Abstract
Background:
Combination antiretroviral therapy (cART) has improved the survival of patients with HIV/AIDS but its impact remains uncertain on the changing prevalence and incidence of neurologic disorders with ensuing effects on mortality.
Methods:
The prevalence and incidence of neurologic disorders were examined in patients receiving active care in a regional HIV care program from 1998 to 2008. The mortality hazard ratio (HR) was calculated by Cox proportional hazard models with adjustment for demographic and clinical variables.
Results:
Of 1,651 HIV-infected patients assessed, 404 (24.5%) were identified as having one or more neurologic disorders, while 41% of AIDS-affected persons exhibited neurologic disease. Symptomatic distal sensory polyneuropathy (DSP, 10.0%) and HIV-associated neurocognitive disorder (HAND, 6.2%) represented the most prevalent disorders among 53 recognized neurologic disorders. Patients with at least one neurologic disorder exhibited higher mortality rates (17.6% vs 8.0%, p < 0.0001), particularly AIDS-related deaths (9.7% vs 3.2%, p < 0.0001), compared with those without neurologic disorders. The highest mortality HR was associated with opportunistic infections of CNS (HR 5.3, 95% confidence interval [CI] 2.5–11.2), followed by HAND (HR 3.1, 95% CI 1.8–5.3) and the presence of any neurologic disorder (HR 2.0, 95% CI 1.2–3.2). The risk of AIDS-related death with a neurologic disorder was increased by 13.3% per 100 cells/mm3 decrement in blood CD4+ T-cell levels or by 39% per 10-fold increment in plasma viral load.
Conclusions:
The burden and type of HIV-related neurologic disease have evolved over the past decade and despite the availability of cART, neurologic disorders occur frequently and predict an increased risk of death.
GLOSSARY
- cART
= combination antiretroviral therapy;
- CI
= confidence interval;
- CNS-OI
= CNS opportunistic infection;
- DSP
= distal sensory polyneuropathy;
- HAD
= HIV-associated dementia;
- HAND
= HIV-associated neurocognitive disorder;
- HR
= hazard ratio;
- IQR
= interquartile range;
- SAC
= Southern Alberta Clinic.

e–Pub ahead of print

CME

Patient Page
The incidence of AIDS and mortality among HIV-infected persons have declined since the introduction of combination antiretroviral therapy (cART).1,2 Nonetheless, the frequency of HIV-associated neurologic syndromes has remained a clinical challenge. Studies from North America and Europe of HIV-infected patients receiving cART report improved outcomes of major neurologic disorders including HIV-associated dementia (HAD),3–6 distal sensory polyneuropathy (DSP),7 and CNS opportunistic infections (CNS-OI),3,6 while other studies showed no change in prevalence of HAD during the early cART period.8 The prevalence of DSP appears higher in the cART era, possibly caused by exposure to neurotoxic antiretroviral therapies.9,10 Unlike these well-recognized complications, the incidence and prevalence of other neurologic disorders, e.g., seizure, headache, and myopathy, have not been prospectively documented in treated HIV infection.11
Prior to widespread use of cART, immunologic and virologic markers were strong predictors of neurologic disease occurrence and survival. Low blood CD4+ T-cell and high plasma viral load levels were regarded as robust risk factors for HAD and DSP.12 In the cART era, the lowest blood CD4+ T-cell level (CD4 nadir) is a predictor of neurologic status of patients with HIV.13,14 Although the survival time of patients with HAD has increased with cART,5 recent studies indicate that the presence of HAD and CNS-OIs were associated with increased mortality rates,15,16 while the contribution of other neurologic disorders to survival of HIV-infected individuals is unclear. Herein, we analyzed the prevalence and incidence of major neurologic disorders in a population-based setting, which included all adult HIV-infected individuals receiving active care in Southern Alberta, Canada, over the past decade. We further examined whether the presence of major neurologic disorders represented an independent risk factor for AIDS-related death.
METHODS
Study patients.
The Southern Alberta Clinic (SAC) is a multidisciplinary, geographically based program for all persons with HIV/AIDS in southern Alberta (total population approximately 1.5 million), Canada. This study includes all HIV-seropositive patients registered with SAC10 (e-Methods on the Neurology® Web site at www.neurology.org) from January 1, 1998, to August 31, 2008, excluding patients with less than 2 clinic visits. AIDS diagnosis was based on Canadian guidelines17 and HIV-related neurologic disorders were diagnosed using established guidelines for symptomatic disorders.10,18,19 Patients were referred from within the clinic to see neurologists. All neurologic disorders were diagnosed and confirmed by 2 certified neurologists (A.H. and C.P.). Neurologic disorder prevalence was measured as the total number of disease cases in all patients during the study period and incidence was defined as the biennial number of first neurologic diagnoses in active-care patients.
