Cerebroretinal vasculopathy (CRV) is an adult-onset autosomal dominant disorder involving the microvessels of the brain and retina due to frameshift mutations in the gene TREX1.1,2 Patients present with vision loss, seizures, hemiparesis, apraxia, dysarthria, or memory loss. There is progression to blindness, a neurovegetative state, and death within 5 to 10 years.1 MRI reveals a tumor-like lesion in approximately half of patients, resembling a primary brain malignancy, while in the other half there are multiple small white matter lesions which may be misdiagnosed as demyelinating disease. The purpose of this report is to heighten awareness of this disease and to note near resolution of the tumor-like lesion.
Case report.
A 44-year-old woman had daily tension-type headaches and chronic sinusitis since 2006. In June 2007, she had transient right nasal visual field blurring. Visual acuity remained intact. Neurologic examination had normal results.
In 2007, a multi-kindred study2 identified the causative mutation of CRV as frameshifts in the C-terminus of the TREX1 gene. The patient was found to carry the V235fs mutation (3688_3689insG). Her father, paternal grandmother, and extended kindred had CRV (n = 27, 4 with active disease, 23 died due to CRV, major family in reference 1). At the age of 43, her father presented with hemiparesis and a tumor-like lesion. He died of progressive disease complicated by steroid-associated bleeding from gastrointestinal ulcers at age 49. Her paternal grandmother had a brain lesion resected, believed to worsen after radiation, and died in her mid-50s.
In January 2008, the patient underwent MRI to evaluate persistent headaches (figure). Retinal examination showed microvascular disease with minimal progression on repeat examinations. Laboratory studies were normal except for mild elevation of liver transaminases (aspartate aminotransferase 57 μ/L, alanine aminotransferase 49 μ/L) and C-reactive protein.
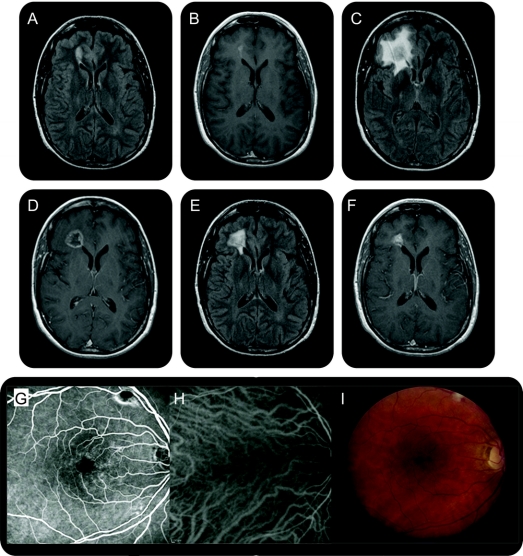
Figure Brain and retinal imaging in a patient with cerebroretinal vasculopathy
Sequential axial MRI showing an ovoid T2-hyperintense (A) and gadolinium-enhancing (B) lesion (2.1 × 1 cm), abutting the frontal horn of the right lateral ventricle, bright on both diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) map. At 6 months, a larger, more aggressive-appearing lesion (2 × 2 × 3 cm) with surrounding edema occupied the right frontal lobe. There was a central zone of presumed necrosis and gadolinium enhancement of the lesion rim (C, D). Diffusion imaging showed more heterogeneous signal, without the characteristic bright DWI and dark ADC signal of acute infarction. At 12 months, the lesion approximated its size on initial imaging (1.4 × 1.2 × 0.9 cm) with a persistent rim pattern of enhancement (E, F). At 18 months, the lesion further decreased in size (1.1 × 0.9 × 0.3 cm) with near resolution of the surrounding edema. Fluorescein angiography and indocyanine green with corresponding color photographs of the retina show views of the macula of the right eye (G–I). Periarteriolar narrowing and sheathing, focal leakage, telangiectasias, and cotton wool spots are present.
Medications included daily fluticasone nasal spray, dexamethasone 8 mg IM once for sinusitis (15 months after the initial MRI), levothyroxine for hypothyroidism, pregabalin for fibromyalgia, buspirone for depression, clonazepam as needed for anxiety, trazodone as needed for sleep, and aspirin for cardiovascular disease prevention and potential benefit in CRV.
Discussion.
This case extends the phenotype of patients with CRV: cerebral tumor-like lesions may improve considerably with time, even if they recur, extend, and progress in later years. Furthermore, neuroimaging findings in CRV may occur without accompanying neurologic impairment.
Nonmalignant lesions that may appear as tumor-like lesions include tumefactive demyelinating disease, cerebritis, abscess, immune reconstitution syndrome in AIDS, Sjögren syndrome, and systemic lupus erythematosus (SLE). In some cases, clinical history and neuroimaging features may narrow the diagnosis. In CRV, cortical sparing is consistent. The proclivity of the tumor-like lesion to occur in the right frontal region, found in different kindreds including this case and her father, remains unexplained.
