Abstract
Objectives:
Antiplatelet therapy (APT) promotes bleeding; therefore, APT might worsen outcome in patients with intracerebral hemorrhage (ICH). We performed a systematic review and meta-analysis to address the hypothesis that pre-ICH APT use is associated with mortality and poor functional outcome following ICH.
Methods:
The Medline and Embase databases were searched in February 2008 using relevant key words, limited to human studies in the English language. Cohort studies of consecutive patients with ICH reporting mortality or functional outcome according to pre-ICH APT use were identified. Of 2,873 studies screened, 10 were judged to meet inclusion criteria by consensus of 2 authors. Additionally, we solicited unpublished data from all authors of cohort studies with >100 patients published within the last 10 years, and received data from 15 more studies. Univariate and multivariable-adjusted odds ratios (ORs) for mortality and poor functional outcome were abstracted as available and pooled using a random effects model.
Results:
We obtained mortality data from 25 cohorts (15 unpublished) and functional outcome data from 21 cohorts (14 unpublished). Pre-ICH APT users had increased mortality in both univariate (OR 1.41, 95% confidence interval [CI] 1.21 to 1.64) and multivariable-adjusted (OR 1.27, 95% CI 1.10 to 1.47) pooled analyses. By contrast, the pooled OR for poor functional outcome was no longer significant when using multivariable-adjusted estimates (univariate OR 1.29, 95% CI 1.09 to 1.53; multivariable-adjusted OR 1.10, 95% CI 0.93 to 1.29).
Conclusions:
In cohort studies, APT use at the time of ICH compared to no APT use was independently associated with increased mortality but not with poor functional outcome.
GLOSSARY
- APT
= antiplatelet therapy;
- CI
= confidence interval;
- GOS
= Glasgow Outcome Scale;
- ICH
= intracerebral hemorrhage;
- mRS
= modified Rankin Scale;
- OR
= odds ratio.

e–Pub ahead of print
Aspirin or other antiplatelet therapy (APT) could worsen outcome from intracerebral hemorrhage (ICH) by promoting bleeding. Published observational studies of outcomes in pre-ICH APT users have yielded conflicting results, however. Some suggest an increased risk of poor outcome1–3 while others suggest no increased risk.4,5 If prior APT worsens outcome, then restoration of normal platelet function could be a therapeutic target.
We hypothesized that pre-ICH APT use would be associated with increased mortality and functional impairment following ICH, and tested this hypothesis by performing a systematic review of the literature. To reduce the likelihood of publication bias, we additionally requested information from established cohort studies that had not previously published on the association between pre-ICH APT and clinical outcomes.
METHODS
Search strategy, selection criteria, and data abstraction.
Using the Meta-analysis of Observational Studies in Epidemiology (MOOSE) criteria as a guide,6 we searched for studies describing mortality or functional outcome of consecutive adults with spontaneous ICH by APT use, excluding ICH due to identified secondary causes such as arteriovenous malformations or thrombolysis. The following keywords were entered into Medline (OVID) and Embase: [intracerebral hemorrhage OR intracerebral hemorrhage OR hemorrhagic stroke OR hemorrhagic stroke] AND [outcome OR mortality OR morbidity OR survival OR death]. The search was limited to English-language human studies. The final search (February 15, 2008) yielded 2,873 articles. A physician investigator (B.B.T.) screened these articles by text, abstract, and then full text (figure e-1 on the Neurology® Web site at www.neurology.org). A permissive approach was used in advancing studies further through the screening process, and any uncertainties were reviewed by a second physician investigator (E.E.S.). Reference lists were hand searched to identify additional relevant studies. Study quality was independently appraised by 2 reviewers (B.B.T. and E.E.S.) using a framework adapted from a recent systematic review.7 Specifically, we only included studies that 1) verified all cases by neuroimaging, 2) included consecutive patients with primary ICH as eligible, rather than select samples (to minimize selection bias), 3) reported odds ratios (ORs) or probabilities of outcomes according to APT use, 4) reported mortality, 5) reported functional outcome using widely accepted validated scales, if functional outcome was reported, and 6) included sufficient information to judge the validity of the statistical methods. APT use was recorded based on medical history and chart review; no studies reported independent verification by pharmacy records. We also evaluated the degree of loss to follow up and whether confounding was considered. Ten articles were selected for inclusion based on this strategy (figure e-1).1–5,8–12
To reduce the likelihood that publication bias would affect our results, we contacted corresponding authors of articles which had been excluded by full text review (mostly for not containing APT data) but reported on ≥100 consecutive ICH patients and were published within the past 10 years (figure e-2). Authors of known cohort studies of ICH and experts in the field were also contacted. Data from an additional 15 cohorts were obtained by these methods.13–27 In many cases, authors contributed more patients than described in the original cohort, which was allowed as long as these patients were collected and characterized according to the same published methods. Study quality was evaluated using the same criteria cited above.
