Biochem. 376, 1–12 [DOI] [PubMed] [Google Scholar]
- 23. Huhn C., Selman M. H., Ruhaak L. R., Deelder A. M., Wuhrer M. (2009) IgG glycosylation analysis. Proteomics 9, 882–913 [DOI] [PubMed] [Google Scholar]
- 24. Selman M. H., McDonnell L. A., Palmblad M., Ruhaak L. R., Deelder A. M., Wuhrer M. (2010) Immunoglobulin G glycopeptide profiling by matrix-assisted laser desorption ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 82, 1073–1081 [DOI] [PubMed] [Google Scholar]
- 25. Skropeta D. (2009) The effect of individual N-glycans on enzyme activity. Bioorg. Med. Chem. 17, 2645–2653 [DOI] [PubMed] [Google Scholar]
- 26. Dennis J. W., Lau K. S., Demetriou M., Nabi I. R. (2009) Adaptive Regulation at the Cell Surface by N-Glycosylation. Traffic 10, 1569–1578 [DOI] [PubMed] [Google Scholar]
- 27. Barisic K., Lauc G., Dumic J., Pavlovic M., Flogel M. (1996) Changes of glycoprotein patterns in sera of humans under stress. Eur. J. Clin. Chem. Clin. Biochem. 34, 97–101 [PubMed] [Google Scholar]
- 28. Bauer D., Haroutunian V., Meador-Woodruff J. H., McCullumsmith R. E. (2010) Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr. Res. 117, 92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baysal B. E., Willett-Brozick J. E., Badner J. A., Corona W., Ferrell R. E., Nimgaonkar V. L., Detera-Wadleigh S. D. (2002) A mannosyltransferase gene at 11q23 is disrupted by a translocation breakpoint that co-segregates with bipolar affective disorder in a small family. Neurogenetics 4, 43–53 [DOI] [PubMed] [Google Scholar]
- 30. Maguire T. M., Thakore J., Dinan T. G., Hopwood S., Breen K. C. (1997) Plasma sialyltransferase levels in psychiatric disorders as a possible indicator of HPA axis function. Biol. Psychiatry. 41, 1131–1136 [DOI] [PubMed] [Google Scholar]
- 31. Sáez-Valero J., Sberna G., McLean C. A., Masters C. L., Small D. H. (1997) Glycosylation of acetylcholinesterase as diagnostic marker for Alzheimer's disease. Lancet 350, 929. [DOI] [PubMed] [Google Scholar]
- 32. Conners C. K. (1998) Rating scales in attention-deficit/hyperactivity disorder: use in assessment and treatment monitoring. J. Clin. Psychiatry. 59 Suppl 7, 24–30 [PubMed] [Google Scholar]
- 33. Knezević A., Polasek O., Gornik O., Rudan I., Campbell H., Hayward C., Wright A., Kolcic I., O'Donoghue N., Bones J., Rudd P. M., Lauc G. (2009) Variability, Heritability and Environmental Determinants of Human Plasma N-Glycome. J. Proteome Res. 8, 694–701 [DOI] [PubMed] [Google Scholar]
- 34. R Development Core Team (2009) R: A Language and Environment for Statistical Computing. Vienna, Austria: Retrieved from http://www.R-project.org [Google Scholar]
- 35. Almasy L., Blangero J. (1998) Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 62, 1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knezevic A., Gornik O., Polasek O., Pucic M., Redzic I., Novokmet M., Rudd P. M., Wright A. F., Campbell H., Rudan I., Lauc G. (2010) Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology 20, 959–969 [DOI] [PubMed] [Google Scholar]
- 37. Partridge E. A., Le Roy C., Di Guglielmo G. M., Pawling J., Cheung P., Granovsky M., Nabi I. R., Wrana J. L., Dennis J. W. (2004) Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306, 120–124 [DOI] [PubMed] [Google Scholar]
- 38. Gornik O., Wagner J., Pucić M., Knezević A., Redzic I., Lauc G. (2009) Stability of N-glycan profiles in human plasma. Glycobiology 19, 1547–1553 [DOI] [PubMed] [Google Scholar]
- 39. Pucic M., Pinto S., Novokmet M., Knezevic A., Gornik O., Polasek O., Vlahovicek K., Wang W., Rudd P. M., Wright A. F., Campbell H., Rudan I., Lauc G. (2010) Common aberrations from normal human N-glycan plasma profile. Glycobiology 20, 970–975 [DOI] [PubMed] [Google Scholar]