Standard protocol approvals and patient consents.
Informed consent approved by the University of Calgary Ethics Committee was obtained from all patients at the outset.
Statistical analysis.
Demographic variables of interest were age at HIV diagnosis, sex, ethnic group (white, First Nations, black, or other), and HIV risk factors (homosexual/bisexual, IV drug use, or other). Clinical variables of interest included baseline and nadir levels of blood CD4+ and CD8+ T cells as well as baseline log viral load. Descriptive statistical analyses were derived from these demographic and clinical variables, as well as the prevalence and incidence of the neurologic disorders. The p values for categorical and continuous variables were calculated by χ2 and nonparametric Mann-Whitney tests, respectively. Causes of death were separated into AIDS-related and non-AIDS-related using the CoDe classification (http://www.cphiv.dk/CoDe/tabid/55/Default.aspx; table e-1). Survival analyses using a Cox proportional hazards model were performed to define the relationships between neurologic diseases with related variables and survival (e-Methods).20
RESULTS
Demographic and clinical features.
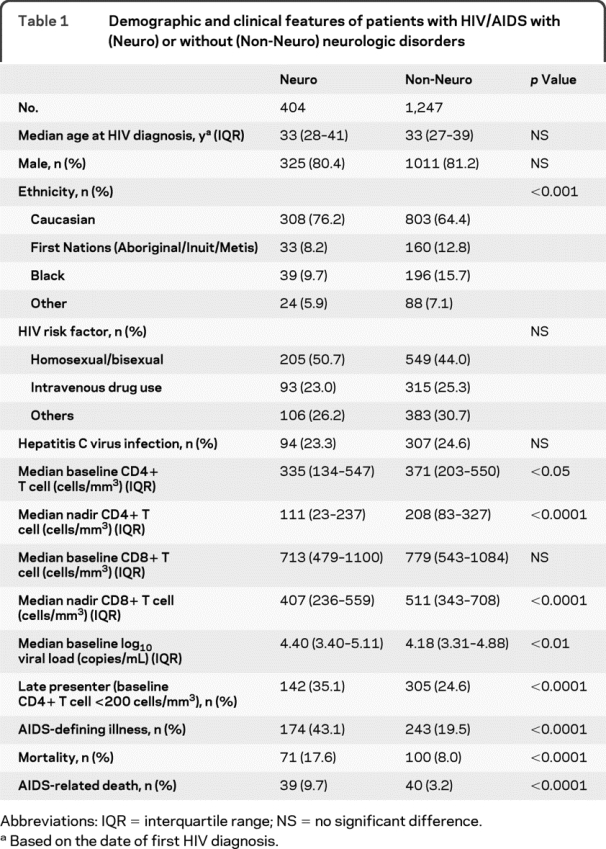
Of 1,651 HIV-infected persons receiving active care at SAC during the study period, 404 patients (24.5%) were identified with at least one symptomatic neurologic disorder (table 1). The median follow-up period was 7.6 years (interquartile range [IQR] 3.7–12.6 years). There were no differences in age, gender, and other comorbidities (e.g., substance abuse, hepatitis C virus coinfection) between patients with or without neurologic disease, although more neurologic disorders were diagnosed in white patients than First Nations patients, black patients, or patients of other ethnicities (table 1). Patients with neurologic disorders were more likely to be late presenters with low CD4+ T-cell levels and high viral loads at baseline (table 1). Patients with neurologic disorders had higher rates of AIDS-defining illnesses, together with lower nadir CD4+ and CD8+ T-cell levels than patients without a neurologic disorder (table 1).
Table 1 Demographic and clinical features of patients with HIV/AIDS with (Neuro) or without (Non-Neuro) neurologic disorders
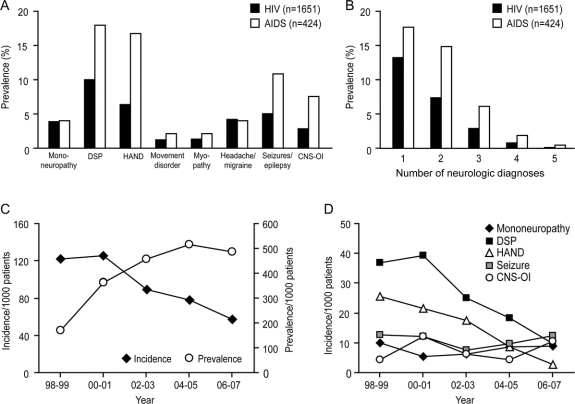
Prevalence and incidence of neurologic diagnoses.