Univariate and multivariable-adjusted ORs for the effect of APT on ICH outcome were extracted. When data were not available or were not clear within the text, corresponding authors were contacted. To harmonize definitions of poor functional outcome across studies, we asked authors to analyze their data using the following scale-specific definitions based, where possible, on previously published recommendations: modified Rankin Scale (mRS) 4–6,28 Barthel Index less than 60,28 or Glasgow Outcome Scale (GOS) 1–3. There is excellent agreement between the mRS and Barthel Index scores for discrimination of the functional severity of stroke (weighted κ 0.85).29 Analysis of our own data11 shows that GOS score 1–3 has excellent agreement with mRS 4–6 (κ 0.87). Because there is evidence that age and previous disability may confound the relationship between pre-ICH APT and outcome,4 we requested that authors contributing multivariate analyses adjusted for both age and premorbid disability, typically defined as mRS >1, if such data were available. We did not request that authors adjust for ICH-related characteristics such as hematoma volume, intraventricular hemorrhage, or stroke severity, because such characteristics may in turn have been influenced by pre-ICH APT, and because methods for measuring hematoma volume and stroke severity varied considerably by study. Whenever possible, results are reported excluding patients taking oral anticoagulants or oral anticoagulants plus APT; studies that did not exclude such patients were noted. The actual covariates for each multivariable analysis differed by study, according to study design and available data, and are listed in detail in the Results.
Statistical analysis.
ORs and 95% confidence intervals (CI) from each study were combined using random effects models according to the method of DerSimonian and Laird, because initial analyses using fixed models showed significant heterogeneity when pooling the univariate ORs (as assessed by the I-squared measure and tested using the χ2 test). Effects in prespecified subgroups were determined. Prespecified meta-regression was used to test for a linear relationship between the OR for poor outcome following ICH and the percentage of pre-ICH APT users taking either 1) nonaspirin APT or 2) multiple APT. We inspected funnel plots of the ORs and performed Egger's test to assess for evidence of publication or reporting bias, that is, bias that could arise if the author's results influenced the likelihood of reporting their data in the literature or for incorporation in this study. Statistical analyses were performed with STATA version 9.2 (StataCorp LP, TX).
RESULTS
Systematic review of published data.
Characteristics of the 10 studies for which the relationship between APT and ICH outcome had previously been published are presented in the table. The relationship between APT and ICH outcome was the primary focus of 5.1–5 Two studies found that APT was an independent predictor of death,2,3 and 1 found that APT was associated with a combined endpoint of hematoma enlargement, surgery, or death within 48 hours, but was not a determinant of death alone.1 By contrast, another study5 failed to find a relationship between APT and either death or disability (defined as mRS >3) at hospital discharge. Finally, another study4 found significant relationships between APT and death and disability (defined as mRS >2) in univariate analysis, but not after controlling for age and premorbid disability (defined as prehospital mRS >1).
Table Characteristics of the 10 studies for which the relationship between APT and ICH outcome had previously been published
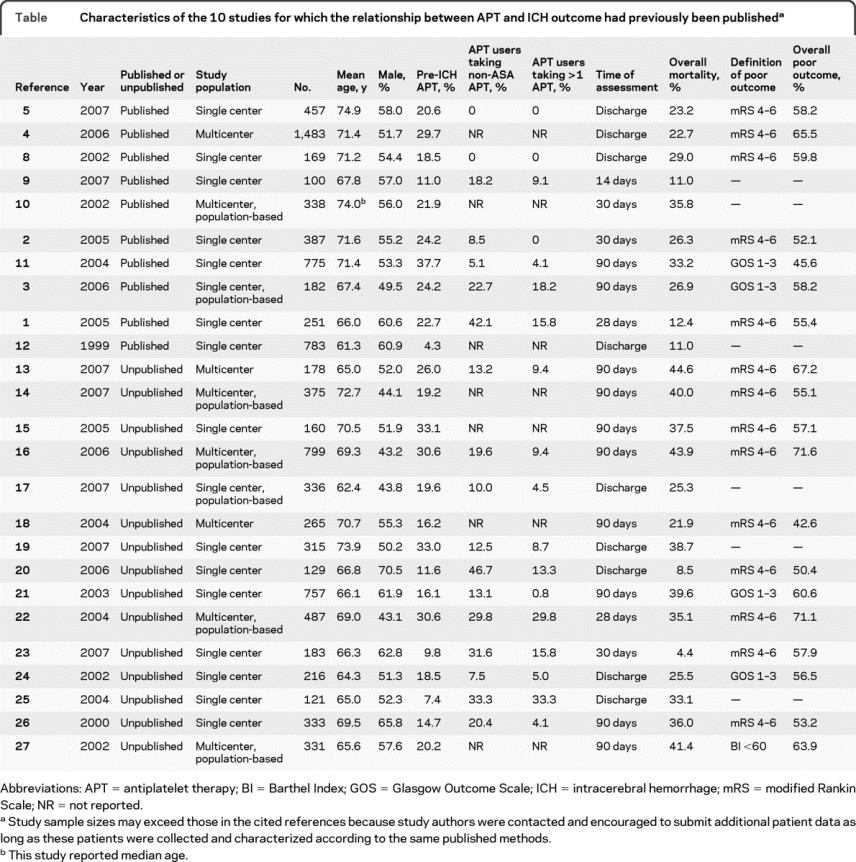
In another 5 studies, data on APT were included as a potentially relevant covariate in analyses of other predictors of ICH outcome (table). One study evaluated APT as a potential contributor to hospital mortality in ICH, and found it was nonsignificant in either univariate analysis or multivariable analysis controlling for age, sex, and other factors.12 The other 4 studies presented only univariate analyses and failed to find associations between APT and outcome (table).8–11
Combined analysis of previously published and unpublished data on APT and outcome.