CRV was first reported in 19881 in a family with an unusual “family history of brain tumors.” After identification of the causative mutation in 2007, 3 diseases—CRV; hereditary endotheliopathy and retinopathy, nephropathy, and stroke; and autosomal dominant vascular retinopathy, migraine, and Raynaud phenomenon—were all found to share C-terminal frameshift mutations in the DNA exonuclease, TREX1.2 These previously separate conditions are now considered the same genetic disorder: autosomal dominant retinal vasculopathy with cerebral leukodystrophy (RVCL; OMIM 192315).3
How TREX1 mutants lead to the pathology observed in RVCL is unknown. TREX1 encodes the major intracellular 3′->5′ DNA exonuclease of mammals4 and responds to oxidative stress by translocation into the nucleus.5,6 Patients with RVCL have truncations of the C-terminal domain due to frameshift insertions in the TREX1 gene which lead to mislocalized but functional exonucleases.2,4–6 Other mutations in TREX1 exist with neurologic consequences. These include the autosomal recessive Aicardi-Goutières syndrome (AGS)7 and some patients who meet criteria for SLE with autoantibody positivity.e1 These conditions do not involve tumor-like lesions but have other signs suggestive of a disrupted blood–brain barrier, including T2 hyperintense lesions on MRI (SLE) and an inflammatory process in the CSF (AGS).
Although our patient did not undergo biopsy of the lesion, the clinical history, ophthalmologic findings, autosomal dominant pattern of inheritance, and identification of the TREX1 mutation were diagnostic. Histopathologic examination of a tumor-like lesion in other patients with CRV,1,2,e2 including her father and grandmother, has shown no evidence of infection, malignancy, granulomas, or amyloid.
Treatment of these tumor-like lesions is unclear. The authors have seen no benefit from plasma exchange, IV immunoglobulin, or chronic immunosuppression. Steroids lessen surrounding edema but are not believed to reduce the underlying lesion, which is progressive in nature. In this patient, the tumor-like lesion substantially regressed without treatment, a previously unreported characteristic of CRV, with near-resolution over an 18-month period.
Supplementary Material
Supplemental data at www.neurology.org
References e1 and e2 are available on the Neurology® Web site at www.neurology.org.
Disclosure: Dr. Mateen served on the editorial board of the Resident & Fellow Section of Neurology® and is supported by the 2010 Practice Research Grant from the American Academy of Neurology. Dr. Krecke, Dr. Younge, and Dr. Ford report no disclosures. Dr. Shaikh received an NIH Rheumatology Training Grant (T32 AR07279). Dr. Kothari receives an NIH/NHLBI Pre-doctoral Training Grant (T32 HL083822). Dr. Atkinson serves on scientific advisory boards for Taligen Therapeutics and Complement Technology, Inc.; serves as an Associate Editor of Molecular Immunology, a Section Editor for Immunobiology, and on the editorial boards of the Journal of Biological Chemistry, the American Journal of Medicine, Biology Direct, The Complement Bulletin, and the Journal of Allergy and Clinical Immunology; holds/has filed patents re: DNA sequences encoding human membrane cofactor protein, Recombinantly produced human membrane cofactor protein: pharmaceutical compositions and methods of inhibiting complement activity, Modified complement system regulators, Complement system regulators, Treatment method using recombinantly produced human membrane cofactor protein, Modified complement system regulators, and Cells expressing a modified regulator of complement activation; receives publishing royalties from UpToDate, Inc.; receives research support from the NIH (5 RO1 AI037618 [PI], RO1 AI041592 [PI], P30 AR48335 [Protein Core Director], U19 AI070489 [Project Leader], R21 AG029907 [Subcontractor], R01 NS062069 [Subcontractor], and T32 AR07279 [PI]), and the American Asthma Foundation (Formerly Sandler Program for Asthma Research); and holds stock in Taligen Therapeutics.
Received October 5, 2009. Accepted in final form May 12, 2010.
Address correspondence and reprint requests to Dr. Farrah J. Mateen, Department of Neurology, Johns Hopkins Hospital, Pathology Room 509, 600 N. Wolfe Street, Baltimore, MD 21287; Fmateen@jhsph.edu
&NA;
- 1.Grand MG, Kaine J, Fulling K, et al. Cerebroretinal vasculopathy: a new hereditary syndrome. Ophthalmology 1988;95:649–659. [DOI] [PubMed] [Google Scholar]
- 2.Richards A, van den Maagdenberg AM, Jen JC, et al. C-terminal truncations in human 3′-5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat Genet 2007; 39:1068–1070. [DOI] [PubMed] [Google Scholar]
- 3.Entrez-Pubmed Gene References into Function. Available at: http://www.ncbi.nlm.nih.gov/sites/entrez?cmd_current=&db=gene&orig_db=genome&term=trex1&cmd=. Accessed August 30, 2009.
- 4.Mazur DJ, Perrino FW. Identification and expression of the TREX1 and TREX2 cDNA sequences encoding mammalian 3′->5′ exonucleases. J Biol Chem 1999;274:19655–19660. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury D, Beresford PJ, Zhu P, et al. The exonuclease TREX1 is in the SET complex and acts in concert with NM23-H1 to degrade DNA during granzyme A-mediated cell death. Mol Cell 2006;23:133–142. [DOI] [PubMed] [Google Scholar]
- 6.Yang YG, Lindahl T, Barnes DE. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 2007;131:873–886. [DOI] [PubMed] [Google Scholar]
- 7.Crow YJ, Hayward BE, Parmar R, et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat Genet 2006;38:917–920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.