At the time of neurologic diagnosis, the median duration of HIV seropositivity was 5.5 years (IQR 1.7–10.0 years). A total of 53 neurologic disorders were identified during the study period (table e-2). The most commonly diagnosed neurologic disorders were symptomatic DSP, followed by symptomatic HIV-associated neurocognitive disorder (HAND), including HAD and minor neurocognitive disorder, seizures/epilepsy, headache/migraine, mononeuropathy, CNS-OI, myopathy, and movement disorder (figure 1A). AIDS-defined persons (n = 424) displayed a higher prevalence of neurologic disorders than persons without AIDS-defining illness (41.0% vs 19.9%, p < 0.0001). The prevalences of DSP, HAND, seizures/epilepsy, and CNS-OI in AIDS-defined persons were 2- to 3-fold higher than in the total HIV-infected group (figure 1A).
Figure 1 Prevalence and incidence of neurologic diagnoses
(A) There were 8 major neurologic disorders that presented in more than 5% (>20 patients) among 404 patients with at least one neurologic diagnosis. The most prevalent neurologic disorder in HIV-infected patients (filled box) was symptomatic distal sensory polyneuropathy (DSP, 10.0%), followed by HIV-associated neurocognitive disorders (HAND, 6.2%). A subset of AIDS-defined patients (open box) exhibited higher prevalences of DSP, HAND, seizures/epilepsy, and CNS opportunistic infection (CNS-OI). (B) Almost half of HIV-infected patients with neurologic disorders (45.7%) showed multiple neurologic disorders. Patients with AIDS had a similar trend in the number of neurologic diagnoses but with more patients manifesting 1 to 3 neurologic disorders. (C) From 1998 to 2007, the incidence of the first neurologic diagnosis declined from 122 to 57 per 1,000 active-care patients (χ2 test, p < 0.05). (D) Although the incidence of DSP or HAND as a first neurologic diagnosis was lower in 2007 compared to 1998 (χ2 test, p < 0.05), there was no change in the incidence of other major neurologic disorders, including mononeuropathy, seizures/epilepsy, and CNS-OI.
Almost 50% of HIV-infected patients with a neurologic disorder exhibited multiple independent neurologic diagnoses (figure 1B). The prevalence of 1 to 3 neurologic disorders was higher in AIDS-defined persons (figure 1B). Patients with 2 or more neurologic disorders had lower baseline CD4+ (250 cells/mm3, IQR 79–480 vs 360 cells/mm3, IQR 164–588, p < 0.01) and nadir CD4+ (81 cells/mm3, IQR 20–188 vs 130 cells/mm3, IQR 29–288, p < 0.01) T-cell levels than patients with only one neurologic disorder without differences in CD8+ T-cell and plasma viral load levels.
The incidence of the first neurologic diagnosis in active-care patients was calculated from 1998 to 2007. The incidence decreased over the study period (figure 1C), from 86 cases among 705 active-care patients in 1998–1999 to 65 cases in 1,134 active-care patients in 2006–2007 (Spearman r = −0.900, p < 0.01), while the cumulative prevalence was increased and reached a plateau in 2004 (figure 1C). Similarly, the incidences of the first diagnosis of DSP and HAND were reduced over time (Spearman r = −0.9000, p < 0.01 and r = −1.000, p < 0.05; figure 1D). However, there was no change in the incidences of mononeuropathy, seizures/epilepsy, or CNS-OIs during this study period.