Characteristics of all the study cohorts, including the cohorts where APT and outcome data were not published in the literature but were contributed by the study authors, are presented in the table. Overall, the studies varied in both design and size. Inclusion criteria were primary ICH (n = 20),1,3,5,10–17,19–27 supratentorial ICH (n = 4),2,8,9,18 or ICH identified by billing codes (n = 1).4 Two cohorts excluded patients admitted later than 24 hours after symptom onset,21,23 2 excluded patients with prior stroke,5,26 1 excluded patients “diagnosed with amyloid angiopathy,”20 and 1 excluded patients with multiple ICH, prior ICH, pre-ICH disability, or need for surgery.30 We contacted study authors to request cohort data excluding patients taking anticoagulation and ultimately received data from 23 cohorts that specifically excluded patients on anticoagulation,1–5,8,9,11,13–27 while one cohort included patients on anticoagulation,10 and one cohort included patients on anticoagulation but controlled for its use in the multivariable model of mortality.12
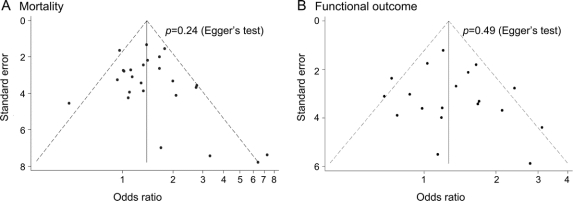
There was no evidence of publication bias in the 10 previously published studies (p = 0.34 for mortality and p = 0.54 for poor outcome, by Egger's test) or when considering all 25 studies (p = 0.24 for mortality and p = 0.49 for poor outcome) (figure 1).
Figure 1 Funnel plots
Funnel plots of univariate odds ratios for mortality (A) and poor functional outcome (B).
The weighted mean age across all studies was 68.9 years (range 61.3–74.9 years for individual cohorts) and 54.3% of patients were male (range 43.1%–70.5%). APT was commonly used prior to ICH (weighted mean proportion 22.9%, range 4.3%–37.7%). The weighted mean proportion of APT users taking nonaspirin APT, either alone or in combination with aspirin, was 15.9% (range 0%–46.7%). The weighted mean proportion taking more than one APT was 8.3% (range 0%–33.3%).
All 25 cohorts (9,910 patients) contributed data for the univariate mortality analysis (table), and 21 cohorts (8,419 patients) provided data for the multivariable mortality analysis.1–4,8,9,11–16,18–24,26,27 Among those providing multivariate data, all models adjusted for age, 7 adjusted for premorbid disability,4,13,15,16,18,19,27 3 adjusted for sex,1,12,31 1 adjusted for diabetes,31 and 1 adjusted for smoking, vascular risk factors, ischemic heart disease, previous cerebrovascular disease, and warfarin use.12 The pooled univariate OR for mortality for the entire cohort was 1.41 (95% CI 1.21 to 1.64, p < 0.001) (figure 2). The pooled multivariable adjusted OR for mortality was attenuated at 1.27 (95% CI 1.10 to 1.47, p = 0.001) (figure 2). Elimination of the single cohort that did not exclude warfarin-treated patients12 made no substantial difference in the estimates (data not shown).
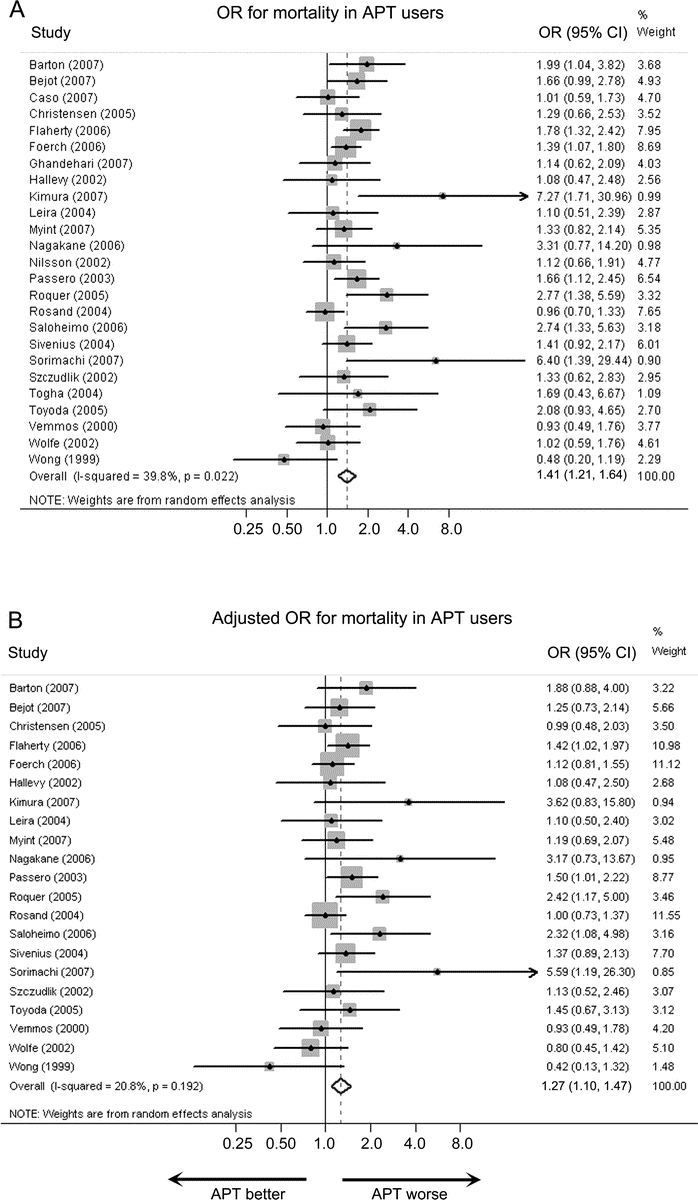
Figure 2 Odds ratios for mortality
Unadjusted (A) and multivariable-adjusted (B) ORs for mortality in pre-ICH antiplatelet therapy (APT) users compared to nonusers. CI = confidence interval; ICH = intracerebral hemorrhage; OR = odds ratio.