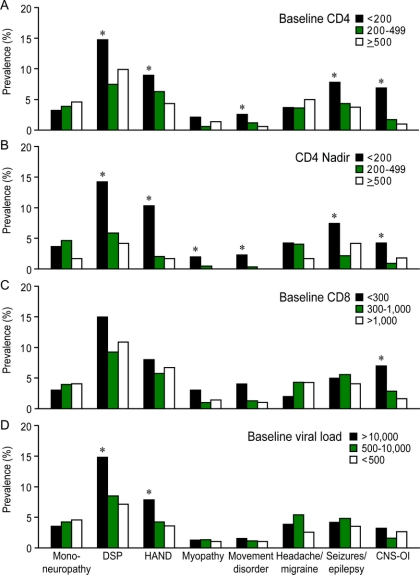
The occurrence of some neurologic disorders including HAD is correlated with the blood CD4+ T-cell levels.4,17 Stratification of baseline CD4+ T-cell levels revealed that late-presenting patients who showed severe immune suppression (CD4+ T-cell levels <200 cells/mm3) also exhibited higher prevalence of DSP, movement disorder, seizures/epilepsy, and CNS-OIs compared with patients showing moderate or minimal immunosuppression (p < 0.05, figure 2A). There was no difference in prevalence of neurologic disorders between patients with no (CD4+ T-cell levels >500 cells/mm3) and moderate (CD4+ T-cell levels 200–500 cells/mm3) immunosuppression. Late-presenter patients displayed a shorter interval between HIV diagnosis and the first neurologic diagnosis compared to patients with higher baseline CD4+ T-cell levels (2.9 years, IQR 0.6–6.8 years vs 7.0 years, IQR 3.0–11.0 years, p < 0.0001). Similarly, HIV-infected patients with nadir CD4+ T-cell levels of <200 cells/mm3 had a greater risk of a neurologic disorder except for mononeuropathy (figure 2B). In contrast, low CD8+ T-cell levels (<300 cells/mm3) were only associated with a higher risk of CNS-OI (p < 0.05, figure 2C) and were not correlated with the prevalence of DSP or HAND. Patients with high plasma viral load (>10,000 copies/mL) displayed an increased prevalence of DSP and HAND (p < 0.05, figure 2D). Unlike other neurologic disorders, the prevalence of mononeuropathy and headache/migraine was not associated with immunologic or virologic variables (figure 2, A–D).
Figure 2 Prevalence of neurologic disorders based on immunologic or virologic status
(A, B) Univariate analyses of prevalence showed that the risks of distal sensory polyneuropathy (DSP), HIV-associated neurocognitive disorders (HAND), movement disorders, seizure, and CNS opportunistic infection (CNS-OI) were greater among persons with baseline and nadir CD4+ T-cell levels below 200 cells/mm3. (C) Low baseline CD8+ T-cell levels were also associated with higher prevalence of HAND and CNS-OI. (D) The prevalence of DSP and HAND was higher in patients with plasma viral load greater than 10,000 copies/mL (χ2 test, *p < 0.05). In contrast to other neurologic disorders, prevalence of mononeuropathy and headache/migraine was not associated with immunologic and virologic variables.
Cause of death and survival of patients with neurologic diagnoses.
During 14,134 person-years of follow-up, 171 deaths were recorded and patients with neurologic diagnoses showed an overall higher mortality rate (p < 0.0001, table 1). The causes of death were categorized into 3 groups: AIDS-related (79 deaths), non-AIDS-related (81 deaths), and unknown cause (11 deaths) (table e-1). A univariate analysis showed that there were more AIDS-related deaths in patients with neurologic diagnoses compared with non-neurologic patients (9.7% vs 3.2%, p < 0.0001, table 1). Cox proportional hazard models revealed that a decrease in baseline CD4+ T cells, an increase of plasma viral load, or the presence of neurologic disorder contributed to a greater mortality rate (p < 0.05) while none of the demographic variables (age, ethnicity, HIV risk factor, gender) influenced this population's survival (table 2 and table e-3). After accounting for covariates, the presence of a neurologic disorder was associated with a 2-fold increased risk of death (hazard ratio [HR] 2.0, 95% confidence interval [CI] 1.2–3.2). The survival plot stratified by the presence and absence of a neurologic disorder showed lower estimated survival rates for HIV-infected persons with neurologic disorders (81.3% vs 92.0% at 20 years after HIV diagnosis, figure 3A). A decrement of 100 cells/mm3 in blood CD4+ T-cell levels at initial presentation increased mortality rate by 13.3% (HR 0.88), while each 10-fold increment in baseline plasma viral load (e.g., from 100 to 1,000 copies/mL) was associated with a 39.0% increase in mortality rate (HR 1.39, table 2).
Table 2 Hazard ratio of AIDS-related death in patients with HIV/AIDS with neurologic disorders (Neuro), HIV-associated neurocognitive disorder (HAND), CNS opportunistic infection (CNS-OI), or distal sensory polyneuropathy (DSP)
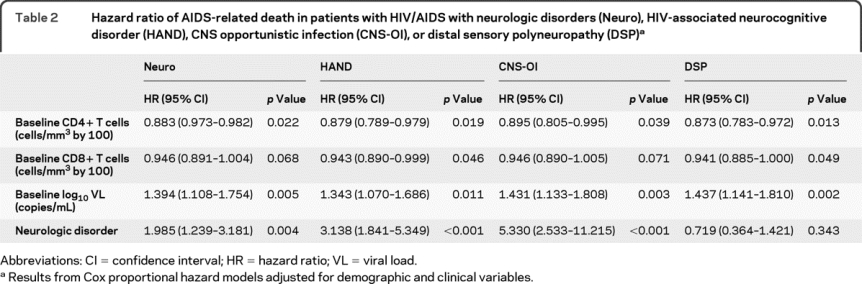
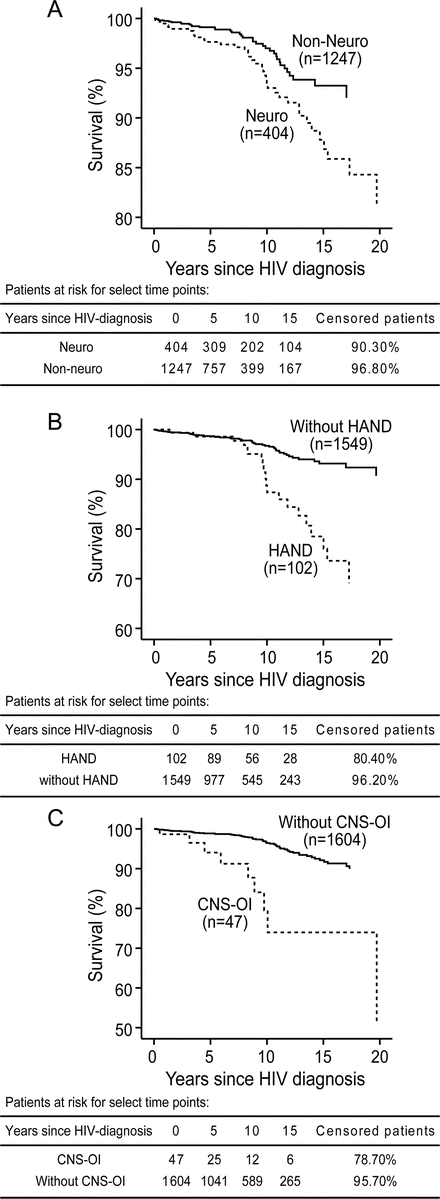
Figure 3 Cox survival curves in HIV-infected persons with or without neurologic disorders
(A) The survival plot obtained by removing the presence of neurologic disorder as a covariate from the model and using it to stratify the survival function showed that patients with at least one neurologic disorder (Neuro) exhibited lower survival times than patients without a neurologic disorder (Non-Neuro). The survival plot was stratified by the presence or absence of (B) HIV-associated neurocognitive disorders (HAND) or (C) opportunistic infection of CNS (CNS-OI). The lower survival time was more evident in patients with HAND or patients who had a history of CNS-OI. All survival plots were estimated at the mean of all covariates including age at HIV diagnosis, gender, ethnicity, HIV risk factors, baseline CD4+ T cells, baseline CD8+ T cells, and baseline viral load.
Of 404 patients with neurologic disorders, HAND, CNS-OI, and DSP were diagnosed in 102, 47, and 165 patients. There were 20 and 10 AIDS-related deaths in the HAND and CNS-OI groups. Similar to the presence of any neurologic disorder, the presence of HAND and CNS-OI increased the risk of mortality by approximately 3- and 5-fold (table 2 and figure 3, B and C), after accounting for demographic, immunologic, and virologic variables in Cox proportional hazard model. In contrast, there were only 10 AIDS-related deaths in 165 patients with DSP and the mortality rate was not related to DSP (table 2). Similar to the analysis with neurologic disorders, HR values of baseline CD4+ T-cell levels or viral load in HAND, CNS-OI, and DSP analyses were associated with the increased mortality (table 2). Each 100 cell/mm3 decrement in baseline CD8+ T-cell levels increased the risk of death in patients with HAND or CNS-OI (table 2). Age, gender, ethnicity, and HIV risk factors did not influence the risk of death (table e-3).
DISCUSSION
During this 10-year study period after the introduction of cART into clinical use, neurologic disorders represented a substantial and cumulative disease burden, which influenced survival among HIV-infected patients. Although the incidence of neurologic disorders, especially DSP and HAND, decreased over time, the incidence of other common disorders, such as CNS-OI, which had a strong negative prognostic effect on mortality, remained unchanged. The low baseline and nadir levels of CD4+ T cells were correlated with the prevalence of more than one neurologic disorder. Patients with severe immunosuppression (CD4 <200) were more likely to develop HIV-related neurologic disorders, especially DSP, HAND, seizures, and CNS-OI. These findings imply that despite the availability of cART, baseline and nadir CD4+ T-cell levels remain strong predictors of neurologic disease, while CD8+ T cells and plasma viral load levels were less powerful predictors. Herein, CNS-OIs had the greatest negative impact on survival of patients followed by HAND and the mere presence of any neurologic disorder. However, DSP, which was the most common neurologic disorder, did not influence mortality rate in HIV-infected patients. In keeping with earlier studies,1 both immunologic and virologic variables were independent risk factors for AIDS-related death.