There were 19 cohorts (7,458 patients) that contributed data for the univariate analyses of poor functional outcome1–5,8,11,13–16,18,20–24,26,27 and 17 studies (6,693 patients) that contributed data for the multivariable-adjusted analysis.2–4,8,11,13–16,18,20–24,26,27 All models adjusted for age, 6 adjusted for premorbid disability,4,13,15,16,18,27 and 1 adjusted for sex and diabetes.3 The pooled univariate OR for poor functional outcome was 1.29 (95% CI 1.09 to 1.53, p = 0.002) (figure 3). The pooled adjusted OR for poor functional outcome was 1.10 (95% CI 0.93 to 1.29, p = 0.32) (figure 3).
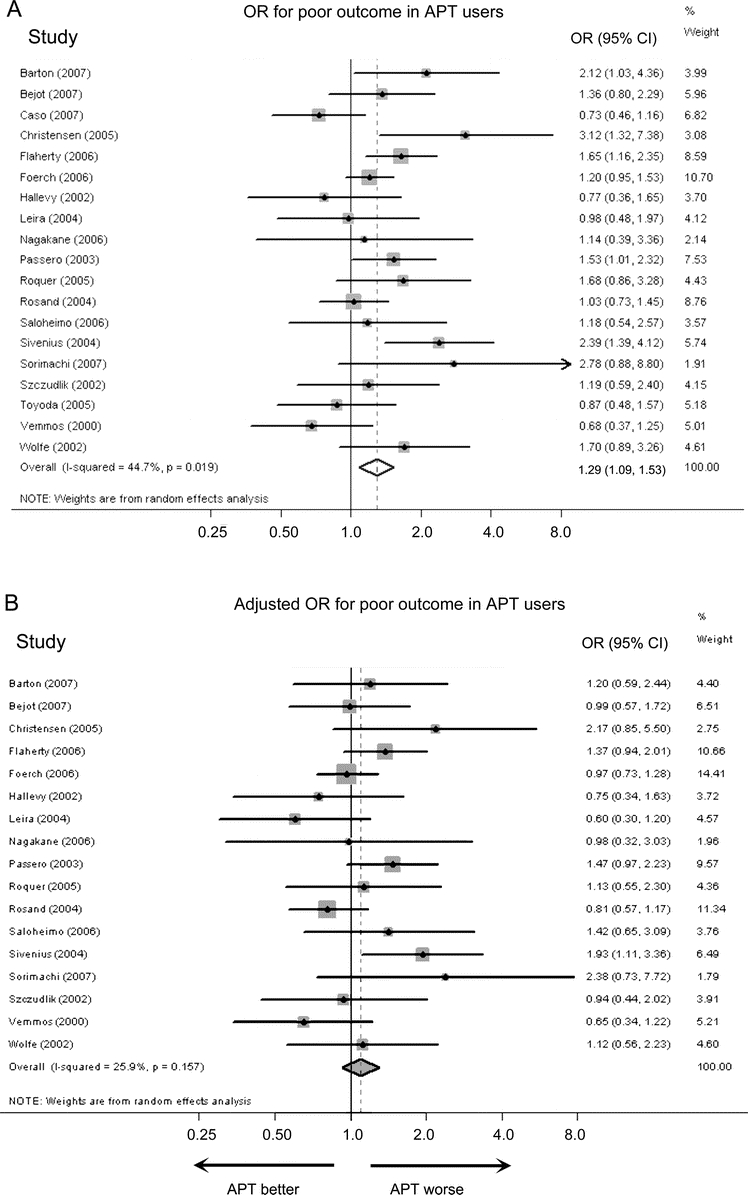
Figure 3 Odds ratios for poor functional outcome
Unadjusted (A) and multivariable-adjusted (B) ORs for poor functional outcome in pre-ICH antiplatelet therapy (APT) users compared to nonusers. CI = confidence interval; ICH = intracerebral hemorrhage; OR = odds ratio.
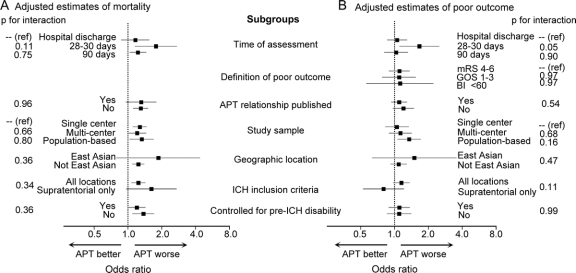
Study heterogeneity was found for univariate analyses (p ≤ 0.02) but not for multivariable analyses (p > 0.15) (figures 2 and 3). To explore sources of heterogeneity among studies, multiple prespecified subgroup analyses were performed using the multivariate-adjusted ORs (figure 4). Adjusted ORs for mortality and poor outcome were similar across most groups (p > 0.05), with the exception that the adjusted odds of poor outcome with APT was higher in studies that measured disability at 30 days compared to hospital discharge (p = 0.05). Using meta-regression, we found that for each 5% absolute increase in the percentage of nonaspirin APT the adjusted OR for mortality for APT, compared to no APT, increased by 0.06 (95% CI −0.01 to 0.13, p = 0.08), and the adjusted OR for poor functional outcome increased by 0.09 (95% CI 0.00 to 0.18, p = 0.04). For each 5% absolute increase in the percentage taking more than one APT the adjusted OR for mortality for APT, compared to no APT, increased by 0.04 (95% CI −0.06 to 0.13, p = 0.45), and the adjusted OR for poor functional outcome increased by 0.12 (95% CI 0.01 to 0.23, p = 0.04).