The current study is the first report of the prevalence and incidence of all neurologic disorders in persons with HIV/AIDS over a decade in the cART era within a population-based setting. Indeed, the overall prevalences of neuropathy and seizures in the present cohort were substantially greater than in the general Canadian population (6.3% and 0.6%).21,22 The occurrence of neurocognitive impairment herein was comparable to the general population who were 20 years older than the current study group.21 However, the prevalence of DSP and HAND was lower than in the pre-cART era and consistent with published reports.3,4,7 The incidences of DSP and HAND as the first neurologic disorder were lower in the latter 5 years compared to the initial 5 years of the study, possibly indicating the increased effectiveness of cART in the prevention of neurologic disorders. The DSP incidence in our study appeared lower than a previous study7 likely because it was the incidence of the first neurologic disorder and there might be differing diagnostic criteria.
The differences in the prevalence of neurocognitive impairment in HIV-infected individuals in different studies lie in the population selection and study design. Tozzi et al.8 reported a 55.1% prevalence of HIV-related neurocognitive impairment in 432 patients undergoing their first neuropsychological tests in an institute which provides care to more than 3,500 HIV-infected individuals. A recent study of HAND prevalence in HIV-infected individuals with suppressed viremia by Simioni et al.23 using questionnaire and neuropsychological tests estimated the prevalence of HAND at 69%; however, the majority of patients with HAND had asymptomatic neurocognitive impairment. In the present analysis, clinical symptoms (e.g., symptomatic neuropathy and neurocognitive impairment) were required for inclusion. Nonetheless, strict criteria were used for excluding other comorbidities which might contribute to peripheral neuropathy or neurocognitive impairment and only the principal confirmed diagnosis was reported. Patients with diabetes or on medications such as metronidazole for protracted periods were excluded from the DSP group. Similarly, patients were excluded from the HAND group if they had previous head injury, drug abuse, or were using opioid analgesics or other medications which might affect cognition. In addition, neurologic diagnoses were made after patients have been referred by SAC physicians, perhaps resulting in underreporting of less severe symptoms. These factors might contribute to the lower prevalences of DSP and HAND compared with other recruited cohorts or studies conducted in neurology clinics.8,9,11,23
Unlike DSP and HAND, the prevalence and incidence of several neurologic disorders herein remain unchanged in the cART era. The incidence of mononeuropathy was consistent during the study period, likely because immunologic and virologic variables were less robust risk factors for this disorder. The prevalence and incidence of seizures/epilepsy in this study were similar to a published report that compared the prevalence and incidence before and after the cART era.24 In contrast to other studies,3,25,26 a reduction of CNS-OI incidence was not observed during this 10-year study, likely because of the relatively low incidence in the present study population. Although the prevalence of headache/migraine herein was lower than previous studies in the pre- or early cART era,27,28 it was not associated with CD4+ T-cell or plasma viral load levels. The prevalence of myopathy and movement disorder was low, similar to previous reports.11,29 Due to limited patient numbers, the incidence of these latter disorders requires further analyses in order to understand fully the benefits of cART.
This report represents a regionally based longitudinal cohort and thus the findings reflect prevalences, incidences, and mortality rates in a broad spectrum (age, sex, ethnocultural) of HIV-infected adults. The annual mortality rates in HIV-infected persons with or without a neurologic disorder were 17.6 and 8.0 per 1,000 people, which were higher than 1.5 per 1,000 people in the general age- and sex-matched Canadian population.30 These data imply that the presence of neurologic disorders at any time during the follow-up period is an important predictor for mortality after accounting for age, sex, ethnicity, HIV risk factor, baseline CD4+, baseline CD8+ T cell, and baseline viral load. The estimated HRs for HAND and CNS-OI in the present studies were similar to recruited cohorts.15,16 This latter finding suggests that the small number of AIDS-related deaths in our patients with CNS-OI is not a limiting factor in our conclusion. Moreover, this report is the first analysis of the survival of HIV-infected patients with all neurologic disorders after accounting for demographic and clinical variables. Previous studies have reported that CD8+ T-cell levels contribute to neurocognitive impairment, the onset of AIDS, and survival in HIV-infected patients,31–33 while another study indicated that the level of CD8+ T cells did not influence patients' survival time.34 Survival analyses which included HAND or CNS-OI as covariates showed that the level of CD8+ T cells at the initial presentation was an independent risk factor for AIDS-related death, implying a substantial effect of CD8+ T-cell levels on HIV pathogenesis.
Nevertheless, the initial CD4+ and CD8+ T-cell levels and plasma viral load did not reflect the change in immunologic and virologic variables over time. Baseline CD4+ T-cell levels and HAND were time-dependent variables. Although lower baseline CD4+ T-cell levels are related to decreased survival rates initially, the relationship becomes less robust after 9 years of survival time. As a result, the estimated HR for baseline CD4+ T-cell levels underestimates the effects during the first 9 years. The relationship between HAND and survival is less evident for the first 7 years of follow-up due to the low number of deaths in this period but thereafter HAND becomes an increasingly strong predictor of mortality. Importantly, the levels of CD4+ T cells and viral load were recorded herein every 3 to 4 months throughout the follow-up period. Future studies of the changes in immunologic and virologic variables in relation to cART use might yield a better understanding of their specific roles in prevalence and severity of neurologic disorders as well as patient survival.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Giseon Heo and Jennifer Gamble.
ACKNOWLEDGMENT
The authors thank Drs. Sumit Majumdar and Tom Marrie for discussions; Leah DeBlock and Krista Nelles for assistance with patients, word processing, and manuscript submission; Danette Mohagen and Ian Cosman for data acquisition; and the staff and patients at Southern Alberta Clinic for their cooperation.
DISCLOSURE
Dr. Vivithanaporn receives postdoctoral fellowship support from the Alberta Heritage Foundation for Medical Research. Dr. Heo, J. Gamble, and Dr. Krentz report no disclosures. Dr. Hoke serves as an Associate Editor of Experimental Neurology and on the editorial board of the Journal of the Peripheral Nervous System; receives research from the NIH (R01 NS43991 [PI]) and the Foundation for Peripheral Neuropathy; and has received license fee payments from Johnson & Johnson for a patent re: Immortalized DRG neuronal cell line. Dr. Gill serves on scientific advisory boards for GlaxoSmithKline, Merck Serono, Gilead Sciences, Inc., and Abbott; and has received speaker honoraria from Abbott. Dr. Power serves on a scientific advisory board for the Canadian Institutes of Health Research (Chair, Institute of Infection and Immunity); serves on the editorial board of the Canadian Journal of Neurological Sciences; holds patents re: Antisense oligodeoxynucleotides regulating expression of TNF-α; and receives research support from the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research.
Supplementary Material
Address correspondence and reprint requests to Dr. Christopher Power, Division of Neurology, Department of Medicine, 6-11 Heritage Medical Research Centre, University of Alberta, Edmonton, AB T6G 2S2, Canada chris.power@ualberta.ca
Supplemental data at www.neurology.org
e-Pub ahead of print on August 25, 2010, at www.neurology.org.
Study funding: Supported by the Canadian Institutes of Health Research (C.P.) and the Alberta Heritage Foundation for Medical Research (C.P. and P.V.).
Disclosure: Author disclosures are provided at the end of the article.
Received December 12, 2009. Accepted in final form May 20, 2010.
REFERENCES
- 1.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet 2003;362:22–29. [DOI] [PubMed] [Google Scholar]
- 2.Authier FJ, Chariot P, Gherardi RK. Skeletal muscle involvement in human immunodeficiency virus (HIV)-infected patients in the era of highly active antiretroviral therapy (HAART). Muscle Nerve 2005;32:247–260. [DOI] [PubMed] [Google Scholar]
- 3.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol 2002;8(suppl 2):115–121. [DOI] [PubMed] [Google Scholar]
- 4.McArthur JC, Haughey N, Gartner S, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol 2003;9:205–221. [DOI] [PubMed] [Google Scholar]
- 5.Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS 2003;17:1539–1545. [DOI] [PubMed] [Google Scholar]
- 6.Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis 2008;197(suppl 3):S294–S306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lichtenstein KA, Armon C, Baron A, Moorman AC, Wood KC, Holmberg SD. Modification of the incidence of drug-associated symmetrical peripheral neuropathy by host and disease factors in the HIV outpatient study cohort. Clin Infect Dis 2005;40:148–157. [DOI] [PubMed] [Google Scholar]
- 8.Tozzi V, Balestra P, Lorenzini P, et al. Prevalence and risk factors for human immunodeficiency virus-associated neurocognitive impairment, 1996 to 2002: results from an urban observational cohort. J Neurovirol 2005;11:265–273. [DOI] [PubMed] [Google Scholar]