Figure 4 Outcomes in specific subgroups
Adjusted odds ratios for mortality (A) and poor functional outcome (B), in pre-ICH antiplatelet user compared to nonusers, according to prespecified study subgroups. APT = antiplatelet therapy; BI = Barthel Index; GOS = Glasgow Outcome Scale; ICH = intracerebral hemorrhage; mRS = modified Rankin Scale; Ref = reference category.
DISCUSSION
APT prior to ICH was common, and there was an association between APT and death and poor functional outcome in univariate analyses. The relationship with increased mortality was attenuated such that the effect size was relatively modest, and the relationship with increased poor functional outcome was reduced to nonsignificance after adjustment for age and other factors. The increased mortality may be related to the antithrombotic effect of APT, which could lead to increased bleeding. We were not able to confirm this hypothesis in the current study because follow-up ICH volumes were not present for most studies.
We found statistical evidence of heterogeneity of the univariate OR estimates, likely reflecting differences in the populations and methods used. Nonetheless, pooling of the OR estimates seems justified for several reasons. We restricted study entry to cohort studies recruiting consecutive patients to minimize selection bias and ensure more homogenous study design. We analyzed mortality as one of the outcomes; mortality can be determined with high interrater reliability and therefore should be less susceptible to ascertainment bias related to study design. Most importantly, statistical evidence of heterogeneity was no longer found when pooling multivariable-adjusted OR, suggesting that some of the heterogeneity of the univariate OR was caused by differences in the prevalence of confounding factors rather than differences in study methods. Therefore these data suggest that normal variation in the effect estimates, rather than heterogeneity of the study methods or populations, appears to be the primary reason for the varying results seen in individual published studies.
With one exception we failed to find differences between prespecified subgroups in the adjusted effect of APT on outcome (figure 4), including no difference when comparing population-based studies with the others. The exception was that studies of 1-month functional outcomes reported a greater effect of APT on poor functional outcome than studies reporting outcomes at hospital discharge (p = 0.05), although this finding is based on only 2 studies measuring functional outcome at 1 month. Given that APT was not associated with poor functional outcome in studies evaluating outcome at discharge or 90 days, this result may be a chance finding.
Because combination APT appears to be associated with a high incidence of intracranial hemorrhage in some populations,32,33 we used meta-regression to determine whether the cohort-specific percentage of combination APT or nonaspirin APT was related to poor outcome. We found some evidence that the rate of combination APT and nonaspirin APT was associated with the adjusted OR for poor functional outcome, but not mortality. These analyses should be interpreted with caution because of the relatively small number of combination therapy and nonaspirin users and the lack of dosage information. Further research is necessary to determine the risk from combination APT therapy, particularly given expanding indications for its use.
A limitation of the reviewed studies is that data on cardiovascular comorbidities, limitation of care orders, and concomitant medications were not always collected or were not collected in a standardized fashion. Thus the effect estimates were not adjusted for all potentially conceivable confounders and should be interpreted with appropriate caution. APT users are expected to have more ischemic cardiovascular disease and might have worse outcomes as a result of these comorbidities, independent of the amount of intracerebral bleeding. Limitation of care orders might have been more frequently used in the APT group, if pre-ICH APT was considered a poor prognostic sign. Treatment practices may have varied according to the presence or absence of pre-ICH APT. The reporting of previous cardiovascular and cerebrovascular disease, concomitant medications, limitation of care orders, and treatment protocols, including whether these data were reported at all, varied substantially by study and therefore we were not able to obtain effect estimates controlling for these factors. We did ask study authors to provide adjusted estimates controlling at minimum for age and previous disability (if such information was available), because these characteristics have been previously shown to confound the relationship between APT and ICH outcome.4 We could not report information on ICH size, ICH growth, presence of intraventricular hemorrhage, or stroke severity, because these data were not systematically collected with comparable methods across the studies. Increased bleeding and increased stroke severity are probable consequences of APT and are therefore more appropriately considered mediating factors between APT and worse outcome, rather than confounders.
Because we could not control for all possible confounders we cannot rule out residual confounding such that the true adjusted effect of APT may be less than reported here. Further, we cannot completely exclude the possibility of bias, although tests of publication bias were negative. The obtained estimates may be viewed as the probable upper bounds of the adjusted odds of poor outcomes associated with APT, however. In other words, we consider it unlikely that the true effect of APT on worse outcome following ICH is greater than that reported here. The pooled adjusted estimates of the APT effect may be fairly accurate, however, for the following reasons. Previous mortality prediction models in ICH have shown that the strongest determinants of ICH outcome are age, ICH size, intraventricular hemorrhage, and clinical stroke severity, with a lesser effect of cardiovascular comorbidities.34,35 Therefore cardiovascular comorbidities may not have strongly confounded these results. Limitation of care orders are independently associated with worse outcome; however, use of such orders is not known to be strongly associated with the presence or absence of APT. There are no proven specific therapies to improve outcome following ICH, or to antagonize APT-related bleeding, therefore treatment practices may not have a large effect on the estimates. Finally, we were able to provide estimates adjusted for age and prior disability. Since increased age is associated with cardiovascular comorbidities and use of limitation of care orders, controlling for age may have accounted for some, but certainly not all, of their effect on outcomes. Prior disability is unlikely to be a significant confounder because a subgroup analysis showed that similar estimates were obtained in the studies that provided ORs adjusted for prior disability, compared to those that did not (figure 4).
Additional limitations of our study include those inherent to any meta-analysis. To mitigate publication bias, we sought out unreported cohorts for inclusion and reviewed funnel plots comparing cohort sizes with effect estimates. Study quality was evaluated using published criteria as a guide.7 No studies reported correlation with pharmacy records; therefore some APT could have been inaccurately reported or missed.
Although this systematic review suggests there is only modestly increased mortality in patients taking pre-ICH APT, and little or no increase in poor functional outcomes, there are a substantial number of ICH patients taking pre-ICH APT who could be at risk. Whether the mortality associated with pre-ICH APT can be ameliorated by therapies designed to restore normal platelet function is uncertain and would require relatively large trials to demonstrate, given the modest increase in risk. Prevention of ICH, by careful attention to risk factors such as hypertension, may be a more easily implemented strategy to reduce the morbidity from APT-associated ICH.
AUTHOR AFFILIATIONS
From the Departments of Neurology and Neurosurgery (B.B.T.), Brown University, Providence, RI; Department of Neurology and Stroke Registry of Dijon (Y.B.), France; Stroke Unit (V.C.), Ospedale Santa Maria della Misericordia, San'Andrea delle Fratte, Perugia; Department of Neurology (J.C., M.R.R.-Y.), Hospital Clínico Universitario de Santiago, Santiago de Compostela, Spain; Department of Neurology (H.C.), Bispebjerg Hospital, University of Copenhagen, Denmark; Department of Neurology (M.L.F., D.W.), University of Cincinnati Academic Health Center, OH; Department of Neurology (C.F.), Johann Wolfgang Goethe University, Frankfurt am Main, Germany; Department of Neurology (K.G.), Ghaem Hospital, Mashhad University of Medical Sciences, Iran; Stroke Registry of Dijon (Inserm and Institut de Veille Sanitaire) (M.G.), France; Department of Neurology (S.M.G., J. Rosand, N.S.R.), Massachusetts General Hospital, Boston; Department of Neurology (H.H.), Sourasky Medical Center, Tel Aviv, Israel; Department of Neurology (J.C.H.), University of California, San Francisco; Division of Health and Social Care Research (P.H.), King's College London, London, UK; Centre for Stroke Research Berlin (P.H.), Charité- Universitaetsmedizin Berlin, Germany; Department of Neurosurgery (S.J.), Turku University Central Hospital, Turku, Finland; Department of Stroke Medicine (K.K.), Kawasaki Medical School, Kurashiki-city, Japan; Department of Medicine for the Elderly (P.K.M.), Norfolk and Norwich University Hospital, and School of Medicine, Health Policy and Practice, University of East Anglia, Norwich, UK; Department of Cerebrovascular Medicine (Y.N., H.N.), National Cerebral and Cardiovascular Center, Suita, Osaka, Japan; Department of Neurosciences (S.P.), Neurology Unit, University of Siena, Italy; Unitat d'Ictus (J. Roquer), Servei de Neurologia, Hospital del Mar, Barcelona, Spain; Department of Neurology (P.S.), University of Oulu, Oulu, and Department of Neuropsychiatry and Psychogeriatrics, Kellokoski Hospital, Tuusula, Finland; Department of Epidemiology and Health Promotion (V.S.), KTL (National Public Health Institute), Helsinki, Finland; University Hospital of Kuopio (J.S.), Brain Research and Rehabilitation Center Neuron, Kuopio, Finland; Department of Neurosurgery (T.S.), Brain Research Institute, University of Niigata, Niigata, Japan; Department of Neurology (M.T.), Sina Hospital, Tehran University of Medical Science, Tehran, Iran; Department of Cerebrovascular Medicine (K.T.), National Cerebral and Cardiovascular Center, Suita, Osaka, Japan; Department of Neurology (W.T.), Collegium Medicum, Jagiellonian University, Krakow, Poland; Acute Stroke Unit (K.N.V.), Department of Clinical Therapeutics, University of Athens School of Medicine, Alexandra Hospital, Athens, Greece; King's College London (C.D.A.W.), Public Health, Division of Health and Social Care Research, R&D Guy's & St Thomas' Foundation Trust, UK; and Department of Clinical Neurosciences (E.E.S.), University of Calgary, Calgary, Alberta, Canada.