- 9.Smyth K, Affandi JS, McArthur JC, et al. Prevalence of and risk factors for HIV-associated neuropathy in Melbourne, Australia 1993–2006. HIV Med 2007;8:367–373. [DOI] [PubMed] [Google Scholar]
- 10.Pettersen JA, Jones G, Worthington C, et al. Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: protease inhibitor-mediated neurotoxicity. Ann Neurol 2006;59:816–824. [DOI] [PubMed] [Google Scholar]
- 11.Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J Neurol Neurosurg Psychiatry 2000;69:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Childs EA, Lyles RH, Selnes OA, et al. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology 1999;52:607–613. [DOI] [PubMed] [Google Scholar]
- 13.Valcour V, Yee P, Williams AE, et al. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection: The Hawaii Aging with HIV Cohort. J Neurovirol 2006;12:387–391. [DOI] [PubMed] [Google Scholar]
- 14.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007;21:1915–1921. [DOI] [PubMed] [Google Scholar]
- 15.Mocroft A, Sterne JA, Egger M, et al. Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal. Clin Infect Dis 2009;48:1138–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tozzi V, Balestra P, Serraino D, et al. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retroviruses 2005;21:706–713. [DOI] [PubMed] [Google Scholar]
- 17.Power C, Boisse L, Rourke S, Gill MJ. NeuroAIDS: an evolving epidemic. Can J Neurol Sci 2009;36:285–295. [DOI] [PubMed] [Google Scholar]
- 18.Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection: report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 1991;41:778–785. [DOI] [PubMed] [Google Scholar]
- 19.Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder: The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurology 1996;47:1247–1253. [DOI] [PubMed] [Google Scholar]
- 20.Therneau T, Grambsch P. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 21.The Burden of Neurological Diseases, Disorders, and Injuries in Canada. Ottawa: Canadian Institute of Health Information; 2007. [Google Scholar]
- 22.Toth C, Lander J, Wiebe S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med 2009;10:918–929. [DOI] [PubMed] [Google Scholar]
- 23.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010;24:1243–1250. [DOI] [PubMed] [Google Scholar]
- 24.Kellinghaus C, Engbring C, Kovac S, et al. Frequency of seizures and epilepsy in neurological HIV-infected patients. Seizure 2008;17:27–33. [DOI] [PubMed] [Google Scholar]
- 25.Engsig FN, Hansen AB, Omland LH, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis 2009;199:77–83. [DOI] [PubMed] [Google Scholar]
- 26.Khanna N, Elzi L, Mueller NJ, et al. Incidence and outcome of progressive multifocal leukoencephalopathy over 20 years of the Swiss HIV Cohort Study. Clin Infect Dis 2009;48:1459–1466. [DOI] [PubMed] [Google Scholar]
- 27.Evers S, Wibbeke B, Reichelt D, Suhr B, Brilla R, Husstedt I. The impact of HIV infection on primary headache: unexpected findings from retrospective, cross-sectional, and prospective analyses Pain 2000;85:191–200. [DOI] [PubMed] [Google Scholar]
- 28.Mirsattari SM, Power C, Nath A. Primary headaches in HIV-infected patients. Headache 1999;39:3–10. [DOI] [PubMed] [Google Scholar]
- 29.Cardoso F. HIV-related movement disorders: epidemiology, pathogenesis and management. CNS Drugs 2002;16:663–668. [DOI] [PubMed] [Google Scholar]
- 30.Tambussi G, Gori A, Capiluppi B, et al. Neurological symptoms during primary human immunodeficiency virus (HIV) infection correlate with high levels of HIV RNA in cerebrospinal fluid. Clin Infect Dis 2000;30:962–965. [DOI] [PubMed] [Google Scholar]
- 31.Madden LJ, Zandonatti MA, Flynn CT, et al. CD8+ cell depletion amplifies the acute retroviral syndrome. J Neurovirol 2004;10(suppl 1):58–66. [DOI] [PubMed] [Google Scholar]
- 32.Stebbing J, Bower M, Mandalia S, Nelson M, Gazzard B. Highly active anti-retroviral therapy (HAART)-induced maintenance of adaptive but not innate immune parameters is associated with protection from HIV-induced mortality. Clin Exp Immunol 2006;145:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez-Ramon S, Bellon JM, Resino S, et al. Low blood CD8+ T-lymphocytes and high circulating monocytes are predictors of HIV-1-associated progressive encephalopathy in children. Pediatrics 2003;111:E168–175. [DOI] [PubMed] [Google Scholar]
- 34.Schellens IM, Borghans JA, Jansen CA, et al. Abundance of early functional HIV-specific CD8+ T cells does not predict AIDS-free survival time. PLoS One 2008;3:e2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.