DISCLOSURE
Dr. Thompson, Dr. Béjot, Dr. Caso, and Dr. Castillo report no disclosures. Dr. Christensen has received funding for travel from Boehringer Ingelheim and receives research support from Augustinusfonden. Dr. Flaherty receives research support from the NIH (NINDS 2P50NS044283 [PI Project 2]: study drug supplied by Novo Nordisk, NINDS R01 NS030678 [coinvestigator], NINDS R01 NS036695 [coinvestigator], NINDS R01 NS42167 [coinvestigator], NINDS R01 NS044876 [coinvestigator], and NINDS R01 NS052220 [coinvestigator]. Dr. Foerch is designated as an inventor of a patent re: Use of GFAP for identification of intracerebral haemorrhage, for which he has received royalty payments from Roche. Dr. Ghandehari and Dr. Giroud report no disclosures. Dr. Greenberg serves on scientific advisory boards for Roche and Alzheimer's Immunotherapy; serves on the editorial boards of Neurology, Stroke, Cerebrovascular Disease, and the Journal of Alzheimer's Disease and Other Dementias; has received speaker honoraria from Merck Serono, Esteve, Medtronics, Inc., and Pfizer Inc.; and has received/receives research support from the NIH (R01AG026484 [PI], K24NS056207 [PI], R01AG021084 [coinvestigator], R01NS042147 [PI], and U54NS057405, 2007–2012 [coinvestigator]), and from the Alzheimer's Association. Dr. Hallevi reports no disclosures. Dr. Hemphill serves on the scientific advisory board for Ornim Inc.; serves on the speakers' bureau of the Network for Continuing Medical Education; receives research support from the NIH/NINDS (U10 NS058931 [PI for hub site for Neurological Emergencies Treatment Trials Network]); holds stock in Ornim Inc.; and has provided multiple case reviews and testimony regarding stroke or neurocritical care. During the prior 2 years he previously served on the scientific advisory board of InnerCool Therapies and held stock and stock options in the previous parent company of InnerCool Therapies (Cardium Therapeutics). He also previously received research support from Novo Nordisk and the University of California. Dr. Heuschmann receives/has received research support from the European Union FP7, the German Stroke Foundation, and the Stanley Thomas Johnson Foundation. Dr. Juvela serves as an Associate Editor for the European Journal of Neurology and on the editorial board of Stroke. Dr. Kimura, Dr. Myint, Dr. Nagakane, Dr. Naritomi, Dr. Passero, Dr. Rodríguez-Yáñez, and Dr. Roquer report no disclosures. Dr. Rosand has received research support from the NIH [R01-NS059727 (PI)] and from the American Heart Association. Dr. Rost serves as an Associate Editor for Frontiers in Hospitalist Neurology and as an Assistant Editor for Stroke and receives/has received research support from the NIH (NINDS 1K23NS064052-01A1 [PI]), the National Stroke Association, and the American Stroke Association-Bugher Foundation. Dr. Saloheimo has received funding for travel from Jassen; served as an Associate Editor for Duodecim Medical Journal and serves as Associate/Managing Editor for the European Journal of Neurology; received a speaker honorarium from Verve; and has received research support from Oulu University Hospital. Dr. Salomaa serves as an Associate Editor for the European Journal of Cardiovascular Prevention and Rehabilitation and receives research support from the Academy of Finland, the Finnish Foundation for Cardiovascular Research, and the Sigrid Juselius Foundation. Dr. Sivenius, Dr. Sorimachi, and Dr. Togha report no disclosures. Dr. Toyoda receives research support from Grants-in-Aid from the Ministry of Health, Labour and Welfare. Dr. Turaj serves as an Associate Editor for Neurologia i Neurochirurgia Polska. Dr. Vemmos and Dr. Wolfe report no disclosures. Dr. Woo receives research support from the NIH (NS36695 [coinvestigator] and NS30678 [coinvestigator]). Dr. Smith serves as an Assistant Editor for Stroke; has received speaker honoraria from the Canadian Consortium on Dementia; serves on speakers' bureaus for QuantiaMD and BMJ Best Practice; and has received/receives research support from the NIH (5R01NS062028 [PI] and K23NS046327 [PI]), the CIHR, the Canadian Stroke Network, and Alberta Innovates-Health Solutions (funded by the Alberta Heritage Fund for Medical Research).
Supplementary Material
Address correspondence and reprint requests to Dr. Eric E. Smith, University of Calgary, Rm C1212, Foothills Medical Centre, 1403 29 Street NW, Calgary, AB, Canada, T2N 2T9 eesmith@ucalgary.ca
Editorial, page 1314
Supplemental data at www.neurology.org
e-Pub ahead of print on September 8, 2010, at www.neurology.org.
Disclosure: Author disclosures are provided at the end of the article.
Received December 23, 2009. Accepted in final form June 3, 2010.
REFERENCES
- 1.Toyoda K, Okada Y, Minematsu K, et al. Antiplatelet therapy contributes to acute deterioration of intracerebral hemorrhage. Neurology 2005;65:1000–1004. [DOI] [PubMed] [Google Scholar]
- 2.Roquer J, Rodriguez Campello A, Gomis M, Ois A, Puente V, Munteis E. Previous antiplatelet therapy is an independent predictor of 30-day mortality after spontaneous supratentorial intracerebral hemorrhage. J Neurol 2005;252:412–416. [DOI] [PubMed] [Google Scholar]
- 3.Saloheimo P, Ahonen M, Juvela S, Pyhtinen J, Savolainen ER, Hillbom M. Regular aspirin-use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke 2006;37:129–133. [DOI] [PubMed] [Google Scholar]
- 4.Foerch C, Sitzer M, Steinmetz H, Neumann-Haefelin T. Pretreatment with antiplatelet agents is not independently associated with unfavorable outcome in intracerebral hemorrhage. Stroke 2006;37:2165–2167. [DOI] [PubMed] [Google Scholar]
- 5.Caso V, Paciaroni M, Venti M, et al. Effect of on-admission antiplatelet treatment on patients with cerebral hemorrhage. Cerebrovasc Dis 2007;24:215–218. [DOI] [PubMed] [Google Scholar]
- 6.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 7.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–437. [DOI] [PubMed] [Google Scholar]
- 8.Hallevy C, Ifergane G, Kordysh E, Herishanu Y. Spontaneous supratentorial intracerebral hemorrhage: criteria for short-term functional outcome prediction. J Neurol 2002;249:1704–1709. [DOI] [PubMed] [Google Scholar]
- 9.Kimura K, Iguchi Y, Inoue T, et al. Hyperglycemia independently increases the risk of early death in acute spontaneous intracerebral hemorrhage. J Neurol Sci 2007;255:90–94. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson OG, Lindgren A, Brandt L, Saveland H. Prediction of death in patients with primary intracerebral hemorrhage: a prospective study of a defined population. J Neurosurg 2002;97:531–536. [DOI] [PubMed] [Google Scholar]
- 11.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med 2004;164:880–884. [DOI] [PubMed] [Google Scholar]
- 12.Wong KS. Risk factors for early death in acute ischemic stroke and intracerebral hemorrhage: a prospective hospital-based study in Asia: Asian Acute Stroke Advisory Panel. Stroke 1999;30:2326–2330. [DOI] [PubMed] [Google Scholar]
- 13.Barton CW, Hemphill JC 3rd. Cumulative dose of hypertension predicts outcome in intracranial hemorrhage better than American Heart Association guidelines. Acad Emerg Med 2007;14:695–701. [DOI] [PubMed] [Google Scholar]
- 14.Bejot Y, Rouaud O, Durier J, et al. Decrease in the stroke case fatality rates in a French population-based twenty-year study: a comparison between men and women. Cerebrovasc Dis 2007;24:439–444. [DOI] [PubMed] [Google Scholar]
- 15.Christensen H, Fogh Christensen A, Boysen G. Abnormalities on ECG and telemetry predict stroke outcome at 3 months. J Neurol Sci 2005;234:99–103. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty ML, Haverbusch M, Sekar P, et al. Long-term mortality after intracerebral hemorrhage. Neurology 2006;66:1182–1186. [DOI] [PubMed] [Google Scholar]
- 17.Ghandehari K, Izadi Z. The Khorasan Stroke Registry: results of a five-year hospital-based study. Cerebrovasc Dis 2007;23:132–139. [DOI] [PubMed] [Google Scholar]
- 18.Leira R, Davalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology 2004;63:461–467. [DOI] [PubMed] [Google Scholar]
- 19.Myint PK, Vowler SL, Woodhouse PR, Redmayne O, Fulcher RA. Winter excess in hospital admissions, in-patient mortality and length of acute hospital stay in stroke: a hospital database study over six seasonal years in Norfolk, UK. Neuroepidemiology 2007;28:79–85. [DOI] [PubMed] [Google Scholar]
- 20.Nagakane Y, Miyashita K, Nagatsuka K, Yamawaki T, Naritomi H. Primary intracerebral hemorrhage during asleep period. Am J Hypertens 2006;19:403–406. [DOI] [PubMed] [Google Scholar]
- 21.Passero S, Ciacci G, Ulivelli M. The influence of diabetes and hyperglycemia on clinical course after intracerebral hemorrhage. Neurology 2003;61:1351–1356. [DOI] [PubMed] [Google Scholar]
- 22.Sivenius J, Tuomilehto J, Immonen-Raiha P, et al. Continuous 15-year decrease in incidence and mortality of stroke in Finland: the FINSTROKE study. Stroke 2004;35:420–425. [DOI] [PubMed] [Google Scholar]
- 23.Sorimachi T, Fujii Y, Morita K, Tanaka R. Predictors of hematoma enlargement in patients with intracerebral hemorrhage treated with rapid administration of antifibrinolytic agents and strict blood pressure control. J Neurosurg 2007;106:250–254. [DOI] [PubMed] [Google Scholar]
- 24.Szczudlik A, Turaj W, Slowik A, Strojny J. Hyperthermia is not an independent predictor of greater mortality in patients with primary intracerebral hemorrhage. Medical Science Monitor 2002;8:CR702–707. [PubMed] [Google Scholar]
- 25.Togha M, Bakhtavar K. Factors associated with in-hospital mortality following intracerebral hemorrhage: a three-year study in Tehran, Iran. BMC Neurol 2004;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vemmos KN, Takis CE, Georgilis K, et al. The Athens stroke registry: results of a five-year hospital-based study. Cerebrovasc Dis 2000;10:133–141. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe CD, Rudd AG, Howard R, et al. Incidence and case fatality rates of stroke subtypes in a multiethnic population: the South London Stroke Register. J Neurol Neurosurg Psychiatry 2002;72:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999;30:1538–1541. [DOI] [PubMed] [Google Scholar]
- 29.Govan L, Langhorne P, Weir CJ. Categorizing stroke prognosis using different stroke scales. Stroke 2009;40:3396–3399. [DOI] [PubMed] [Google Scholar]
- 30.Roquer J. Previous antiplatelet treatment and mortality in patients with intracerebral hemorrhage [comment]. Stroke 2007;38:863; author reply 864. [DOI] [PubMed]
- 31.Saloheimo P, Lapp TM, Juvela S, Hillbom M. The impact of functional status at three months on long-term survival after spontaneous intracerebral hemorrhage. Stroke 2006;37:487–491. [DOI] [PubMed] [Google Scholar]
- 32.Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364:331–337. [DOI] [PubMed] [Google Scholar]
- 33.Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903–1912. [DOI] [PubMed] [Google Scholar]
- 34.Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 35.Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 2008;39:2304–2309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.